Introduction
Hepatocellular carcinoma (HCC) is the second most
common cause of male cancer-related mortalities worldwide, with
~700,000 cancer mortalities due to HCC were reported in 2008
(1). Despite progress in early
diagnosis and surgical interventions, the long-term survival rate
of patients with HCC remains unsatisfactory due to the high rate of
recurrence and metastasis (2).
Thus, it is important to explore prognostic factors for HCC and to
develop effective therapeutic schemes.
Studies have highlighted important features of the
nucleocytoplasmic transport of RNAs and proteins (3). A number of the transport factors have
been demonstrated to be dysregulated in primary tumor specimens and
linked to poor prognosis (4,5).
Nuclear RNA export factor 3 (NXF3) is a member of the nuclear RNA
export factor (NXF) family of proteins, which plays a role in
mediating the export of cellular mRNA from the nucleus to the
cytoplasm for translation (6). NXF3
has been identified as a nucleocytoplasmic shuttle protein and
demonstrated to induce RNA export by recruitment of Crm1, which is
overexpressed in various types of human cancer (6,7). A
study has shown that NXF3 may mediate the downregulation of the
levels of transforming growth factor β3 (TGF-β3) mRNA expression
and protein secretion in Sertoli cells (8), and TGF-β3 is considered to be involved
in tumor progression (9). However,
the roles of NXF3 in human tumor development and/or progression
remain undetermined and no association between NXF3 and the
clinical significance of tumors has been established. In the
present study, the expression levels and the clinical relevance of
NXF3 were investigated in a cohort of 112 patients with primary
HCC. To the best of our knowledge, this is the first time such a
study has been conducted. The aim was to establish the association
between NXF3 and HCC and identify novel prognostic factors for
HCC.
Materials and methods
Study population
Immunohistochemistry (IHC) was performed on tumor
and peritumoral tissue samples from 112 patients who had undergone
curative resection for HCC at Zhongshan Hospital, Fudan University
(Shanghai, China) between February and September 2005. All patients
without distant metastasis or any form of anticancer treatment
prior to the surgical resection were selected on the basis of
complete clinicopathological and follow-up data for the patients.
The clinical typing of tumors followed the tumor-node-metastasis
(TNM) classification system of the American Joint Committee on
Cancer and the Union for International Cancer Control (7th edition)
(10). The histological grade of
tumor differentiation was assigned using the Edmondson grading
system (11). Ethical approval was
obtained from the Zhongshan Hospital Research Ethics Committee and
written informed consent was obtained from all patients.
Follow-up after surgery
Patient follow-up was completed in March, 2010 with
a median observation time of 48.7 months. A diagnosis of recurrence
was based on the typical imaging appearance of hepatic lesions in
computed tomography and/or magnetic resonance imaging scans at
elevated α-fetoprotein (AFP) levels. The overall survival (OS) time
was defined as the interval between surgery and when the patient
succumbed to the disease or between surgery and the last
observation for surviving patients. Time to recurrence (TTR) was
defined as the interval between the date of surgery and that of
recurrence. The data were censored at the last follow-up for living
patients and patients without any sign of recurrence.
Evaluation of immunohistochemical
findings
Tissue sections (4 μm) were stained with hematoxylin
and eosin for histological analysis and with specific primary
anti-human antibodies against NXF3 (1:150; LS-C31687; LifeSpan
Biosciences, Inc., Seattle, WA, USA) for IHC. Following microwave
antigen retrieval, the tissues were incubated with the primary
antibodies overnight at 4°C followed by a 30-min incubation with
the secondary antibody (Dako EnVision kit, Dako, Glostrup,
Denmark). The reaction was visualized with diaminobenzidine and the
tissues were counterstained with hematoxylin.
The tissue sections were viewed at ×200
magnification using a Leica DMI6000B inverted microscope (Leica
Microsystems, Heidelberg, Germany) and images were captured. Two
experienced pathologists independently assessed all IHC staining.
The scoring for nuclear NXF3 expression was based on the staining
proportion and intensity. The staining proportion was scored as
follows: 0–25% staining, 1; 26–50% staining, 2; 51–75% staining, 3;
and 76–100% staining, 4. The staining intensity was scored as
follows: Negative intensity, 0; weakly positive, 1; moderately
positive, 2; and strongly positive, 3, according to a previous
study (12). The sum of the
proportion and intensity scores was used to calculate the final
staining score, which was then categorized as low (1–5) or
high (6–7).
