Introduction
Vaginal neoplasms account for ~2% of all female
genital neoplasms (1) and are among
the rarest malignancies. Thus, there is little information in the
literature concerning this type of cancer, particularly with regard
to therapy (2). Since the etiology
of vaginal carcinoma is similar to that of carcinoma of the cervix,
patients with advanced vaginal cancer can be treated with the
standard cervical cancer regimen of cisplatin-based chemotherapy.
If chemotherapy lacks efficacy or the patient is in poor physical
condition, then determining the best treatment strategy can be
difficult. Erlotinib is an epidermal growth factor receptor (EGFR)
tyrosine kinase inhibitor (TKI). This drug is widely used in the
treatment of advanced non-small cell lung cancer (NSCLC) and
pancreatic cancer, primarily to disrupt the EGFR signaling pathway.
EGFR is also expressed in cervical cancer and serves as a strong
prognostic indicator (3). Previous
studies have also shown that TKIs may be suitable for use as second
or third lines of therapy for cervical cancer (4). The current report presents the case of
a patient with metastatic vaginal carcinoma who was administered
erlotinib and remained stable for 9 months following the failure of
first-line chemotherapy.
Case report
A 48-year-old female was diagnosed with vaginal
carcinoma in 2009. The patient underwent 15 rounds of radiation
therapy prior to surgery and an additional 15 rounds following
surgery. Later, the patient underwent 6 cycles of chemotherapy with
paclitaxel plus carboplatin. During this period, the patient
experienced grade 2 bone marrow suppression on two separate
occasions. After 6 cycles, imaging indicated remission. The patient
underwent routine disease surveillance, but developed hemoptysis
without fever or chest pain 1 year after diagnosis. Chest computed
tomography (CT) revealed multiple nodules in the lungs. A pair of
dominant soft tissue masses measuring 5 and 3 cm were located in
the left lower lung and right upper lung, respectively. Mediastinal
lymph nodes were enlarged. Carcinoembryonic antigen levels were
elevated to 35.9 mg/dl. Following percutaneous lung biopsy,
pathological examination confirmed a diagnosis of squamous cell
carcinoma associated with the vaginal carcinoma. The patient went
on to receive chemotherapy with paclitaxel (135 mg/m2 on
day 1) plus carboplatin (area under the curve, 5 × 500 mg on day 1)
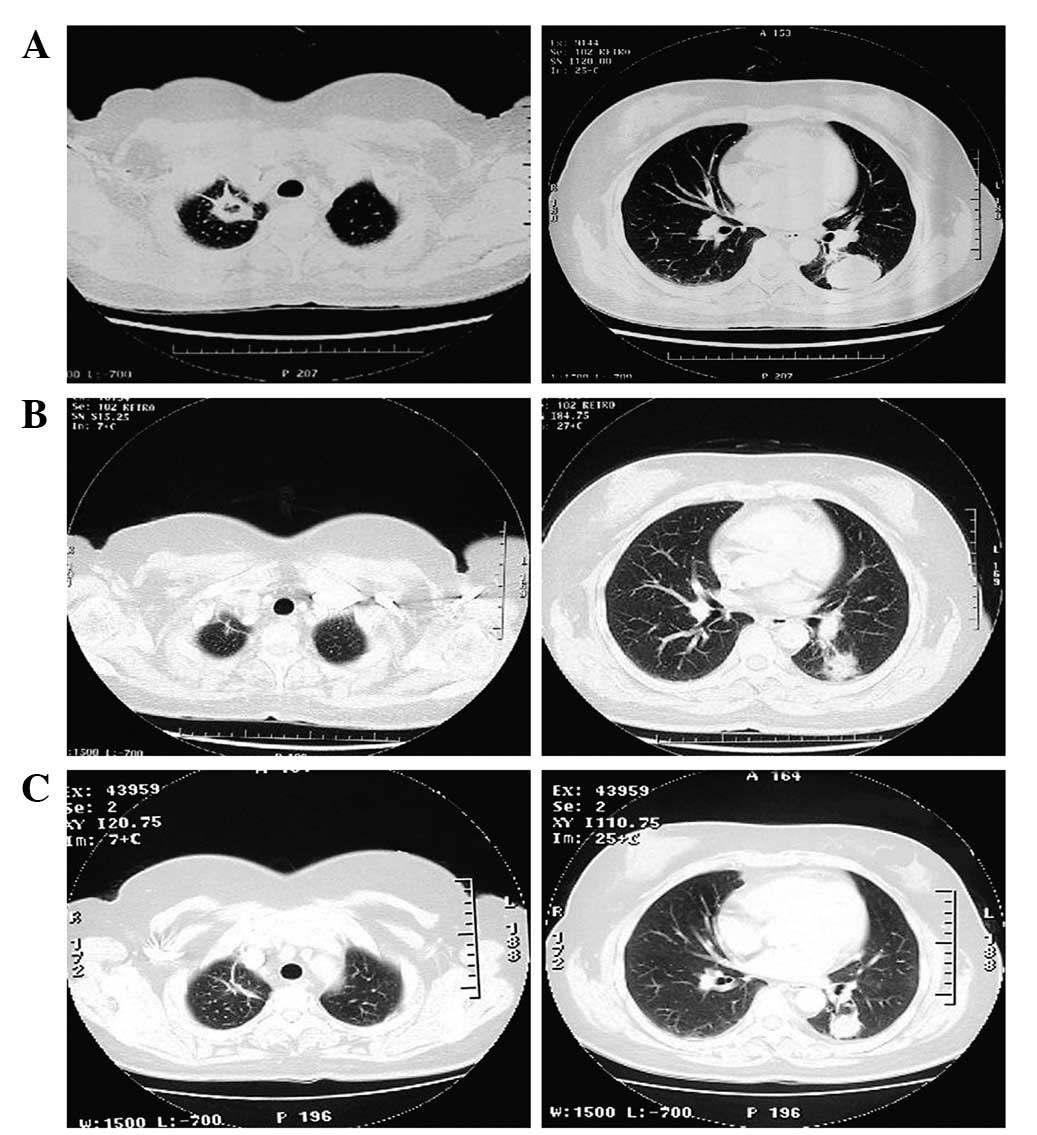
every 21 days. After 3 cycles, the lesions became stable (Fig. 1), but the patient began to feel
nauseous and fatigued from fourth-degree bone marrow depression,
preventing tolerance of further treatment. The patient refused
