Introduction
Prostate cancer (PCa) is a complex and biologically
heterogeneous disease (1) and there
is currently no cure for advanced, hormone-refractory PCa (2). PCa is the second leading cause of
cancer mortality in males >40 years of age in the USA (3) and the third most common cause of
cancer-related mortality in males (4). PCa is generally a slow developing type
of cancer, and 5- and 10-year relative survival rates of early
stage PCa are 99 and 95%, respectively (5). Compared with western countries, the
Chinese population exhibits a lower incidence of PCa, however, it
is increasing annually, in addition to an increased average life
expectancy, improved dietary patterns and enhanced diagnosis
technology. There are numerous risk factors that induce PCa, such
as specific hormones, age, ethnicity and family history. Regardless
of other factors, a family history of PCa is the strongest known
risk factor (6).
Speculation regarding an association between
inflammation and cancer has been considered for some time and
epidemiological studies have established that numerous tumors occur
alongside chronic infectious diseases (7). Prostatitis is a common clinical
disease, which is associated with renal surgery and it is also
known that diet and sexually transmitted infections increase the
risk of PCa (8). During the
development of PCa, tumor cells and the microenvironment of the
local host tissue interact and form a tumor-host microenvironment
(9). The tumor-host
microenvironment is composed of tumor cells, numerous types of host
cells, extracellular matrices and various sources of secretory
factors, which can modify the local extracellular matrix (ECM),
stimulate migration and promote proliferation and survival
(10). Proteases are fundamental to
numerous biological processes and are associated with a wide
variety of pathological conditions, including cancer (11). Matrix metalloproteinases (MMPs) are
a large family of calcium-dependent zinc-containing endopeptidases,
which are responsible for tissue remodeling and degradation of the
ECM (12). As digestion of the ECM
is essential for tumor invasion and metastasis, the role of MMPs in
the later stages of tumor development has been studied (13).
A disintegrin and metalloproteinases (ADAMs) are a
family of proteins with a sequence that exhibits similarities to
the reprolysin family of snake venom metalloproteinases (14); ADAMs share the metalloproteinase
domain with the MMPs (15).
Functional ADAMs are involved in ectodomain shedding of diverse
growth factors, cytokines, receptors and adhesion molecules
(16). Furthermore, pathologies,
such as inflammation and cancer, involve specific ADAM family
members, including ADAM10 (17).
Dysregulation of ADAM10 in inflammation and disease has lead to the
use of the catalytic domain of the protein as a therapeutic target;
however, ADAM10 also appears to play important roles in normal
states (18). In vitro,
ADAM10 has been implicated in E-cadherin cleavage within
keratinocytes and gastric cancer cell lines (19,20).
Moreover, in the prostate, the membranous ADAM10 expression was
observed to be high in benign prostatic hyperplasia patient
samples, and the nuclear translocation of ADAM10 coupled with the
androgen receptor was involved in human PCa tumor growth and
progression (21).
Previous studies have indicated that ADAM10
participates in PCa development, however, the mechanism has not
been investigated. In the present study, TNF-α was identified as a
specific inducer of ADAM10 protein expression in the PCa cell line,
PC-3, and demonstrated the regulatory function that exists between
tumor necrosis factor (TNF)-α and ADAM10 regarding gene expression
and protein levels. Furthermore, it was identified that TNF-α
regulates ADAM10 through the p38 mitogen activated protein
(MAPK)/necrosis factor (NF)-κB signaling pathway. In addition, the
effects of ADAM10 on Fas ligand (FasL) and cell apoptosis were
investigated.
Materials and methods
Reagents and antibodies
Recombinant human TNF-α, anti-TNF-α and anti-ADAM10
antibody were purchased from Sigma-Aldrich Chemie B.V.
(Zwijndrecht, Netherlands). An RNeasy kit was provided by Gibco-BRL
(Gaithersburg, MD, USA) and an enhanced chemiluminescence (ECL) kit
was provided by Boehringer Ingelheim (Berlin, Germany). The
following were obtained from Invitrogen Life Technologies
(Carlsbad, CA, USA): Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection kit, expression vector pGEX-4T-1,
pcDNA™3.1/myc-His, fetal calf serum (FCS), RPMI-1640 medium,
nitrocellulose membranes for western blot analysis, a polymerase
chain reaction (PCR) kit and Lipofectamine™ 2000. FITC-labeled goat
anti-mouse IgG (H+L), anti-p38MAPK, anti-phospho-p38MAPK and
anti-NF-κB antibodies were obtained from Millipore (Billerica, MA,
USA) and SB 203580 and pyrrolidine dithiocarbamate (PDTC) were
purchased from Calbiochem (San Diego, CA, USA). The primers that
were used in the present study were synthesized by Shanghai Sangon
Company (Shanghai, China) and small interfering RNA (siRNA), small
interfering ADAM10 (si-ADAM10) and non-silencing siRNA (si-control)
were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Unless otherwise specified, all of the other reagents were of
an analytical grade.