Statistical analysis
Statistical analysis was performed with SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). The
cumulative survival time was calculated using the Kaplan-Meier
method and analyzed with the log-rank test. Univariate and
multivariate analyses were performed based on the Cox proportional
hazards regression model. The Student’s t-test and χ2
test were used as appropriate. P<0.05 was considered to indicate
a statistically significant difference.
Results
NXF3 expression in HCC tissues
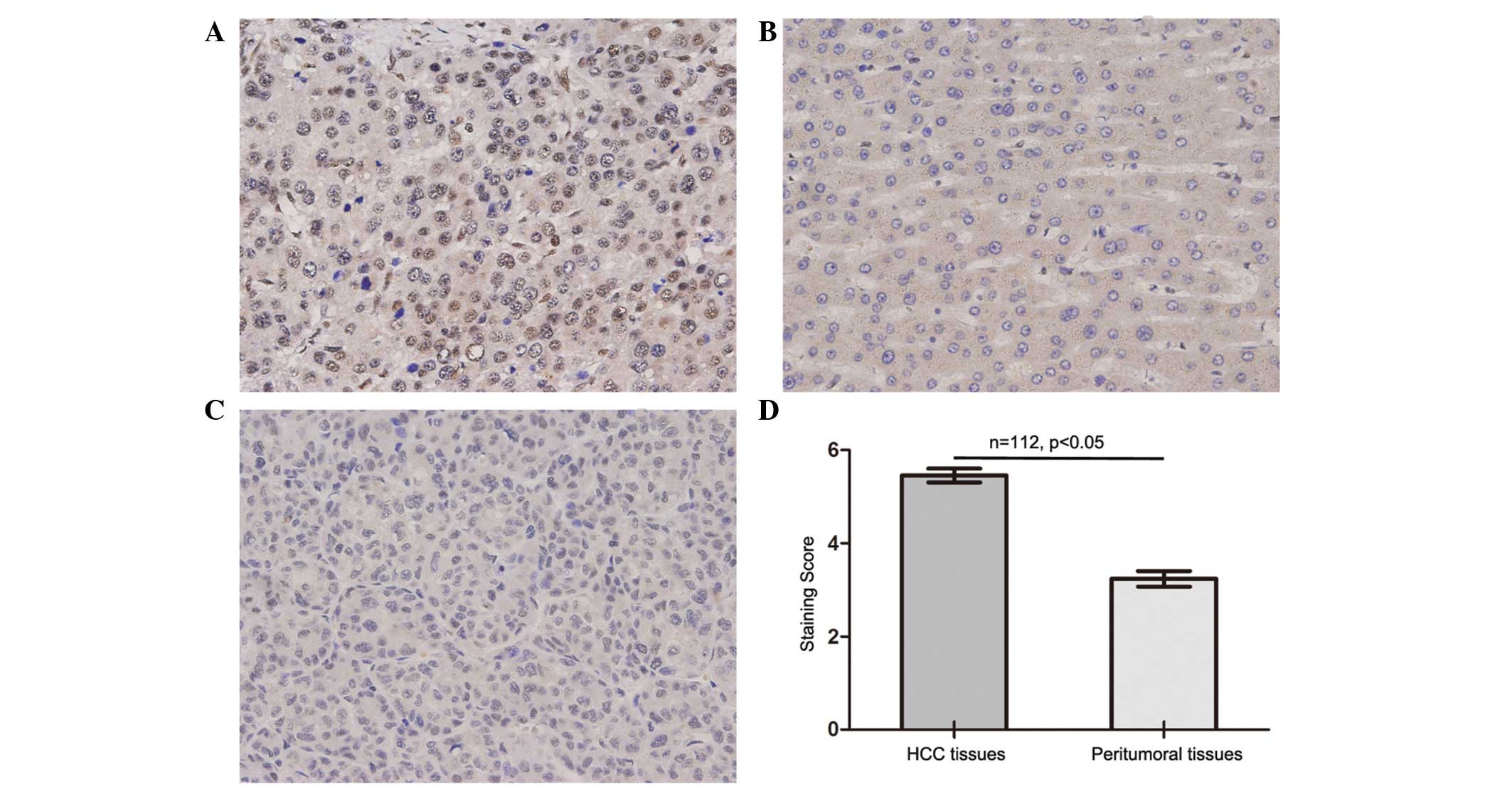
NXF3 staining was mainly observed in the nucleus of
the tumor cells. Significantly higher expression levels of NXF3
were identified in the HCC tissues compared with those in the
paired peritumoral liver tissues, with intense expression of NXF3
in the cancerous tissues and weak expression in the normal liver
tissues (P<0.001, Fig. 1). High
expression levels of NXF3 in the HCC tissues were prevalent in the
patients, and were observed in 78 (~70%) of the total 112 patients
(Table I).