second-line chemotherapy. After being fully informed about the uses
of erlotinib and the lack of evidence for the efficacy of the drug
in this setting, the patient was orally administered 150 mg/day
erlotinib. Following six weeks of erlotinib therapy, the target
lesions were assessed and found to have reduced in size by 40–50%.
This was consistent with a partial response. The target lesions
continued to decrease in size, and stabilized three months
following therapy (CT showed maximum diameters of 2.8 and 1.5 cm,
respectively). The patient’s condition remained stable for a total
of nine months. Subsequently, the patient decided to terminate
therapy with the exception of palliative care. Progressive disease
was evident in the lungs 16 months later, and eventually the
patient succumbed to respiratory failure. This study was approved
by the Ethics Committee of Tianjin Medical University (Tianjin,
China). The patient provided written informed consent.
Discussion
Primary vaginal cancer is a rare condition
accounting for 1–3% of all gynecological malignancies. While the
major relapse pattern for vaginal carcinoma is local recurrence,
the development of lesions outside the pelvis has also been
reported (5,6). The patient of the present case report
had metastatic lesions in the lungs. Since the patient could not
tolerate additional courses of carboplatin and paclitaxel and
refused second-line chemotherapy, erlotinib was administered in an
attempt to control the disease.
Erlotinib is the standard therapy for advanced NSCLC
with EGFR mutation and has also been approved for the treatment of
unselected chemorefractory advanced NSCLC and for maintenance
therapy following first-line chemotherapy (7). While it is known that EGFR-TKIs,
including erlotinib, mainly benefit EGFR-mutated adenocarcinomas, a
pooled analysis was conducted that demonstrated an efficacy of
gefitinib for non-adenocarcinoma NSCLC patients harboring the EGFR
mutation (8). A phase III trial
(BR.21) evaluated the effects of erlotinib in patients who had been
treated for NSCLC. Among the participants, 30% had squamous cell
histology. Patients with wild-type EGFR showed some survival
benefit when treated with erlotinib alone (9). Subgroup data from the global,
multicenter, randomized, double-blind, placebo-controlled study,
SATURN, showed that erlotinib can be beneficial regardless of
patient age, race, histology or smoking history. Squamous cell
carcinoma patients, who had not previously been considered suitable
for EGFR-TKI treatment, showed some benefit from treatment with
EGFR-TKIs. Patients who received erlotinib maintenance therapy
following chemotherapy had a 24% lower risk of disease progression.