Cell culture
The human PCa cells, PC-3 and Epstein-Barr
virus-transformed B371 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). The cells were maintained
in RPMI-1640 medium, which was supplemented with 10% (v/v)
heat-inactivated FCS and penicillin-streptomycin-mixed solution in
a humidified incubator with 95% air and 5% CO2. The
medium was changed every 2–3 days and the cell concentration was
~106 cell/ml. The experiments were conducted on cells in
exponential growth. The study was approved by the ethics committee
of The People’s Liberation Army Mount Lu Sanatorium (Jiujiang,
China).
RNA preparation and PCR
Total RNA was isolated from the untreated control
cells and the TNF-α-treated cells using the RNeasy kit according to
the manufacturer’s instructions. PCR was performed according to the
manufacturer’s instructions and the PCR products were resolved on a
100 g/l agarose gel and visualized using ethidium bromide
transillumination under ultraviolet light. β-actin served as an
internal control to evaluate the efficiency of cDNA synthesis and
the PCR amplification. The primer sequences were as follows: ADAM10
forward, 5′-TCCACAGCCCATTCAGCAA-3′ and reverse,
5′-AGGCACTAGGAAGAACCAA-3′; and β-actin forward,
5′-TCACCCACACTGTGCCCATCTACGA-3′ and reverse,
5′-CAGCGGAACCGCTCATTGCCAATGG-3′
Flow cytometric analyses of ADAM10
surface expression
Flow cytometry was employed to detect the ADAM10
protein expression on the surface of the cells. Following treatment
with TNF-α, the nonspecific antibody-binding sites of the PC-3
cells were blocked via incubation with 5% rabbit serum in
Dulbecco’s phosphate-buffered saline. Anti-ADAM10 antibody was
subsequently added and incubated at 4°C for 30 min. After washing,
the cells were incubated with FITC-labeled goat anti-mouse IgG and
analyzed by flow cytometry (FACSCalibyr flow cytometer, BD
Biosciences, San Jose, CA, USA).
Western blot analysis
The samples were separated using 10% SDS-PAGE and
transferred on to a nitrocellulose membrane. The western blot
analyses were performed as previously described (22) with minor modifications. The blot was
incubated using anti-p38MAPK, anti-phospho-p38MAPK and anti-NF-κB
antibodies, and visualized with horseradish peroxidase-conjugated
anti-rabbit IgG and an ECL-Plus chemiluminescence detection system
(GE Healthcare, Pittsburgh, PA, USA).
DNA transfection
The segments of FasL and ADAM10 in the human PC-3
cells were amplified using PCR. The PCR-amplified ADAM10 and FasL
segments were subcloned into pGEX-4T-1 and pcDNA3.1/myc-His,
respectively and the B371 cells were seeded into a 24-well plate at
a density of 105 cells/well. B371 cells were transfected
with an ADAM10 and/or FasL expression vector for 5 h in serum-free
media using Lipofectamine 2000, according to the manufacturer’s
instructions.
Measurement of FasL using ELISA
The concentrations of soluble FasL (sFasL) were
measured using ELISA (R&D Systems, Minneapolis, MN, USA)
according to the manufacturer’s instructions. All samples were run
in duplicate and the average was obtained.
