Introduction
Hyperthermia is a type of cancer treatment in which
tissue is exposed to high temperatures. Previous studies have shown
that high temperatures can damage and kill cancer cells, usually
with minimal injury to normal tissues (1). As hyperthermia can kill cancer cells
and damage proteins and cellular structures, it is able to shrink
tumors (1–3). Conventional hyperthermia cannot
precisely focus on tumors and results in fat hyperpyrexia (2–4). In
order to achieve an improved clinical outcome, magnet-mediated
hyperthermia was developed to induce localized heating in response
to focused radio waves (3–4). This method has become a novel
antitumor therapy. In this method, thermoseeds are implanted inside
the tumor, followed by the application of a magnetic field to heat
the thermoseeds. As a result, the heat is transferred from the
thermoseeds to the surrounding tissue, causing a rise in
temperature that is necessary for treating the tumor. Compared with
traditional hyperthermia, thermoseed-induced hyperthermia is a
reproducible process, which offers the capability to control the
local temperature in vivo (3–4).
The abscopal effect, or remote effects, were
identified during a study of hyperthermia-induced treatment of
tumors. In this phenomenon, local treatment of a tumor can affect
tumor growth at distant sites in the body. Previous studies using
animal models have demonstrated that hyperthermia treatment for the
primary tumor caused the ablation of metastatic tumors (4–7).
Similarly, following the treatment of metastatic tumors, the
primary tumor could also be ablated (4–7).
In 1965, Strauss et al (8) described the abscopal effect of tumor
thermotherapy. Subsequently, it became clear that the immune system
was also involved in this phenomenon (4–5). The
magnetite thermoseed-induced hyperthermia method has been applied
to investigate the effects of local hyperthermia therapy (4–7). In
our previous study, we showed that magnetic induction of
hyperthermia not only promoted local tumor-cell killing, but also
significantly induced the metastatic tumor-cell killing effects of
radiotherapy in breast cancer (4).
In the present experiment, we established an experimental model of
Walker-256 carcinosarcoma cells in Wistar rats. Sarcomas were
chosen, as they are more resistant to radiotherapy and chemotherapy
than carcinomas.
The present study aimed to analyze the thermodynamic
and antitumor characteristics of magnet-mediated hyperthermia at
two different temperature ranges (42–46°C and 50–55°C). We
hypothesized that a high therapeutic temperature of
magnetic-mediated hyperthermia may improve the effectiveness of
hyperthermia treatment on carcinosarcomas.
Materials and methods
Materials
RPMI-1640 culture medium was purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA), calf blood serum
was obtained from Sigma-Aldrich (St. Louis, MO, USA) and
formaldehyde solution was from Beijing Dongxu Factory (Beijing,
China). Immunohistochemistry reagents, mouse anti-proliferating
cell nuclear antigen (PCNA) primary antibody, PV6002 secondary
antibody and enzyme-linked immunosorbent assay (ELISA) kits for rat
interferon (IFN)-γ and interleukin (IL)-2 were purchased from Wuhan
Boshide Biological Engineering Co., Ltd. (Wuhan, China), Zhongshan
Jinqiao Biotechnology Co., Ltd. (Beijing, China) and Invitrogen
Life Technologies, respectively. Flow cytometry reagents,
phycoerythrin (PE)-conjugated anti-CD4 and -CD8 single-staining
antibodies were provided from Zhongshan Jinqiao Biotechnology Co.,
Ltd. Thermoseeds, comprised of nickel-copper alloy (72:27%) with a
Curie point of 57°C (0.9 mm in diameter and 1.1 cm in length), were
fabricated by the Beijing University of Science and Technology
(Beijing, China) in cooperation with the Research Laboratory of
Metal Physics, Tsinghua University (Beijing, China). This study was
approved by the Ethics Committee of Xiangya Medical School of
Central South University (Changsha, China).
Equipment
A magnet-mediated prototype machine for clinical
care was provided by Shuangping Instrument Technology, Co., Ltd.
(Shenzhen, China). The temperature survey and recording system were
purchased from Physitemp Instruments Inc. (Clifton, NJ, USA). Other
equipment included: Forma™ 3111 CO2 incubator (Thermo
Fisher Scientific, Inc., Waltham, MA, USA), DL-CJ-1N high
performance aseptic laboratory bench (Harbin Donglian Electronic
Technology Development Co., Ltd., Harbin, China), electronic
balance (Mettler-Toledo Instruments, Columbus, OH, USA), a vernier
caliper and a Sigma 3–18K high-speed centrifuge 3–18 K (G&M
Scientific, Ltd., Livingston, UK).
Establishment of the experimental animal
model
Walker-256 carcinosarcoma cells were purchased from
the Institute of Pharmacology, Chinese Academy of Medical Sciences
(Beijing, China) and were preserved in liquid nitrogen until use.
Forty healthy male Wistar rats (age, 6 weeks; body weight, 110–130
g) were purchased from Beijing Weitong Lihua Laboratory Animal
Center (Beijing, China), used under license number SCXK (Beijing)
2007-0001 and housed at a constant temperature of 23±2°C. For
routine recovery of cells, the Walker-256 carcinosarcoma cells were
centrifuged at 225 × g for 7 min and the supernatant was discarded.