 | Table ISummary of the clinicopathological
data of the 112 patients in the NXF3 protein expression study. |
Table I
Summary of the clinicopathological
data of the 112 patients in the NXF3 protein expression study.
| Variable | Cases, n (%) |
|---|
| Gender |
| Male | 96 (85.7) |
| Female | 16 (14.3) |
| Age (years) |
| ≤51 | 58 (51.8) |
| >51 | 54 (48.2) |
| HBsAg |
| Negative | 13 (11.6) |
| Positive | 99 (88.4) |
| AFP (ng/ml) |
| ≤20 | 42 (37.5) |
| >20 | 70 (62.5) |
| GGT (U/l) |
| ≤54 | 56 (50) |
| >54 | 56 (50) |
| Liver cirrhosis |
| No | 19 (17.0) |
| Yes | 93 (83.0) |
| Tumor size (cm) |
| ≤5 | 55 (49.1) |
| >5 | 57 (50.9) |
| Tumor number |
| Single | 100 (89.3) |
| Multiple | 12 (10.7) |
| Tumor
encapsulation |
| Complete | 72 (64.3) |
| None | 40 (35.7) |
| Tumor
differentiation |
| I–II | 84 (75.0) |
| III–IV | 28 (25.0) |
| Vascular
invasion |
| No | 69 (61.6) |
| Yes | 43 (38.4) |
| TNM stagea |
| I | 61 (54.5) |
| II–III | 51 (45.5) |
| NXF3 expression |
| Low | 34 (30.4) |
| High | 78 (69.6) |
Clinical relevance of NXF3 expression in
HCC
Table I summarizes
the clinicopathological data of the patients in the present study.
The one-, three- and five-year overall and recurrence-free survival
rates of the 112 patients were 81.2, 53.9 and 47.2% and 69.3, 42.6
and 35%, respectively. The univariate analysis revealed that the
AFP levels, γ-glutamyltransferase levels, tumor size and TNM stage
were correlated with the OS time and TTR, and that the tumor
differentiation and vascular invasion were correlated with the OS
time rather than TTR (Table II).
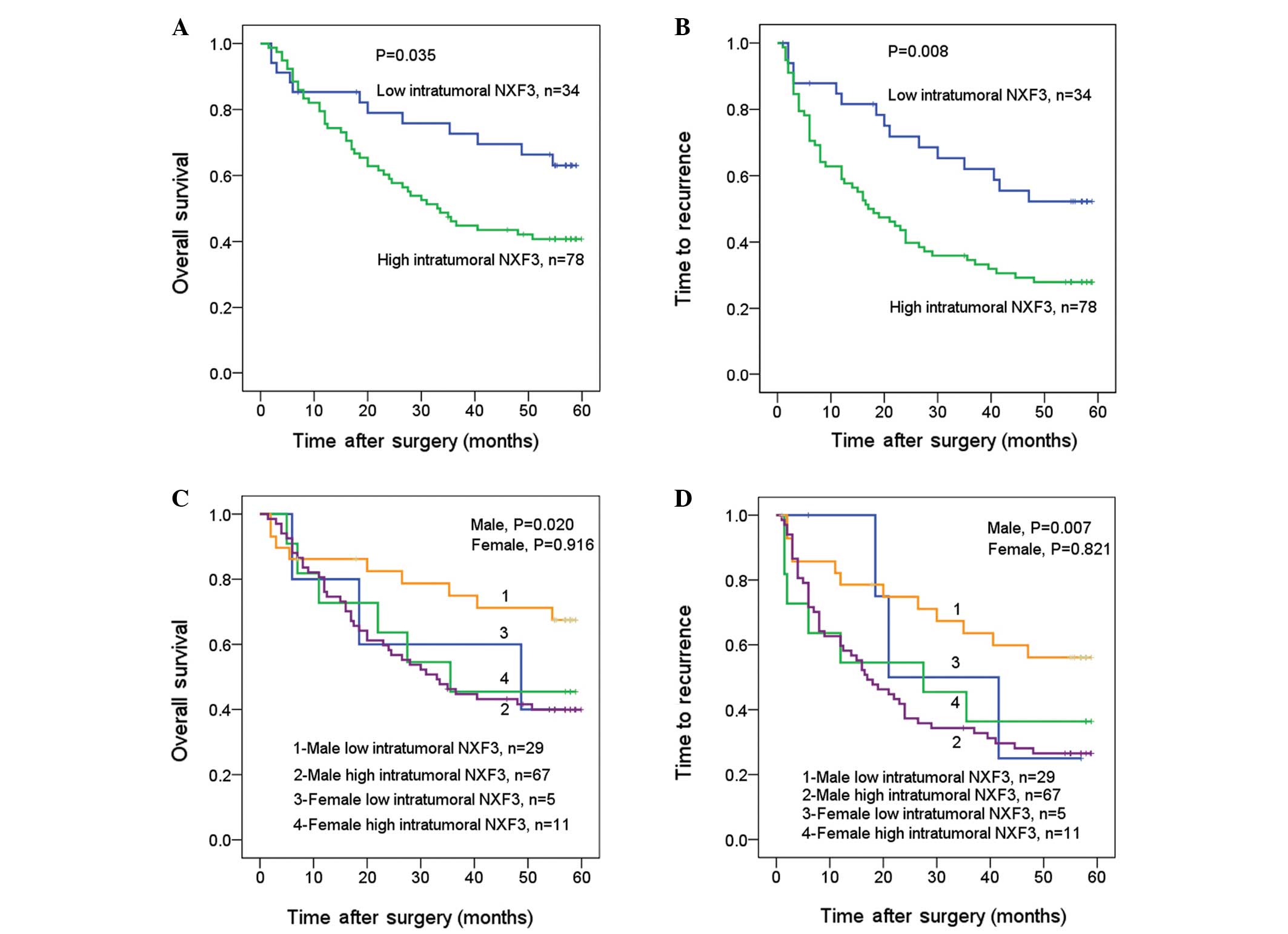