RNA interference
To knock down ADAM10 expression at the mRNA level,
5×105 cells/well were seeded in complete medium in a
24-well plate. ADAM10 siRNA was transfected using Lipofectamine
2000 according to the manufacturer’s instructions.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Data were compared by one-way analysis of variance and
pairwise comparison procedures were conducted using Tukey’s test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
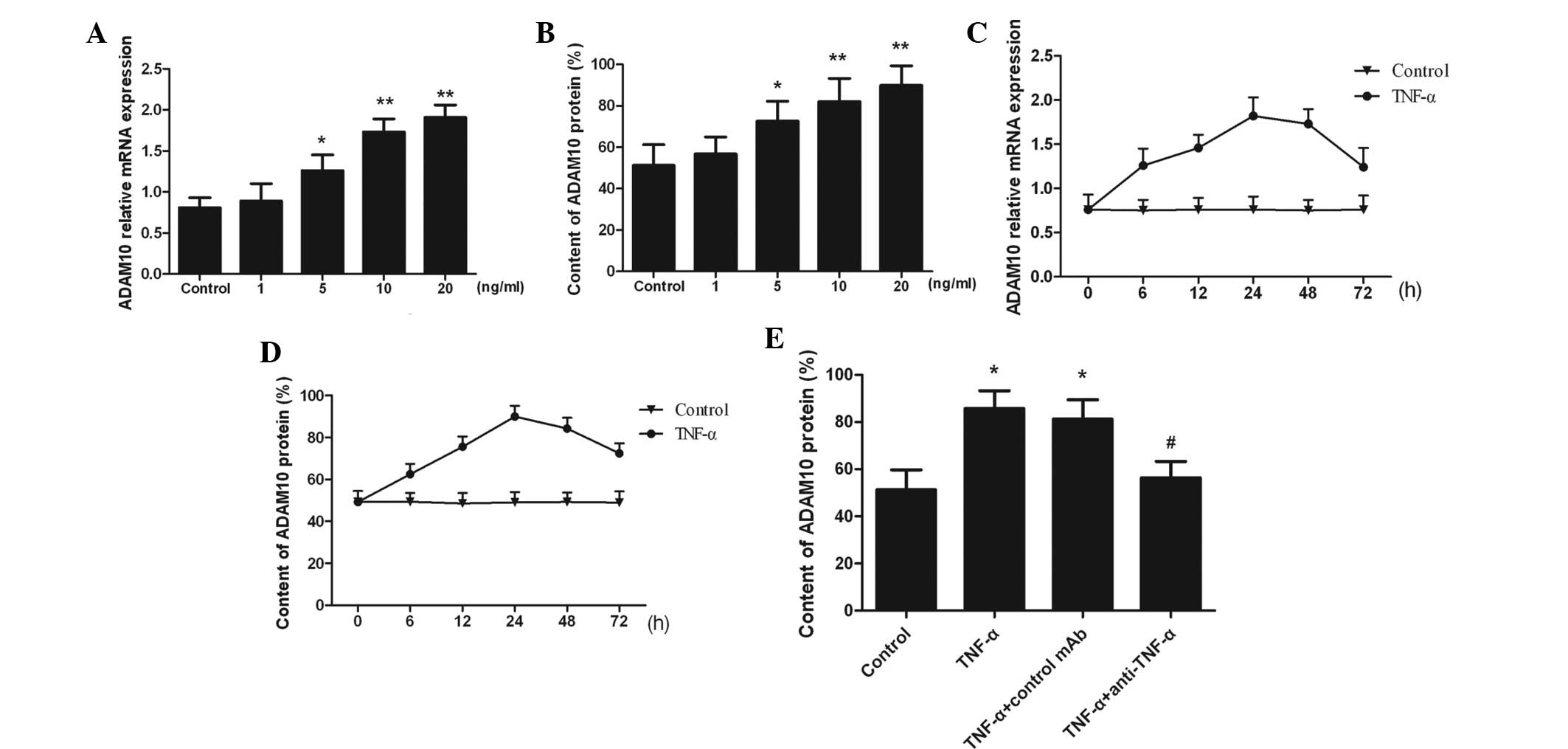
TNF-α induces ADAM10 expression
ADAM10 expression was observed in untreated PC-3
cells, however, expression was significantly increased following
the addition of TNF-α in a dose-dependent manner (P<0.05;
Fig. 1A and B). The ADAM10
expression increased in a time-dependent manner and peaked at 24 h
in response to 10 ng/ml TNF-α stimulation (Fig. 1C and D). Moreover, the addition of
anti-TNF-α neutralized TNF-α, and TNF-α-induced ADAM10 expression
was attenuated (P<0.05; Fig.
1E). The results indicated that ADAM10 expression was markedly
increased as a result of TNF-α stimulation in a time- and
dose-dependent manner.