The pellet was washed twice in phosphate-buffered saline (PBS),
suspended and 1 ml of the suspension was injected into the rat
abdominal cavity. For tumor inoculation, ascetic fluid was removed
from the rats and centrifuged at 225 × g for 7 min, and the
supernatant was discarded. The cells were then resuspended in
serum-free RPMI-1640 culture medium, centrifuged at 225 × g for 7
min and the supernatant was discarded. Cell pellets were
resuspended in normal saline and counted; cell numbers were
adjusted to 1×107 and 2×107 per ml. Cell
suspension (0.2 ml) was administered via subcutaneous injection
into the hind legs of the rats; the left leg received a higher
concentration, whereas the right leg received a lower
concentration. After 7–10 days, tumor growth was evident and the
rats were randomly divided into five experimental groups.
Experimental groups
The rats were randomly divided into three different
control groups, groups C, M and T. Group C served as the untreated
control group. Group M was the magnetic field control group and was
used to compare the magnetic field treatment samples. The magnetic
field was applied to the back or shoulder of the rats for 30 min.
The thermoseed control group (group T) received two thermoseeds (~1
cm in length) implanted into the largest tumor on the surface of
the shoulder or back of the rat. Group T was not exposed to a
magnetic field to heat the thermoseeds. For the two magnet-mediated
hyperthermia treatment groups (groups H1 and H2), several
thermoseeds were implanted into one of the hind legs of the rats.
We then randomly divided the rats into group H1, to which a
magnetic field was applied to heat the thermoseeds to 42–46°C for
30 min (Curie point, 57°C), and group H2, in which the thermoseeds
were heated to 50–55°C for 10 min (Curie point, 70°C). Between 42
to 45°C, apoptosis is the main form of cell death, when the
temperature is >46°C, the number of necrotic cells is markedly
increased. Basic thermal dose biological effects correlate with
temperature and time. In order to allow a comparison between the
conventional temperature hyperthermia group and the high
temperature hyperthermia group, the Arrhenius equation, which
describes the correlation between temperature and the chemical
reaction rate, was used to calculate the temperature ranges for the
two groups which are capable of achieving the biological heating
effect at the same level: H1, thermoseeds heated to 42–46°C for 30
min; H2, thermoseeds heated to 50–55°C for 10 min. As these groups
are capable of maintaining the same biological effects, they were
used in the experiments. All control and experimental groups
contained eight rats per group.
Implantation of the thermoseeds
The thermoseeds (1.0 mm in diameter and 1.0 cm in
length) were implanted in parallel and ~0.5 cm apart in a tumor
(~1.5 cm in diameter) in each rat. Following implantation, X-rays
were performed to verify the location and direction of the
implanted thermoseeds.
Magnet-mediated hyperthermia
The rats were anesthetized with 2% barbital sodium
(2.3 ml/kg; Sigma-Aldrich) prior to hyperthermia treatment. The
rats were then placed under the magnetic field and the body
temperatures were measured rectally.
Rats in group M were exposed to the magnetic field
for 30 min; rats in groups H1 and H2 were placed under the magnetic
field to ensure that the major axis of the thermoseeds and the
reversal magnetic field direction were parallel. Three electric
thermocouples were inserted in order to monitor the temperature at
the center of the tumor, at the tumor edge and the body
temperature, separately. A fixed electric current (50 Hz) was
applied to groups H1 and H2 to heat the thermoseeds (for ~2 min to
heat to the desired temperature). The treatment was maintained for
30 min for the H1 group and 10 min for the H2 group,
respectively.
Pathological observations
Fourteen days after the treatment, four rats were
randomly selected from each group and sacrificed by an
intrapertoneal injection of barbital sodium (Sigma-Aldrich). The
tumors were removed, fixed in 10% formalin and paraffin-embedded
sections were prepared. Hematoxylin and eosin staining of the tumor
tissue was performed and the pathological changes were visualized
under a microscope (Nikon Eclipse Ci-E, Nikon, Beijing, China).
Immunohistochemistry
Paraffin-embedded tumor tissues were then examined
for PCNA protein expression. Tissue sections (5-μm thick) were
prepared and dewaxed using conventional techniques. Sections were
incubated in 0.01 mol/l citrate buffer and microwaved for 15 min,
after which, they were incubated with an anti-PCNA antibody at 4°C
overnight. The subsequent immunohistochemistry steps were performed
in accordance with the SP kit instructions. We performed nuclear
hematoxylin staining. PCNA expression was detected in the nucleus
(brown staining indicated positive cells). We counted the number of
positive cells in 10 random high-power fields. The PCNA index was
calculated as the number of positive tumor cells divided by the
total number of tumor cells.
Flow cytometry for determination of T
lymphocyte subsets
An additional four rats were randomly selected from
each group and sacrificed. Peripheral blood was collected in
EDTA-coated tubes and separated into three samples for incubation
with CD4+ or CD8+ antibodies and with one
sample as the control (2 μl). The samples were incubated at room
temperature in the dark for 20 min and shaken once every 3 min.
Samples were then incubated with 1 ml of 1X erythrocyte lysis for
10 min and centrifuged at 626 × g for 2 min. The supernatant was
discarded and the pellet was washed twice with PBS containing 2%
FBS (Gibco, Beijing, China) and centrifuged at 626 × g for 2 min.
The supernatant was discarded and 500 μl of 4% polyformaldehyde was
added to the tubes and the samples were analyzed by flow cytometry
(F500, Beckman-Coulter, Inc., Beijing, China).