Patients with wild-type EGFR had a 22% lower risk of progression
(10).
The use of EGFR-TKIs outside of lung and pancreatic
cancer is uncommon. A multicenter, open-label, non-comparative,
phase II trial was previously performed to evaluate the clinical
outcomes of gefitinib in cervical cancer in 28 patients. The
condition of 6 patients (20%) was stabilized for a median period of
111.5 days. The median time to progression was 37 days and the
median overall survival time was 107 days. Disease control did not
appear to be correlated with levels of EGFR expression. Gefitinib
was well tolerated. The use of gefitinib (or other EGFR-TKI
therapies) in cases of recurrent disease resistant to standard
treatment may warrant further investigation (4).
To the best of our knowledge, the present study is
the first report of the use of erlotinib in a patient with vaginal
cancer with metastatic disease in the lungs. Although the evidence
is somewhat subjective, the results are promising. Further
investigation of the use of this class of drugs in this setting is
warranted.
References
|
1
|
Grigsby PW: Vaginal cancer. Curr Treat
Options Oncol. 3:125–130. 2002. View Article : Google Scholar
|
|
2
|
Creasman WT: Vaginal cancers. Curr Opin
Obstet Gynecol. 17:71–76. 2005. View Article : Google Scholar
|
|
3
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37(Suppl 4): S9–S15. 2001.
View Article : Google Scholar
|
|
4
|
Goncalves A, Fabbro M, Lhommé C, Gladieff
L, Extra JM, Floquet A, Chaigneau L, Carrasco AT and Viens P: A
phase II trial to evaluate gefitinib as second- or third-line
treatment in patients with recurring locoregionally advanced or
metastatic cervical cancer. Gynecol Oncol. 108:42–46. 2008.
View Article : Google Scholar
|
|
5
|
Tran PT, Su Z, Lee P, Lavori P, Husain A,
Teng N and Kapp DS: Prognostic factors for outcomes and
complications for primary squamous cell carcinoma of the vagina
treated with radiation. Gynecol Oncol. 105:641–649. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeh AM, Marcus RB Jr, Amdur RJ, Morgan LS
and Million RR: Patterns of failure in squamous cell carcinoma of
the vagina treated with definitive radiotherapy alone: what is the
appropriate treatment volume? Int J Cancer. 96(Suppl): 109–116.
2001.
|
|
7
|
Piperdi B and Perez-Soler R: Role of
erlotinib in the treatment of non-small cell lung cancer: clinical
outcomes in wild-type epidermal growth factor receptor patients.
Drugs. 72(Suppl 1): 11–19. 2012. View Article : Google Scholar
|
|
8
|
Shukuya T, Takahashi T, Kaira R, Ono A,
Nakamura Y, Tsuya A, Kenmotsu H, Naito T, Kaira K, Murakami H, et
al: Efficacy of gefitinib for non-adenocarcinoma non-small-cell
lung cancer patients harboring epidermal growth factor receptor
mutations: a pooled analysis of published reports. Cancer Sci.
102:1032–1037. 2011. View Article : Google Scholar
|
|
9
|
Bezjak A, Tu D, Seymour L, Clark G,
Trajkovic A, Zukin M, Ayoub J, Lago S, de Albuquerque Ribeiro R,
Gerogianni A, et al: Symptom improvement in lung cancer patients
treated with erlotinib: quality of life analysis of the National
Cancer Institute of Canada Clinical Trials Group Study BR.21. J
Clin Oncol. 24:3831–3837. 2006. View Article : Google Scholar
|
|
10
|
Cappuzzo F, Ciuleanu T, Stelmakh L,
Cicenas S, Szczésna A, Juhász E, Esteban E, Molinier O, Brugger W,
Melezínek I, et al; SATURN investigators. Erlotinib as maintenance
treatment in advanced non-small-cell lung cancer: a multicentre,
randomised, placebo-controlled phase 3 study. Lancet Oncol.
11:521–529. 2010. View Article : Google Scholar
|