Notably, the univariate analysis also revealed that individuals
with high intratumoral expression levels of NXF3 had a
significantly worse prognosis than those with low intratumoral
expression levels of NXF3, as reflected by the OS and TTR (P=0.035
and P=0.008, respectively; Fig. 2A and
B). The median OS and TTR for patients with low intratumoral
expression levels of NXF3 were 55 and 42 months, respectively, as
compared with 33 and 17 months for patients with high intratumoral
expression levels of NXF3. Notably, high intratumoral expression
levels of NXF3 correlated with a poor prognosis in men (P=0.020 and
P=0.007 for the OS and TTR, respectively), while the prognostic
value in women requires further analysis with a larger number of
samples since no significant correlation was identified (P=0.916
and P=0.821 for the OS and TTR, respectively) (Fig. 2C and D). It may be concluded that
intratumoral NXF3 represents a promising prognostic variable for
the prediction of HCC pathogenesis, particularly in male patients
with HCC.
 | Table IIUnivariate analyses of factors
associated with HCC survival and recurrence. |
Table II
Univariate analyses of factors
associated with HCC survival and recurrence.
| OS | TTR |
|---|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years; ≤51 vs.
>51) | 1.185 | 0.707–1.984 | 0.520 | 1.392 | 0.871–2.224 | 0.166 |
| Gender (female vs.
male) | 0.909 | 0.446–1.850 | 0.791 | 0.996 | 0.510–1.944 | 0.990 |
| HBsAg (negative vs.
positive) | 1.106 | 0.475–2.576 | 0.816 | 1.055 | 0.506–2.202 | 0.886 |
| AFP (ng/ml; ≤20 vs.
>20) | 2.520 | 1.397–4.546 | 0.002 | 2.385 | 1.418–4.010 | 0.001 |
| GGT (U/l; ≤54 vs.