Assessment of tumor growth and rat
survival time
A vernier caliper was used to determine the mean
diameter of the tumors every 2 days. The tumor’s largest diameter
was measured in horizontal (a) and vertical (b) directions. The
tumor volume was calculated as follows: V = (a × b2)/2.
Subsequently, tumor growth curves were calculated for each group of
rats. Changes in the survival were compared based on the number of
days each group of tumor-bearing rats survived.
Data processing and statistical
analysis
We used SPSS software, version 10.0 (SPSS, Inc.,
Chicago, IL, USA) for data processing and statistical analysis. The
size of the tumor in each group was compared using analysis of
variance and data are expressed as the means ± standard deviation.
The CD4+, CD8+,
CD4+/CD8+ subsets were analyzed by the
log-rank test with two-sided P-values. P<0.05 was considered to
indicate a statistically significant difference.
Results
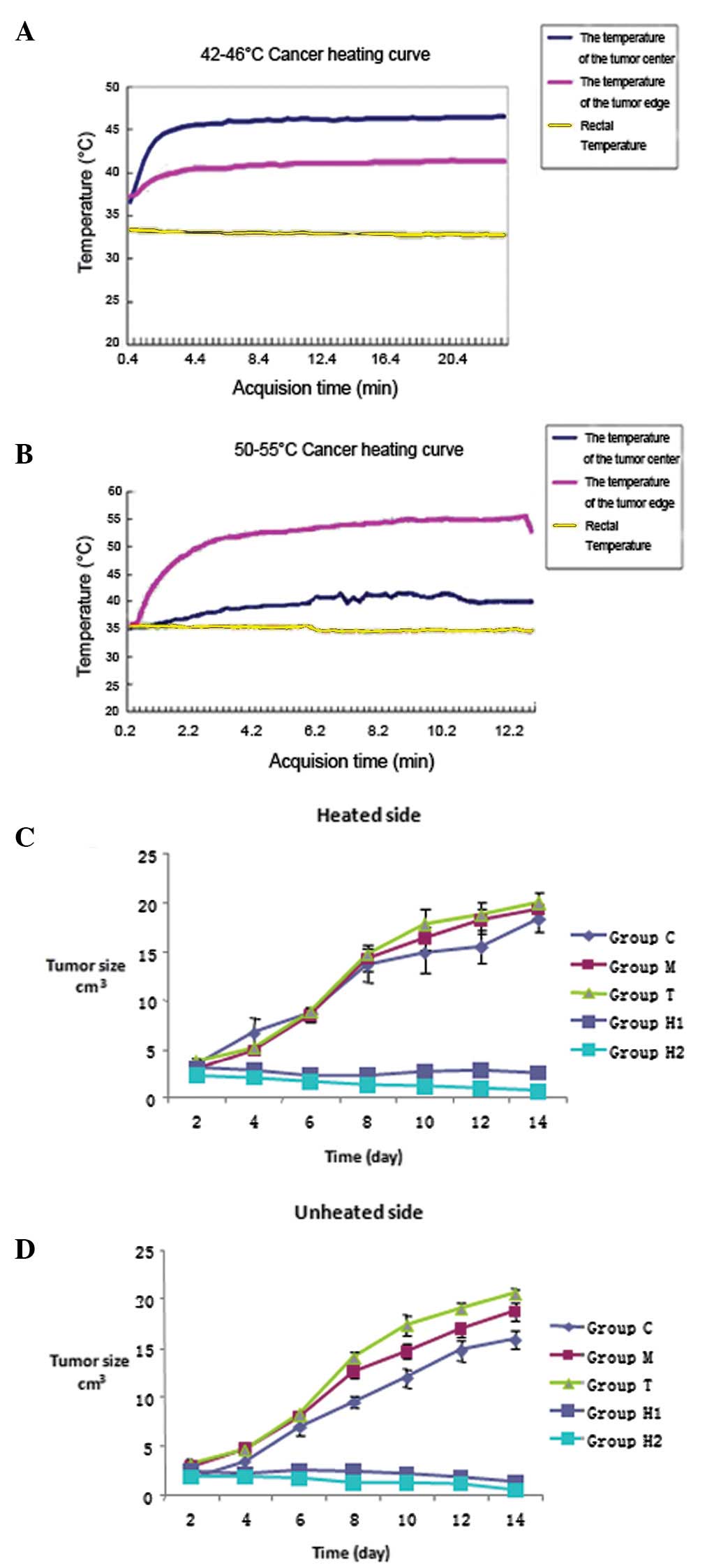
Effects of applying an alternating
magnetic field to thermoseeds on local temperature
By using the different Curie temperatures of the
thermoseeds, it was possible to increase the temperature within the
tumor to 46 or 50°C within 5 min. By controlling the electric
current of the alternating magnetic fields, we were able to
maintain the temperature within the ranges of 42–46°C and 50–55°C.
For the H1 and H2 groups, the rectal temperature was maintained at
35–37°C (Fig. 1A and B).
