Introduction
A number of primary breast cancers arise from the
mammary gland; however, occasionally tumors exhibit metaplastic
features with a variety of histologic findings (1–8) or
apocrine metaplasia which is termed apocrine carcinoma (AC)
(9,10). Squamous cell carcinomas (SCCs) of
metaplasic breast cancers and ACs contain cancer cells with
eosinophilic cytoplasm, which complicates the pathomorphological
differentiation, although the tumor grade varies between the two
cancer types. Moreover, SCCs are often triple-negative (TN)
cancers, negative for the expression of the estrogen receptor (ER),
progesterone receptor (PgR) and human epidermal growth factor
receptor 2 (HER2) and certain ACs are TN cancers (11). However, the prognosis of AC is
favourable (12), whereas the
prognosis for SCCs is generally poor (13). In the present report,
pathomorphological and immunohistochemical (IHC) data that was
obtained from SCC exhibiting apocrine features were analyzed with a
focus on the histological characteristics of the cancer cells.
Case report
Clinical summary
A 68-year-old female attended St. Mary’s Hospital
(Kurume, Japan) to undergo examination of an ulcerated lesion of
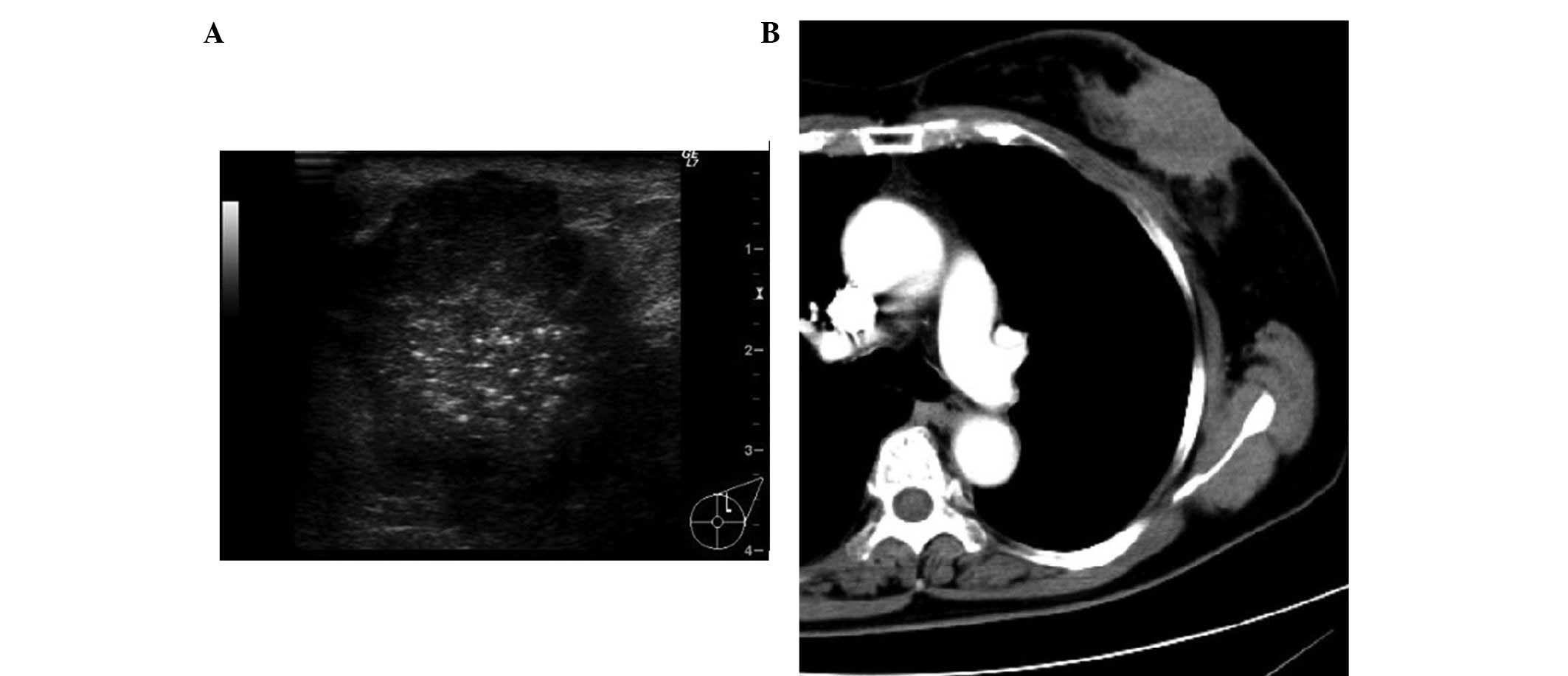
the right breast. Ultrasound imaging demonstrated a lobulated solid
tumor ~40×30 mm containing multiple stippled calcifications in the
left mammary gland (Fig. 1A). A
computed tomography scan displayed a heterogeneously hypodense mass
lesion in the left gland and indicated multiple enlarged lymph
nodes in the left axilla (Fig. 1B).