TNF-α upregulates ADAM10 expression via
the p38MAPK/NF-κB pathway
As TNF-α-induced ADAM10 expression was upregulated
via the p38MAPK/NF-κB pathway, phosphorylated-p38MAPK, p38MAPK and
intranuclear NF-κB protein expression was measured in the PC-3
cells. Phosphorylated-p38MAPK and intranuclear NF-κB were rarely
observed in the untreated control PC-3 cells, however, upon TNF-α
stimulation for 15 min, the expression of the two significantly
increased (Fig. 2A). The expression
of ADAM10 was markedly reduced following addition of the p38MAPK
inhibitor, SB 203580, and the NF-κB inhibitor, PDTC (P<0.05;
Fig. 2B). These observations
indicated that the ADAM10 expression was upregulated by TNF-α
through the p38MAPK/NF-κB pathway.
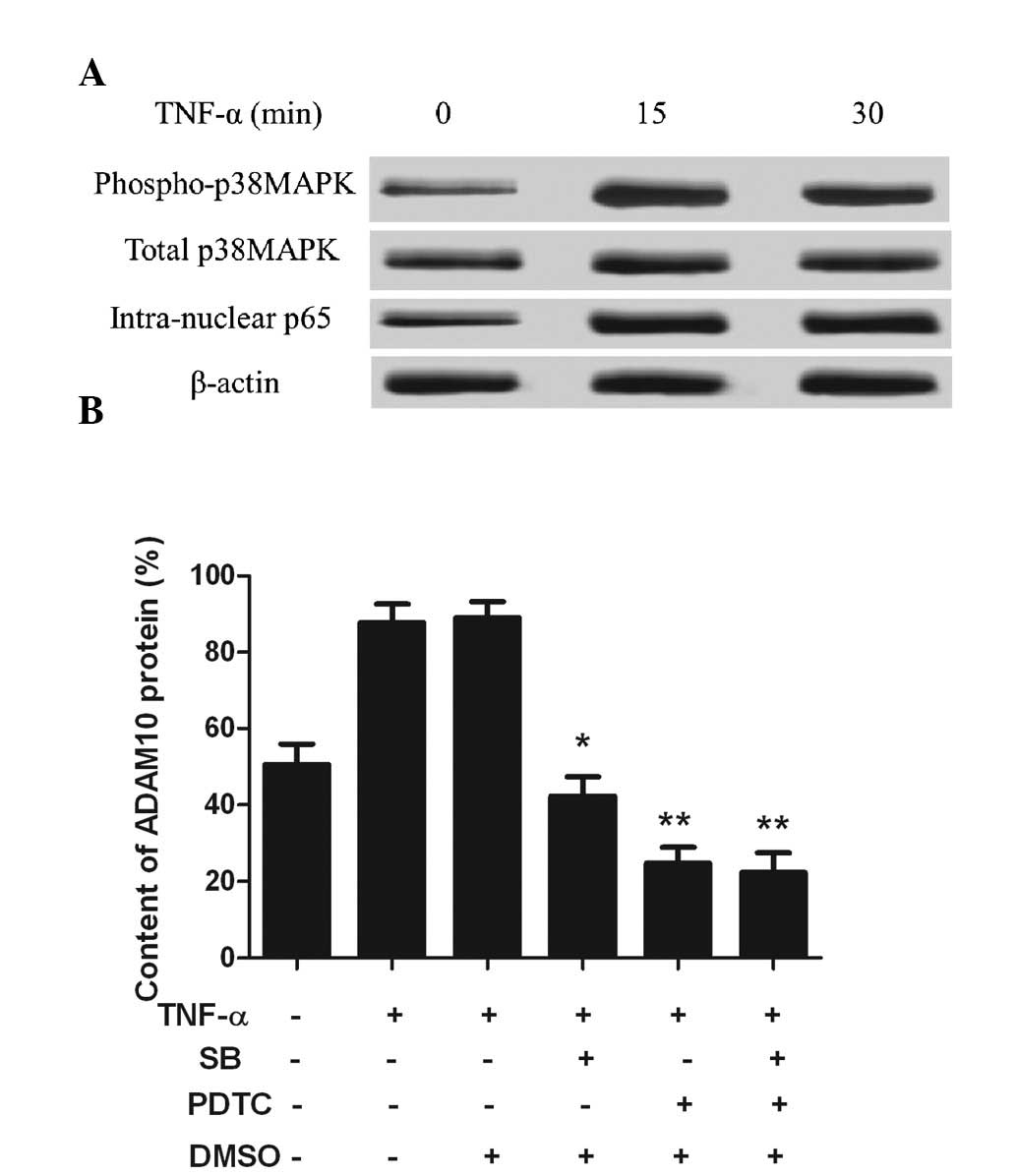 | Figure 2TNF-α upregulates ADAM10 expression
via the p38MAPK/NF-κB pathway. (A) TNF-α (10 ng/ml) was
administered to treat the PC-3 cells at three time-points. Western
blot analysis showed that phosphorylated-p38MAPK and
intranuclear-NF-κBp65 were markedly increased from 15 min. (B) PC-3
cells were pretreated with the p38MAPK inhibitor, SB, or the NF-κB
inhibitor, PDTC, for 1 h and incubated with 10 ng/ml TNF-α for 24
h. Flow cytometry of the ADAM10 cell surface protein expression was
conducted. *P<0.05 and **P<0.01, vs.