>54) | 2.362 | 1.380–4.042 | 0.002 | 2.400 | 1.481–3.889 | <0.001 |
| Liver cirrhosis (no
vs. yes) | 1.683 | 0.764–3.710 | 0.196 | 1.750 | 0.869–3.523 | 0.117 |
| Tumor size (cm; ≤5
vs. >5) | 1.766 | 1.042–2.993 | 0.034 | 1.615 | 1.006–2.593 | 0.047 |
| Tumor number
(single vs. multiple) | 1.127 | 0.511–2.485 | 0.767 | 1.405 | 0.697–2.832 | 0.342 |
| Tumor encapsulation
(complete vs. none) | 1.207 | 0.685–2.126 | 0.514 | 1.366 | 0.854–2.208 | 0.203 |
| Tumor
differentiation (I–II vs. III–IV) | 1.865 | 1.111–3.131 | 0.018 | 1.263 | 0.758–2.105 | 0.370 |
| Vascular invasion
(no vs. yes) | 2.112 | 1.260–3.540 | 0.005 | 1.586 | 0.992–2.537 | 0.054 |
| TNM stage (I vs.
II–III) | 2.201 | 1.307–3.707 | 0.003 | 1.892 | 1.185–3.021 | 0.008 |
| Intratumoral NXF3
(low vs. high) | 1.954 | 1.034–3.695 | 0.039 | 2.101 | 1.186–3.722 | 0.011 |
To further confirm the prognostic significance of
NXF3 expression levels, Cox multivariate proportional hazards
regression analysis was performed with all the variables that were
identified as significantly associated with the OS and/or TTR in
the univariate analysis to control for confounders. The
multivariate analysis showed that the patients with high
intratumoral expression levels of NXF3 were 2.68- and 2.79-fold
more likely to succumb to the disease [95% confidence interval (CI)
= 1.28–5.60, P=0.009] and experience recurrence (95% CI =
1.44–5.37, P=0.002; Table III),
respectively, than those with low intratumoral expression levels of
NXF3. Other characteristics, including the AFP levels,
γ-glutamyltransferase levels, tumor size, tumor differentiation,
vascular invasion and TNM stage, were also assessed and the results
are presented in Table III.
 | Table IIIMultivariate analyses of factors
associated with HCC survival and recurrence. |
Table III
Multivariate analyses of factors
associated with HCC survival and recurrence.
| Survival | HR | 95% CI | P-value |
|---|
| OS |
| AFP (ng/ml; ≤20
vs. >20) | 2.451 | 1.316–4.563 | 0.005 |
| GGT (U/l; ≤54 vs.
>54) | 1.887 | 1.041–3.423 | 0.036 |
| Tumor size (cm; ≤5
vs. >5) | 1.318 | 0.720–2.415 | 0.371 |
| Tumor
differentiation (I–II vs. III–IV) | 0.771 | 0.413–1.441 | 0.416 |
| Vascular invasion
(no vs. yes) | 2.205 | 0.817–5.951 | 0.118 |
| TNM stage (I vs.
II–III) | 1.111 | 0.417–2.962 | 0.833 |
| Intratumoral NXF3
(low vs. high) | 2.680 | 1.282–5.603 | 0.009 |
| TTR |
| AFP (ng/ml; ≤20
vs. >20) | 2.088 | 1.234–3.534 | 0.006 |
| GGT (U/l; ≤54 vs.
>54) | 1.745 | 1.030–2.958 | 0.039 |
| Tumor size (cm; ≤5
vs. >5) | 1.419 | 0.835–2.413 | 0.196 |
| TNM stage (I vs.
II–III) | 1.771 | 1.073–2.924 | 0.025 |
| Intratumoral NXF3
(low vs. high) | 2.785 | 1.444–5.372 | 0.002 |
Discussion
The proteins of the NXF family play roles in the
transport of mRNAs from the nucleus to the cytoplasm, which is
fundamental for gene expression (13). Yang et al (6) demonstrated that the expression of NXF3
was tissue-specific, that NXF3 was detected at high levels in the
testes and that the RNA export induced by NXF3 could be inhibited
by leptomycin B (an antibiotic that specifically blocks Crm1
function), indicating that NXF3 is an adapter for Crm1-dependent
nuclear mRNA export. Although Crm1 is overexpressed in various
types of human cancer, including glioma, cervical cancer and renal
cell carcinoma, and has the potential to be a prognostic marker for
cancer (14–16), the clinical relevance of NXF3 in
human cancer remains undetermined. In the present study, the data
revealed that NXF3 expression levels were markedly elevated in
primary human HCC tissues compared with those in peritumoral liver
tissues. Furthermore, the clinically relevant data presented showed
that patients with HCC and high tumor NXF3 expression levels had
decreased OS times and earlier TTR compared with those of patients
with low tumor NXF3 expression levels. These data indicate that
NXF3 protein expression may be a promising prognostic biomarker for
HCC and raises the possibility that NXF3 may play a role in
promoting the transformation of hepatocytes to tumor cells,
possibly by mediating the dysregulation of nucleocytoplasmic
transport.