Magnetic resonance imaging identified a lesion of irregular mass,
measuring 66 × 68 × 47 mm in addition to skin infiltration, located
in the left breast. Furthermore, a contrast-enhanced dynamic study
of the lesion exhibited hyperintensities during the early phase and
was marginally washed out in the delayed phase, six min
post-contrast. The lesion was therefore considered to be advanced
breast cancer and a mastectomy was performed.
Postoperative period
In the postoperative period, the patient did not
consent to receiving chemotherapy; however, the follow-up
procedures were continued. A mass lesion resulted from the surgical
wound three months postoperatively and was subsequently removed.
The pathological findings of the mass were consistent with those of
tumor relapse. The patient was informed repeatedly concerning
chemotherapy, however, did not provide consent. Eight months
following surgery, the patient developed radiographically evident
metastasis of the lung and the liver. The patient was informed
again regarding chemotherapy and consented to treatment; the
patient received epirubicin (75 mg/m2), fluorouracil
(500 mg/m2) and cyclophosphamide (500 mg/m2)
for four, 21-day cycles. At the time of writing, the patient was
receiving the first cycle of docetaxel (60 mg/m2), was
classified as Performance Status 1 (http://ecog.dfci.harvard.edu/general/perf_stat.html)
and was leading an independent life.
Pathological findings
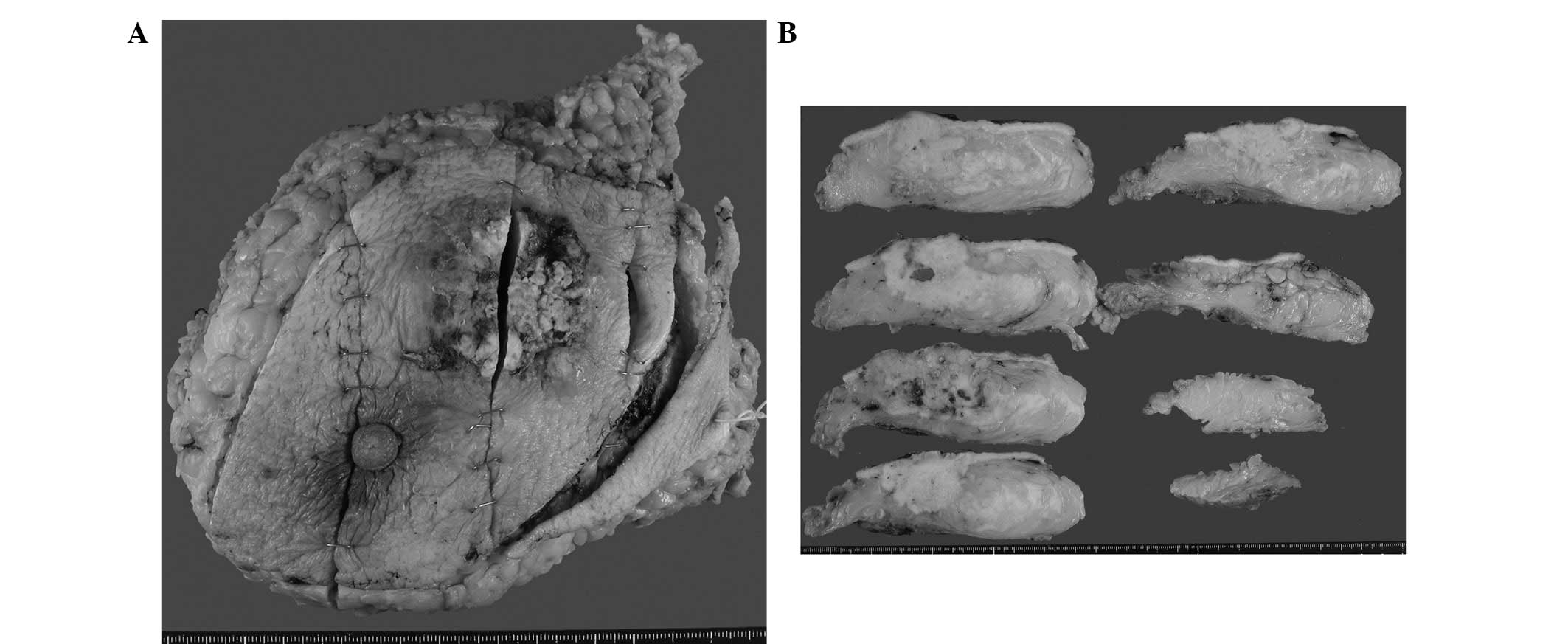
Macroscopic investigations identified the lesion as
white and solid, measuring 61×27 mm, which exhibited an extensive
area of necrosis (Fig. 2A and B).