TNF-α treatment group. TNF, tumor necrosis factor; MAPK,
mitogen-activated protein kinase; ADAM10, a disintegrin and
metalloprotease 10; NF-κB, nuclear factor-κB; SB, SB 203580; PDTC,
pyrrolidine dithiocarbamate; DMSO, dimethyl sulfoxide. |
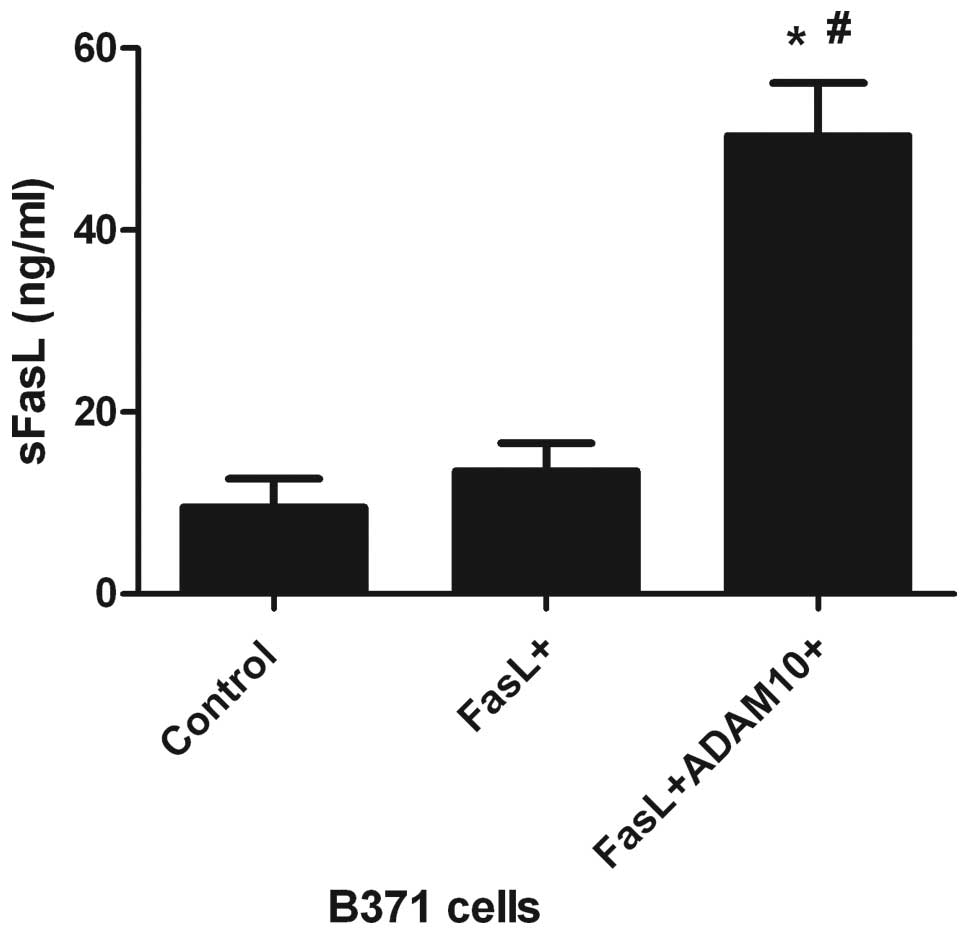
ADAM10 involvement in the cleavage of
FasL
To determine whether ADAM10 is responsible for FasL
cleavage, the release of sFasL into the culture medium of
FasL-transfected B371 cells (FasL-6X His) was investigated using
ELISA. A marked effect on FasL shedding was observed in
FasL+ADAM10+B371 cells, when compared with
the B371 and FasL+B371 cells (P<0.05; Fig. 3). These data indicate that ADAM10
may be responsible for the cleavage of FasL.