Effects of hyperthermia treatment on
tumor growth in rats
Following thermal treatment, tumor growth on both
sides of the rats was inhibited in the H1 and H2 groups. We
measured the tumor diameter every 2 days to determine the growth
curves of the tumors on both sides. We identified that compared
with groups C, M and T, tumor growth in groups H1 and H2 was
significantly inhibited (P<0.05) (Table I). Furthermore, compared with the
control group, the inhibition of tumor growth was more effective in
the H2 group than in group H1 (P<0.01). However, there were no
significant differences in tumor growth between groups C, M and T
(P>0.05) (Fig. 1C and D,
Table I).
 | Table ITumor size in each experimental group
(n=10 per group). |
Table I
Tumor size in each experimental group
(n=10 per group).
| | Day |
|---|
| |
|
|---|
| Groups | Location | 2 | 4 | 6 | 8 | 10 | 12 | 14 |
|---|
| C | Left side | 3.33±0.75 | 6.64±1.52 | 8.61±0.71 | 13.62±1.71 | 14.89±1.96 | 15.51±1.64 | 18.33±1.43 |
| Right side | 1.66±0.39 | 3.33±1.05 | 6.88±1.80 | 9.46±0.57 | 11.90±0.85 | 14.68±0.99 | 15.79±0.97 |
| M | Left side | 2.86±0.42 | 4.88±0.44 | 8.37±0.55 | 14.19±1.16 | 16.32±1.24 | 18.15±1.28 | 19.35±0.41 |
| Right side | 2.86±0.42 | 4.72±0.43 | 7.94±0.27 | 12.52±0.64 | 14.67±0.81 | 16.86±0.79 | 18.70±0.91 |
| T | Left side | 3.72±0.28 | 5.04±0.42 | 8.76±0.43 | 14.75±2.43 | 17.85±1.46 | 18.85±1.56 | 20.08±0.94 |
| Right side | 3.12±0.45 | 4.45±0.51 | 8.24±0.38 | 14.95±0.56 | 17.31±1.10 | 19.02±0.58 | 20.53±0.43 |
| H1 | Left side | 2.98±0.35 | 2.74±0.48 | 2.18±0.31 | 2.27±0.14a | 2.64±0.22a | 2.78±0.13a | 2.45±0.22a |
| Right side | 2.44±0.36 | 2.12±0.50 | 2.48±0.25 | 2.42±0.17 | 2.21±0.16c | 1.78±0.16c | 1.31±0.07c |
| H2 | Left side | 2.18±0.41 | 2.03±0.33 | 1.62±0.34 | 1.22±0.30 | 1.10±0.31a | 0.91±0.27a | 0.60±0.13b |
| Right side | 1.87±0.39 | 1.81±0.13 | 1.75±0.16c | 1.25±0.41c | 1.20±0.14c | 1.12±0.03c | 0.43±0.17d |
Histological observation
In the control group, the tumor tissues on both
sides contained typical tumor cells; the nuclei were large and
deeply stained. Tumor cells were mostly round or oval-shaped,
showing expansive growth. The tumor showed vascular invasion in the
control group (Fig. 2A1 and B1). In
the H1 group, tumor cells, which were directly exposed to heat,