The lesion was identified as neoplastic, via histological analysis,
and was predominantly composed of tumor cells arranged in nests
with solid regions of varying size and shape. The lesion exhibited
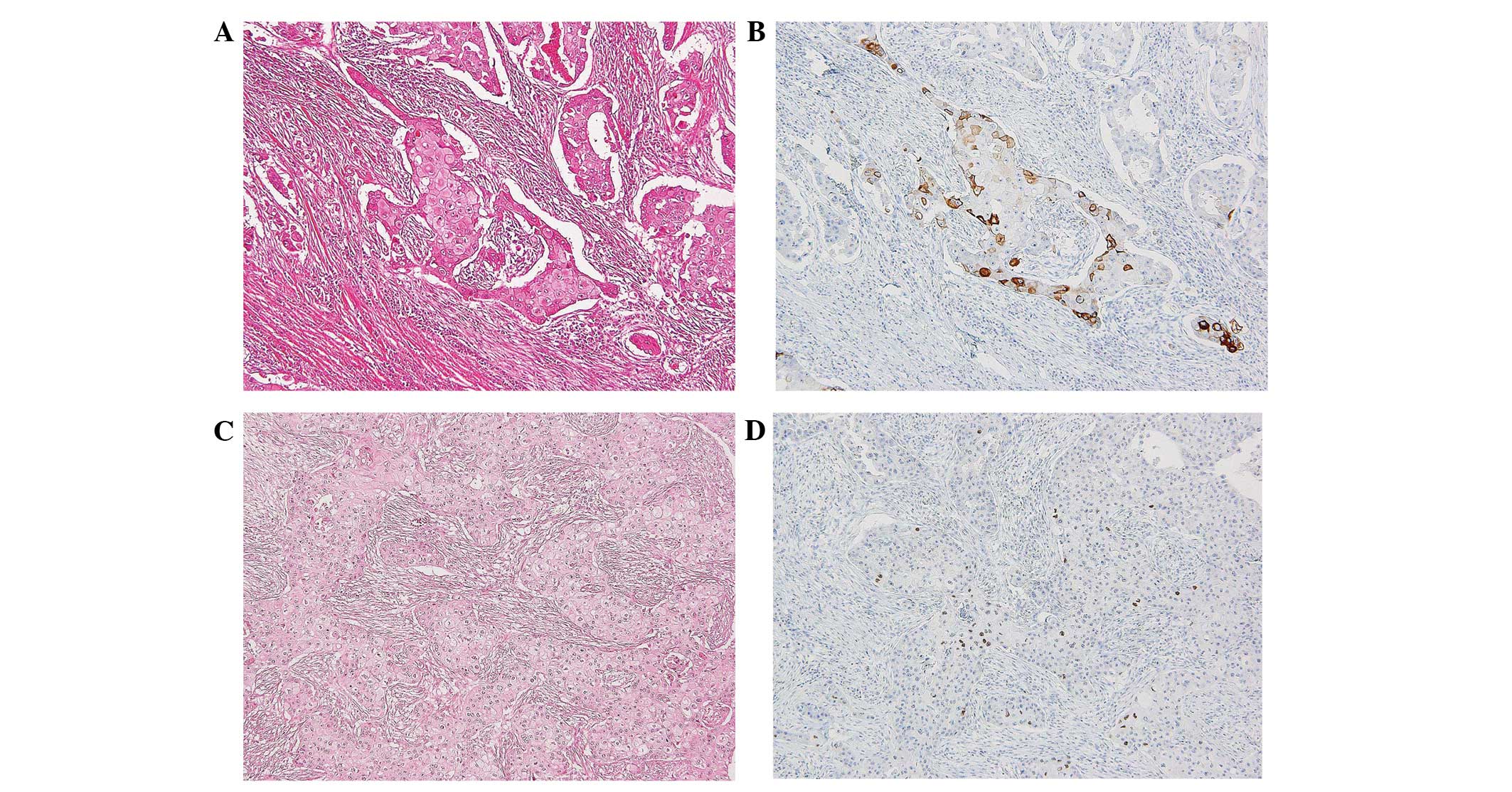
moderate glandular lumen formation and comedo-like necrosis
(Figs. 3A and 4B), which extended into the surrounding
ducts and infiltrated the adipose tissue and skin. The numerous
cancer cells that formed such nests exhibited nuclear enlargement,
nuclear pleomorphism and an increased nuclear to cytoplasmic ratio,
in addition to intracellular keratinization and intercellular
bridges. The IHC analysis identified the cancer cells as positive
for cytokeratin (CK) 5/6, 34βE12 and P63. The lesion exhibited
squamous features (Fig. 3A–D) with
a histological grade of three and was negative for the expression
of ER, PgR and HER2; the tumor was a triple-negative breast cancer.