Additionally, NXF3 expression levels showed
prognostic value in male patients with HCC but not in female ones.
This finding may be due to the human X chromosome having the
property that one X chromosome undergoes inactivation in females
(17,18). This feature leads to a genetic
situation unique to females in which mutations in oncogenes
(possibly including NXF3) or tumor suppressor genes on the inactive
chromosome are not expressed. As males have only one maternally
inherited X chromosome, they are expected to be more susceptible to
oncogenic alterations on the X chromosomes inherited from their
mothers (19). Epidemiological data
have revealed that the incidence of HCC shows a gender discrepancy,
with males accounting for more than two-thirds of HCC cases
worldwide (20,21). The factors that contribute to this
gender discrepancy remain unclear. Sex hormones and environmental
factors are considered to be important in the gender discrepancy in
the process of hepatocarcinogenesis (22), while the present study indicates
that the X-linked genes (based on data of NXF3) may also play a
role in this gender discrepancy.
Nucleocytoplasmic transport maintains the balance of
spatial regulation of protein activity and thus is critical for
normal cell function (23). The
transport of oncogenes and tumor suppressors is disrupted in
various types of cancer cells. The forkhead box O (FOXO)
transcription factors, including FOXO1a, FOXO3a and FOXO4, are
located in the cell nucleus and negatively regulate cell growth,
proliferation, differentiation and survival (24). The inactivation of these factors may
be induced by inappropriate nuclear export and cytoplasmic
mislocalization that is mediated by Crm1 and contributes to the
development of glioblastoma multiforme, renal cancer and colon
cancer (23,25). Any change in the subcellular
localization of oncoproteins and tumor suppressors has the
potential to affect the regulation and activity of FOXO
transcription factors. Based on the theory that a variety of vital
proteins are mislocalized in cancer cells, strategies for
redirecting these proteins to the correct subcellular location may
be developed to provide effective cancer therapies (26). Therefore, such factors (including
Crm1 and NXF3) that are involved in RNA or protein export may yield
novel therapeutic targets for cancer treatment.
Although a number of target proteins, including p53,
p21, FOXO and NF-κB, have been identified that undergo nuclear
export in a Crm1-dependent manner (27), few mRNAs or proteins for
NXF3-dependent nuclear export have been identified thus far. A
study has shown that NXF3 mediates the downregulation of the levels
of TGF-β3 mRNA expression and protein secretion in Sertoli cells
(8), but additional studies are
required to confirm whether TGF-β3, which is considered to be
involved in tumor progression, may be a candidate target gene
mediated by NXF3-dependent nuclear export. In the present study,
the findings provide a preliminary connection between NXF3
expression levels and HCC. Further studies are required to identify
the specific mRNAs or proteins for NXF3-dependent nuclear export
and to establish the exact role of NXF3 in the pathogenesis of
HCC.
In summary, NXF3, as an NXF family member, has for
the first time, to the best of our knowledge, been demonstrated to
be linked to human cancer, extending its role into tumor
development. Furthermore, NXF3 was identified to be correlated with
the overall and recurrence-free survival time in postoperative
patients with HCC, suggesting that NXF3 may be a promising
prognostic marker for HCC as well as a novel RNA export pathway for
targeting with cancer therapies.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 30872503 and
81071992).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar
|
|
3
|
Grünwald D, Singer RH and Rout M: Nuclear
export dynamics of RNA-protein complexes. Nature. 475:333–341.