exhibited large areas of necrosis and karyorrhexis. On the unheated
side, necrosis of the tumor cells was also visible (Fig. 2C1 and D1). In the H2 group, tumor
cells also showed a large area of necrosis. Notably, the unheated
side exhibited increased necrosis compared with that of the heated
side (Fig. 2E1 and F1).
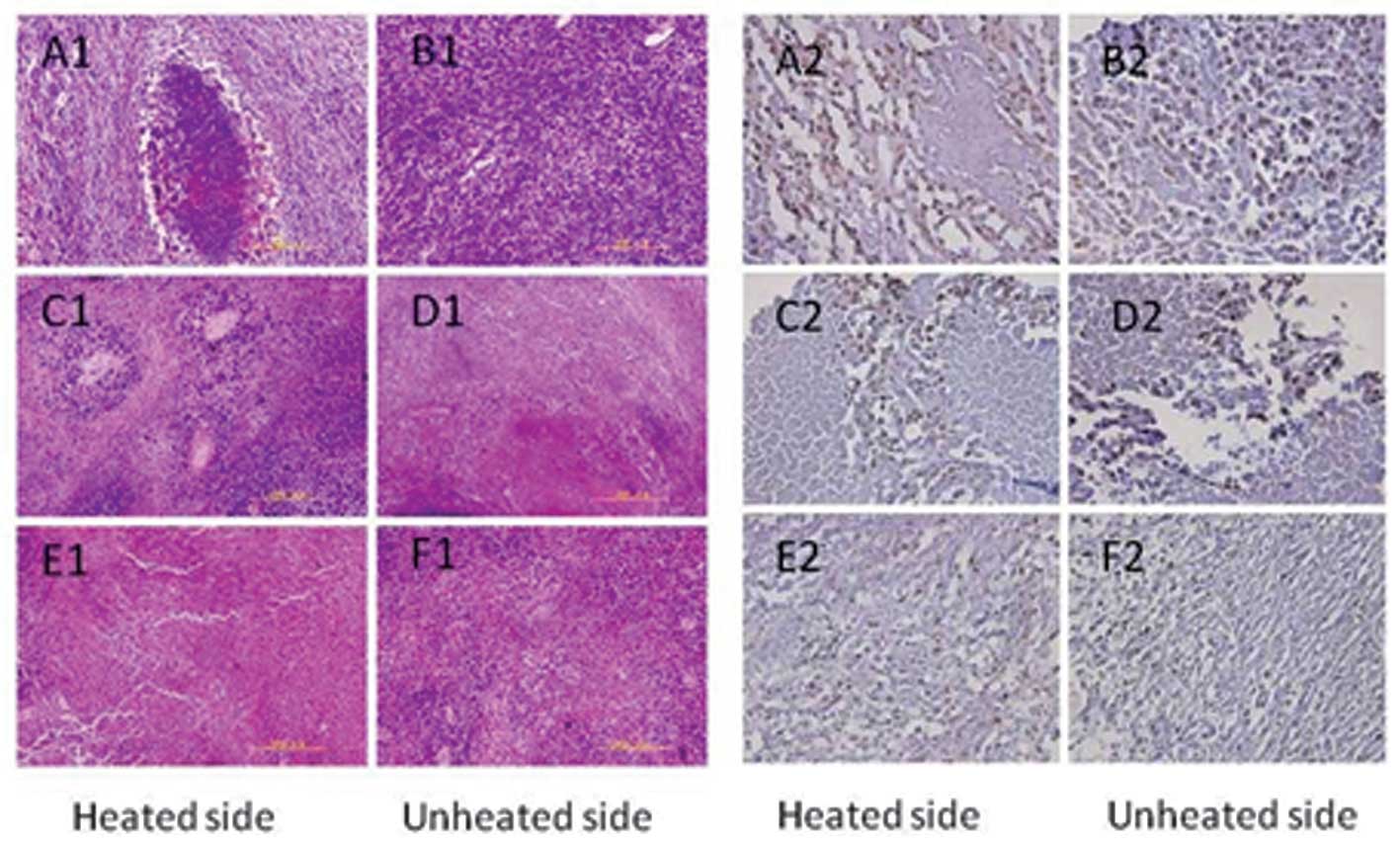 | Figure 2Pathological observation and
immunohistochemistry in the control group (C), and hyperthermia
groups 1 (H1) and 2 (H2). In the control group, both sides of the
tumor tissue contained typical tumor cells (A1, B1, both unheated);
in the H1 group, the tumor cells exposed to heat exhibited large
areas of necrosis and karyorrhexis (C1, heated side; D1, unheated
side); in the H2 group, tumor cells showed a large area of
necrosis. The unheated side showed more necrosis than the heated
side (E1, heated side; F1, unheated side). Compared with groups C,
M and T, the PCNA index was significantly decreased in the H1 group
(C2, heated side; D2, unheated side) and the H2 group (E2, heated
side; F2, unheated side) (P<0.05). Compared with the H1 group,
the PCNA index in the H2 group was significantly decreased
(P<0.01), but there was no significant difference in the PCNA
indexes between groups C, M and T (P>0.05). PCNA, proliferating
cell nuclear antigen. |
Immunohistochemistry
Compared with groups C, M and T, the PCNA index was
significantly decreased in the H1 and H2 groups (P<0.05). In
addition, compared with the H1 group, the PCNA index in the H2
group was significantly decreased (P<0.01), but there were no
significant differences in the PCNA indexes between groups C, M and
T (P>0.05) (Table II, Figs. 2A2–F2 and 3A).
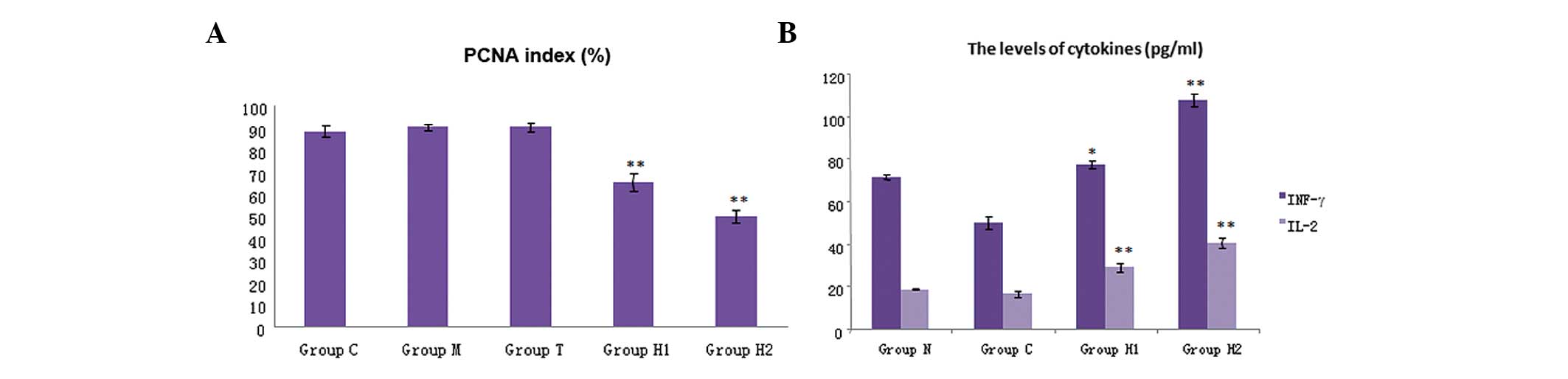 | Figure 3Immunohistochemistry analysis
indicated that (A) PCNA expression was decreased significantly in
the hyperthermia groups, H1 and H2, compared with that of the
control. Compared with groups C, M and T, the PCNA index was
significantly decreased (P<0.05). Compared with the H1 group,
the PCNA index in the H2 group was significantly decreased
(P<0.05). (B) The levels of cytokines in the five groups. IFN-γ
and IL-2 were significantly higher in the H1 and H2 groups compared
with the three control groups (P<0.05); and there was also a
significant difference between the H1 and H2 group (P<0.01).