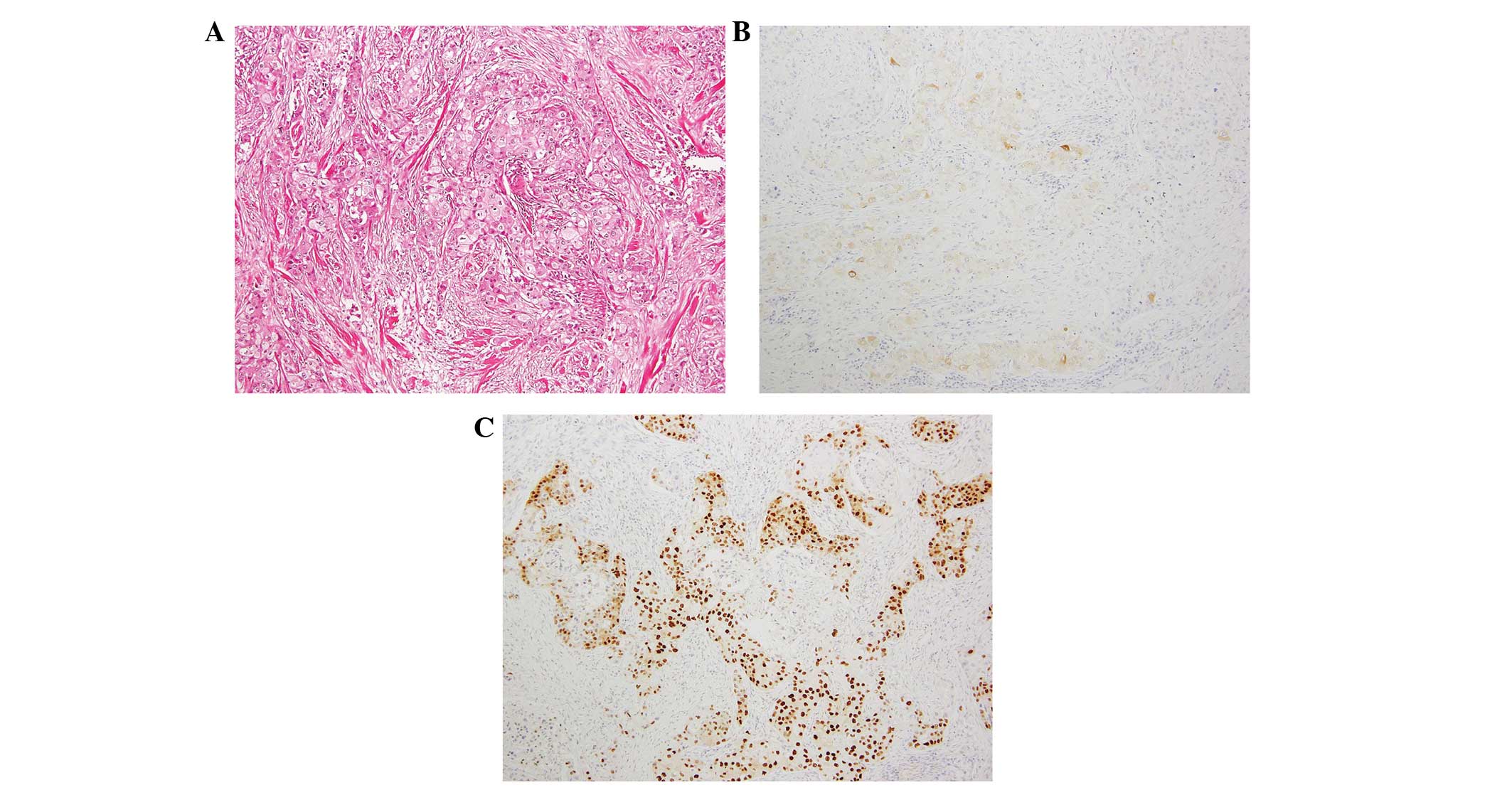
In contrast to the pathomorphology of pure squamous features,
approximately one-third of the tumor area was invaded by cancer
cells with abundant eosinophilic cytoplasm and a positive
expression for gross cystic disease fluid protein-15 (GCDFP-15).