2011.PubMed/NCBI
|
|
4
|
Siddiqui N and Borden KL: mRNA export and
cancer. Wiley Interdiscip Rev RNA. 3:13–25. 2012. View Article : Google Scholar
|
|
5
|
Chow KH, Factor RE and Ullman KS: The
nuclear envelope environment and its cancer connections. Nat Rev
Cancer. 12:196–209. 2012.PubMed/NCBI
|
|
6
|
Yang J, Bogerd HP, Wang PJ, Page DC and
Cullen BR: Two closely related human nuclear export factors utilize
entirely distinct export pathways. Mol Cell. 8:397–406. 2001.
View Article : Google Scholar
|
|
7
|
van der Watt PJ and Leaner VD: The nuclear
exporter, Crm1, is regulated by NFY and Sp1 in cancer cells and
repressed by p53 in response to DNA damage. Biochim Biophys Acta.
1809:316–326. 2011.PubMed/NCBI
|
|
8
|
Yin Y, Wang G, Liang N, et al: Nuclear
export factor 3 is involved in regulating the expression of
TGF-beta3 in an mRNA export activity-independent manner in mouse
Sertoli cells. Biochem J. 452:67–78. 2013.PubMed/NCBI
|
|
9
|
Petrella BL, Armstrong DA and Vincenti MP:
Interleukin-1 beta and transforming growth factor-beta 3 cooperate
to activate matrix metalloproteinase expression and invasiveness in
A549 lung adenocarcinoma cells. Cancer Lett. 325:220–226. 2012.
View Article : Google Scholar
|
|
10
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: a study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ke AW, Shi GM, Zhou J, et al: Role of
overexpression of CD151 and/or c-Met in predicting prognosis of
hepatocellular carcinoma. Hepatology. 49:491–503. 2009. View Article : Google Scholar
|
|
13
|
Cullen BR: Nuclear mRNA export: insights
from virology. Trends Biochem Sci. 28:419–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen A, Wang Y, Zhao Y, Zou L, Sun L and
Cheng C: Expression of CRM1 in human gliomas and its significance
in p27 expression and clinical prognosis. Neurosurgery. 65:153–160.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van der Watt PJ, Maske CP, Hendricks DT,
et al: The Karyopherin proteins, Crm1 and Karyopherin beta1, are
overexpressed in cervical cancer and are critical for cancer cell
survival and proliferation. Int J Cancer. 124:1829–1840. 2009.
|
|
16
|
Inoue H, Kauffman M, Shacham S, et al:
CRM1 blockade by selective inhibitors of nuclear export attenuates
kidney cancer growth. J Urol. 189:2317–2326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lyon MF: Gene action in the X-chromosome
of the mouse (Mus musculus L.). Nature. 190:372–373. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lyon MF: Sex chromatin and gene action in
the mammalian X-chromosome. Am J Hum Genet. 14:135–148.
1962.PubMed/NCBI
|
|
19
|
Spatz A, Borg C and Feunteun J:
X-chromosome genetics and human cancer. Nat Rev Cancer. 4:617–629.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen JG and Zhang SW: Liver cancer
epidemic in China: past, present and future. Semin Cancer Biol.
21:59–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang N, Zheng Y, Yu X, Lin W, Chen Y and
Jiang Q: Sex-modified effect of hepatitis B virus infection on
mortality from primary liver cancer. Am J Epidemiol. 169:990–995.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kau TR, Way JC and Silver PA: Nuclear
transport and cancer: from mechanism to intervention. Nat Rev
Cancer. 4:106–117. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
van der Horst A and Burgering BM:
Stressing the role of FoxO proteins in lifespan and disease. Nat
Rev Mol Cell Biol. 8:440–450. 2007.PubMed/NCBI
|
|
25
|
Kau TR, Schroeder F, Ramaswamy S, et al: A
chemical genetic screen identifies inhibitors of regulated nuclear
export of a Forkhead transcription factor in PTEN-deficient tumor
cells. Cancer Cell. 4:463–476. 2003. View Article : Google Scholar
|
|
26
|
Sakakibara K, Saito N, Sato T, et al:
CBS9106 is a novel reversible oral CRM1 inhibitor with CRM1
degrading activity. Blood. 118:3922–3931. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao L and Yang Y: Targeting the nuclear
transport machinery by rational drug design. Curr Pharm Des.
19:2318–2325. 2013. View Article : Google Scholar : PubMed/NCBI
|