Levels of cytokines, IFN- γ and IL-2, were markedly increased in
the H2 group. PCNA, proliferating cell nuclear antigen; IFN-γ,
interferon-γ; IL-2, interleukin-2; group C, untreated control;
group M, magnetic field control; group T, thermoseed control; group
H1, thermoseeds heated to 42–46°C for 30 min; group H2, thermoseeds
heated to 50–55°C for 10 min. |
 | Table IIPCNA index in each group (n=10). |
Table II
PCNA index in each group (n=10).
| Groups | PCNA index (mean ±
SD) |
|---|
| C | 88.12±2.69 |
| T | 89.86±1.24 |
| M | 89.63±1.87 |
| H1 | 65.15±3.93b |
| H2 | 49.55±2.62b |
Flow cytometry of T lymphocyte
subpopulations
Results of the flow cytometry for subpopulations of
T lymphocytes are shown in Fig. 3B.
We demonstrated that the levels of CD4+ and
CD8+ were significantly increased in the H1 and H2
groups compared with those of the three control groups; the
increase in CD8+ cells was higher than that of the
CD4+ cells. The CD4+/CD8+ ratio
decreased in the H1 and H2 groups compared with that of the control
groups. CD8+ T cells play a major role in immune
regulation, particularly in the cytotoxic response to tumor
tissues. The ratio of CD4+/CD8+ T lymphocyte
subsets in the H1 and H2 groups was significantly increased
(particularly in the H2 group) compared with that of groups M and
C. There were also significant differences in the ratio of
CD4+/CD8+ T lymphocytes between the H1 and H2
groups (Table III).
 | Table IIIFlow cytometry for subpopulation of T
lymphocytes. |
Table III
Flow cytometry for subpopulation of T
lymphocytes.
| Groups | CD4+T (%) | CD8+T (%) | CD4+T/CD8+T
(%) |
|---|
| M | 39.56±0.59 | 34.61±0.93 | 1.14±0.04 |
| C | 26.01±2.68 | 61.07±2.04 | 0.43±0.04 |
| H1 | 38.36±1.36a | 50.96±2.17a | 0.75±0.03a |
| H2 | 62.21±1.77b | 45.32±1.63b | 1.37±0.02b |
Cytokine levels
The cytokine levels in the five groups are shown in
Fig. 3B. The levels of IFN-γ and
IL-2 were significantly higher in the H1 and H2 groups compared
with those of the three control groups (P<0.05). A significant
difference was also identified between groups H1 and H2 (P<0.01)
(Table IV); the levels of IFN-γ
and IL-2 in the H2 group were higher than those in the H1 group
(Fig. 3B). These results indicated
that magnetic-induced hyperthermia can stimulate the immune system
to release cytokines.
 | Table IVLevel of cytokines in each group. |
Table IV
Level of cytokines in each group.
| Groups | IFN-γ | IL-2 |
|---|
| M | 71.37±0.94 | 18.72±0.36 |
| C | 49.91±2.71 | 16.71±1.61 |
| H1 | 77.33±1.83a | 28.97±2.03b |
| H2 | 107.74±2.93b | 40.41±2.44b |
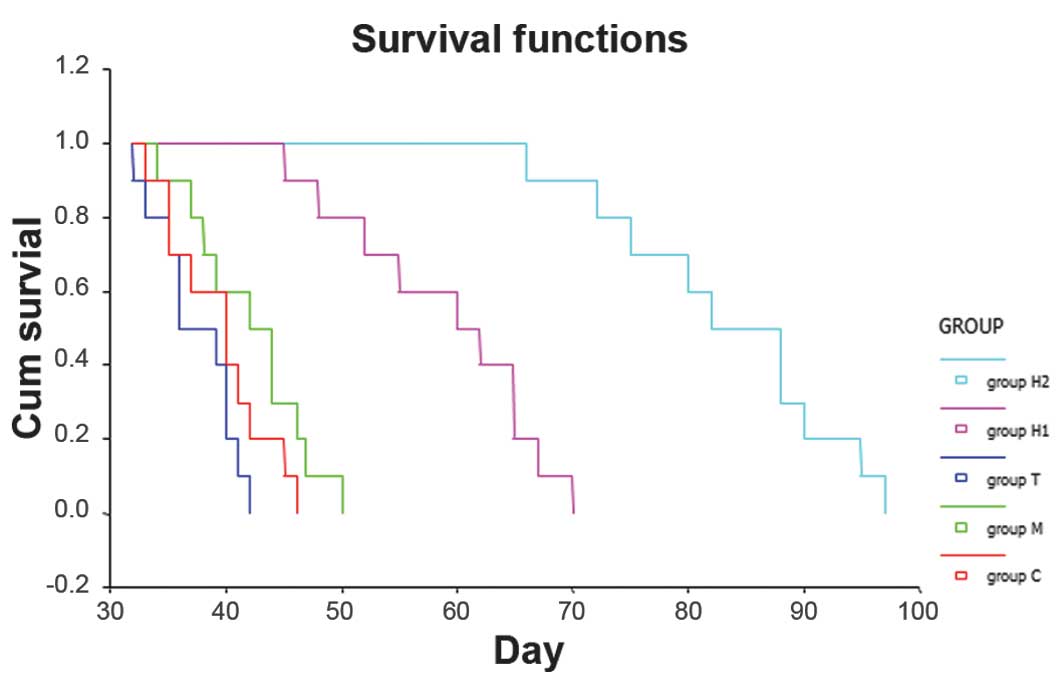
Effects on the survival of the rats after
treatment
The mean survival of groups C, M and T was
27.33±1.40, 42.10±4.10 and 37.40±3.00 days, respectively. There
were no significant differences between the three control groups
(P>0.05). Compared with group C, the survival time of the rats
in the H1 and H2 groups was significantly prolonged (P<0.05).