The androgen receptor (AR) was expressed in the cytoplasm and/or
the nucleus within the tumor cells (Fig. 4A–C); thus, the tumor appeared to
exhibit apocrine features. Based on the histological analysis, the
lesion was diagnosed as an SCC of the breast, with apocrine
features and documented lymph node metastasis. Written informed
consent was obtained from the patient.
Discussion
SCC is generally characterized by histologically
identified keratinization and intercellular bridges (2,14). AC
is defined as a carcinoma in which >90% of tumor cells exhibit
abundant eosinophilic cytoplasm (15,16).
Although histological classification is generally based on the
structure and status of a tumor, AC is classified according to the
tumor cell findings rather than the tumor structure. In the present
case, the cancer cells exhibited abundant eosinophilic cytoplasm
containing eosinophilic granules, thus demonstrating apocrine
differentiation. These cells, however, did not account for >90%
of total cells, thus the findings were considered to indicate
apocrine features occurring within an SCC.
The IHC analysis identified that more than half of
the squamous cell features expressed high molecular weight keratins
(CK14, CK5/6 and 34βE12) and P63 (14). The present case may, therefore, be
diagnosed as an SCC due to the presence of keratinization and
intercellular bridges, as well as the positive expression of high
molecular weight keratin and P63 (Fig.
3A–D). In addition to the SCC, the apocrine features were
documented pathomorphologically and using IHC staining (Fig. 4A–C). Generally in AC, a
characteristic steroid receptor expression profile defines these
tumors as consistently ER-negative, PR-negative and AR-positive
(17–19), and previous IHC studies identified
that GCDFP-15 was expressed in ~75% of AC cases (19). The patient in the present case was
positive for GCDFP-15, which was consistent with the apocrine
features within the pathomorphologically abundant eosinophilic
cytoplasm. Furthermore, the IHC analysis identified that the lesion
was consistent with a tumor exhibiting apocrine features. With
regard to invasive carcinomas, GCDFP-15 expression has been
identified as significantly lower in small tumors or tumors
exhibiting lymph node metastasis (20). Previous studies have suggested that
compared with invasive carcinomas, ACs are not associated with a
significant difference in survival rate; however, they exhibit
reduced lymph node metastasis and lymphatic invasion, and an
improved prognosis and treatment response (21,22).
Thus, it was suggested that the patient in the present case, may
experience an improved treatment response as the tumor exhibited
apocrine features (pathomorphologically and in the IHC analysis);
however, tumor relapse and metastasis occurred in the patient at an
early stage. The factors that contribute to tumor relapse and
metastasis include a large tumor diameter, the presence of lymph
node metastasis and the dominance of a high-grade SCC (2,14).
Identification of histological subtypes is required for tumor
grading in cases where differentiation between squamous and
apocrine features is complex.
AR, in addition to GCDFP-15, is an effective IHC
marker for identifying apocrine features and is frequently
expressed in AC (11,18,23);
in the present case, the AR was expressed in the nucleus and/or the
cytoplasm of the tumor cells. Although the analysis performed was
not able to explicitly distinguish between the squamous and
apocrine features, the expression of AR may be significant in
tumors that exhibit squamous and apocrine features. Further
investigation is required to clarify this observation for pre- and
post-operative adjuvant treatments.
In conclusion, although the differentiation between
SCC and AC using hematoxylin and eosin staining is complex,
particularly when eosinophilic cells are predominant within the
tumor, performing IHC staining for markers (such as GCDFP-15 and
AR) may be essential for the grading of tumors and the development
of appropriate treatment.
References
|
1
|
Rosen PP: Carcinoma with metaplasia.