The mean survival time in the H1 and H2 groups was 58.90±7.12 and
83.30±8.30 days, respectively, which were significantly different
(Fig. 4).
Discussion
Magnet-mediated hyperthermia is the process of
directly implanting thermoseeds into tumors and then increasing the
temperature by alternating the magnetic field through the Neel
relaxation mechanism. When exposed to a magnetic field, the
implanted thermoseeds can be specifically heated. The heat can then
be transferred to surrounding tissues and used to increase the
temperature of the tumor tissue, while leaving the normal tissue
mostly unaffected. To date, magnet-mediated hyperthermia may have
several advantages over the conventional techniques currently
employed for regional hyperthermia, including radiofrequency,
microwave or ultrasound methods, which are often limited by their
inability to selectively target tumor tissue. Moreover, the
temperature during hyperthermia can be controlled by altering the
intensity of the magnetic field, which can solidify the tumor
without damaging the surrounding normal tissue. The majority of
previous clinical research regarding hyperthermia treatment has
focused on its effects for prostate cancer and brain cancer
(9–10).
Izawa et al (11) demonstrated thermotherapy experiments
at 60°C for bone tumors. An induction activity of albumen occurred
in terms of bone shape, but there was no change within 10 h.
Therefore, in order to treat bone tumors and kill the tumor cells,
it was vital to maintain temperatures at 50–65°C for 30 min. A
number of biological mechanisms have been proposed to explain these
effects, in particular the role of T cells, which are closely
associated with the function of natural killer (NK) cells and cell
factors. It has been suggested that the p53 gene is particularly
important and can stimulate inflammatory pathways to release tumor
antigen and inflammatory factors, which stimulate cell death. In
addition, lymphocytes, including NK cells, bind with the tumor
antigens and play a major role in stimulating the abscopal effect
(5,12,13).
Experiments focusing on the abscopal effect are increasing in scope
and corresponding changes in treatment options may introduce novel
treatment methods that will greatly aid the current techniques of
tumor therapy. Previous studies have reported that thermotherapy
directly damages tumor cells; however, it can also stimulate the
immune system to inhibit or dispel the microenvironment of the
tumor and introduce antitumor immunity (14–16).
Currently, it has been indicated that hyperthermia stimulates the
re-emergence of immunity via the activity of heat shock protein
(HSP), which is important in antigen processing, antigen binding
and the formation of tumor HSP-peptide complexes. As a result of
the formation of HSP-peptide complexes with major
histocompatibility complex class I molecules, macrophage processing
can occur and the cells become available for cytotoxic T cell
recognition and, thus, generate specific immunity (17–20).
It has previously been demonstrated that hyperthermia significantly
increased the levels of CD4+ and CD8+ T cells
compared with those of the control group, although the
CD4+/CD8+ ratio was lower in the hyperthermia
groups than that of the control group (21). In the present study, we focused on
the ectopic effects of hyperthermia on the growth of
carcinosarcomas and the resulting effects on the immune system. As
characterized by the increased levels of CD4+ and
CD8+ cells and the elevated levels of IFN-γ and IL-2, we
found that hyperthermia stimulated a specific immune response and
enhanced the cellular immune function. The results also showed that
by applying localized hyperthermia, particularly at 50–55°C, the
growth of Walker-256 hypodermic sarcomas was inhibited, with ideal
abscopal effects and upregulation of the immune system. The results
showed that the application of magnet-mediated hyperthermia was an
effective treatment for carcinosarcomas, particularly at 50–55°C.
At this temperature, the growth of the primary and ectopic tumors
was better controlled compared with that of hyperthermia treatment
at 42–46°C. Moreover, hyperthermia at 50–55°C improved the
CD4+/CD8+ ratio, further improved the
cellular immune function and increased the level of immune factors,
fully stimulating the organism’s antineoplastic immune response to
inhibit the primary tumor and ectopic metastases.
In conclusion, the use of magnet-mediated
hyperthermia offers an exciting and novel therapeutic approach for
carcinosarcomas. Magnet-mediated hyperthermia induced the direct
ablation of the target tumor, which was achieved by the heated
thermoseeds and the abscopal effect with induction of the
endogenous antitumor immunity. In this study, we investigated the
effects of two temperatures using magnetic induction, which
suppressed the growth of the carcinosarcoma. We found that the
inhibition of tumor growth was greater in rats exposed to
temperatures of 50–55°C for 10 min compared with that in rats
exposed to 42–46°C for 30 min. We identified that magnet-mediated
hyperthermia was effective in treating carcinosarcomas at a high
temperature (50–55°C) for 10 min and improved the abscopal
antitumor effects as well as stimulating significant endogenous
immune responses in sarcoma-bearing rats.
This study provided novel data indicating that
magnet-mediated hyperthermia can improve abscopal antitumor effects
and stimulate more significant endogenous immune responses in
sarcoma-bearing rats at the higher temperature of 50–55°C.
Acknowledgments
This study was supported by a the National Natural
Science Foundation of China (grant nos. 10775085 and 30571779), the
Science Committee Fund of Beijing (grant no. Z07000200540704) and
the Yuyuan Fund of Tsinghua University (grant no. 20240000519).
References
|
1
|
van der Zee J: Heating the patient: a
promising approach? Ann Oncol. 13:1173–1184. 2002.PubMed/NCBI
|
|
2
|
Hildebrandt B, Wust P, Ahlers O, et al:
The cellular and molecular basis of hyperthermia. Crit Rev Oncol
Hematol. 43:33–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moroz P, Jones SK and Gray BN:
Magnetically mediated hyperthermia: current status and future
directions. Int J Hyperthermia. 18:267–284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Li X, Xi X, Hu B, Zhao L, Liao Y
and Tang J: Effects of magnetic induction hyperthermia and
radiotherapy alone or combined on a murine 4T1 metastatic breast
cancer model. Intl J Hyperthermia. 27:563–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Camphausen K, Moses MA, Ménard C, et al:
Radiation abscopal antitumor effect is mediated through p53. Cancer
Res. 63:1990–1993. 2003.PubMed/NCBI
|
|
6
|
Blanquicett C, Saif MW, Buchsbaum DJ, et
al: Antitumor efficacy of capecitabine and celecoxib in irradiated
and lead-shielded, contralateral human BxPC-3 pancreatic cancer
xenografts: clinical implications of abscopal effects. Clin Cancer
Res. 11:8773–8781. 2005. View Article : Google Scholar
|
|
7
|
Sgouros G, Knox SJ, Joiner MC, Morgan WF
and Kassis AI: MIRD continuing education: Bystander and low
dose-rate effects: are these relevant to radionuclide therapy? J
Nucl Med. 48:1683–1691. 2007. View Article : Google Scholar
|
|
8
|
Strauss AA, Appel M, Saphir O and
Rabinovitz AJ: Immunologic resistance to carcinoma produced by
electrocoagulation. Surg Gynecol Obstet. 121:989–996.
1965.PubMed/NCBI
|
|
9
|
Park BH, Koo BS, Kim YK and Kim MK: The
induction of hyperthermia in rabbit liver by means of duplex
stainless steel thermoseeds. Korean J Radiol. 3:98–104. 2002.
View Article : Google Scholar
|
|
10
|
Tucker RD: Use of interstitial temperature
self-regulating thermal rods in the treatment of prostate cancer. J
Endourol. 17:601–607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Izawa H, Hachiya Y, Kawai T, Muramatsu K,
Narita Y, Ban N and Yoshizawa H: The effect of heat-treated human
bone morphogenetic protein on clinical implantation. Clin Orthop
Relat Res. 390:252–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Demaria S, Ng B, Devitt ML, Babb JS,
Kawashima N, Liebes L and Formenti SC: Ionizing radiation
inhibition of distant untreated tumors (abscopal effect) is immune
mediated. Int J Radiat Oncol Biol Phys. 58:862–870. 2004.
View Article : Google Scholar
|
|
13
|
Van der Meeren A, Monti P, Vandamme M,
Squiban C, Wysocki J and Griffiths N: Abdominal radiation exposure
elicits inflammatory responses and abscopal effects in the lungs of
mice. Radiat Res. 163:144–152. 2005.PubMed/NCBI
|
|
14
|
Srivastava PK and Maki RG: Stress-induced
proteins in immune response to cancer. Curr Top Microbiol Immunol.
167:109–123. 1991.PubMed/NCBI
|
|
15
|
Wells AD, Rai SK, Salvato MS, Band H and
Malkovsky M: Hsp72-mediated augmentation of MHC class I surface
expression and endogenous antigen presentation. Int Immunol.
10:609–617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wells AD and Malkovsky M: Heat shock
proteins, tumor immunogenicity and antigen presentation: an
integrated view. Immunol Today. 21:129–132. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ito A, Shinkai M, Honda H, Yoshikawa K,
Saga S, Wakabayashi T, Yoshida J and Kobayashi T: Heat shock
protein 70 expression induces antitumor immunity during
intracellular hyperthermia using magnetite nanoparticles. Cancer
Immunol Immunother. 52:80–88. 2003.
|
|
18
|
Todryk S, Melcher AA, Hardwick N,
Linardakis E, Bateman A, Colombo MP, Stoppacciaro A and Vile RG:
Heat shock protein 70 induced during tumor cell killing induces Th1
cytokines and targets immature dendritic cell precursors to enhance
antigen uptake. J Immunol. 163:1398–1408. 1999.
|
|
19
|
Ito A, Shinkai M, Honda H, Wakabayashi T,
Yoshida J and Kobayashi T: Augmentation of MHC class I antigen
presentation via heat shock protein expression by hyperthermia.
Cancer Immunol Immunother. 50:515–522. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dressel R, Lübbers M, Walter L, Herr W and
Günther E: Enhanced susceptibility to cytotoxic T lymphocytes
without increase of MHC class I antigen expression after
conditional overexpression of heat shock protein 70 in target
cells. Eur J Immunol. 29:3925–3935. 1999. View Article : Google Scholar
|
|
21
|
Srivastava PK, Udono H, Blachere NE and Li
Z: Heat shock proteins transfer peptides during antigen processing
and CTL priming. Immunogenetics. 39:93–98. 1994. View Article : Google Scholar : PubMed/NCBI
|