Rosen’s Breast Pathology. 3rd edition. Lippincott Williams and
Wilkins; Philadelphia, PA: pp. 470–505. 2009
|
|
2
|
Yamaguchi R, Horii R, Maeda I, et al:
Clinicopathologic study of 53 metaplastic breast carcinomas: their
elements and prognostic implications. Hum Pathol. 41:679–685. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wargotz ES and Norris HJ: Metaplastic
carcinomas of the breast. I Matrix-producing carcinoma. Hum Pathol.
20:628–635. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wargotz ES, Deos PH and Norris HJ:
Metaplastic carcinomas of the breast. II Spindle cell carcinoma.
Hum Pathol. 20:732–740. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wargotz ES and Norris HJ: Metaplastic
carcinomas of the breast. III Carcinosarcoma. Cancer. 64:1490–1499.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wargotz ES and Norris HJ: Metaplastic
carcinomas of the breast. IV Squamous cell carcinoma of ductal
origin. Cancer. 65:272–276. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wargotz ES and Norris HJ: Metaplastic
carcinomas of the breast: V. Metaplastic carcinoma with
osteoclastic giant cells. Hum Pathol. 21:1142–1150. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weigelt B and Reis-Filho JS: Histological
and molecular types of breast cancer: is there a unifying taxonomy?
Nat Rev Clin Oncol. 6:718–730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmitt FC and Reis-Filho JS: Oncogenes,
granules and breast cancer: what has c-myc to do with apocrine
changes? Breast. 11:463–465. 2002. View Article : Google Scholar
|
|
10
|
Selim AG and Wells CA: Immunohistochemical
localisation of androgen receptor in apocrine metaplasia and
apocrine adenosis of the breast: relation to oestrogen and
progesterone receptors. J Clin Pathol. 52:838–841. 1999. View Article : Google Scholar
|
|
11
|
Tsutsumi Y: Apocrine carcinoma as
triple-negative breast cancer: novel definition of apocrine-type
carcinoma as estrogen/progesterone receptor-negative and androgen
receptor-positive invasive ductal carcinoma. Jpn J Clin Oncol.
42:375–386. 2012. View Article : Google Scholar
|
|
12
|
Japaze H, Emina J, Diaz C, et al: ‘Pure’
invasive apocrine carcinoma of the breast: a new
clinicopathological entity? Breast. 14:3–10. 2005.
|
|
13
|
Toikkanen S: Primary squamous cell
carcinoma of the breast. Cancer. 48:1629–1632. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamaguchi R, Tanaka M, Kondo K, et al:
Immunohistochemical study of metaplastic carcinoma and central
acellular carcinoma of the breast: central acellular carcinoma is
related to metaplastic carcinoma. Med Mol Morphol. 45:14–21. 2012.
View Article : Google Scholar
|
|
15
|
Frable WJ and Kay S: Carcinoma of the
breast. Histologic and clinical features of apocrine tumors.
Cancer. 21:756–763. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abati AD, Kimmel M and Rosen PP: Apocrine
mammary carcinoma. A clinicopathologic study of 72 cases. Am J Clin
Pathol. 94:371–377. 1990.PubMed/NCBI
|
|
17
|
Vranic S, Tawfik O, Palazzo J, et al: EGFR
and HER-2/neu expression in invasive apocrine carcinoma of the
breast. Mod Pathol. 23:644–653. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gatalica Z: Immunohistochemical analysis
of apocrine breast lesions. Consistent over-expression of androgen
receptor accompanied by the loss of estrogen and progesterone
receptors in apocrine metaplasia and apocrine carcinoma in situ.
Pathol Res Pract. 193:753–758. 1997.
|
|
19
|
Niemeier LA, Dabbs DJ, Beriwal S, Striebel
JM and Bhargava R: Androgen receptor in breast cancer: expression
in estrogen receptor-positive tumors and in estrogen
receptor-negative tumors with apocrine differentiation. Mod Pathol.
23:205–212. 2010. View Article : Google Scholar
|
|
20
|
Honma N, Takubo K, Akiyama F, et al:
Expression of GCDFP-15 and AR decreases in larger or node-positive
apocrine carcinomas of the breast. Histopathology. 47:195–201.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanaka K, Imoto S, Wada N, Sakemura N and
Hasebe K: Invasive apocrine carcinoma of the breast:
clinicopathologic features of 57 patients. Breast J. 14:164–168.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogiya A, Horii R, Osako T, et al: Apocrine
metaplasia of breast cancer: clinicopathological features and
predicting response. Breast Cancer. 17:290–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sapp M, Malik A and Hanna W: Hormone
receptor profile of apocrine lesions of the breast. Breast J.
9:335–336. 2003. View Article : Google Scholar : PubMed/NCBI
|