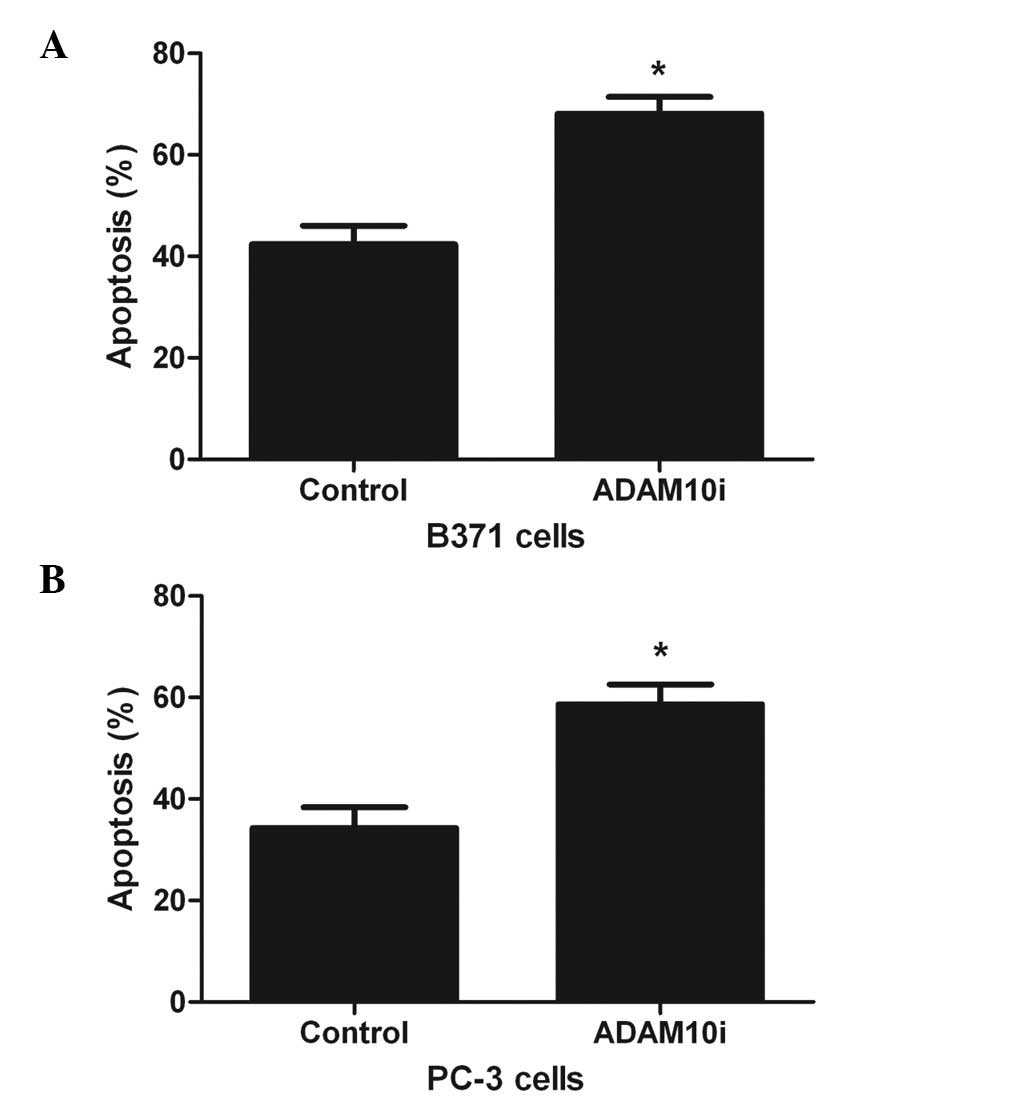
ADAM10 expression inhibits FasL-mediated
apoptosis
To further certify the involvement of ADAM10 in
shedding, B371 (ADAM10+FasL+) and PC-3 cells
were treated with ADAM10 siRNA and cell apoptosis was analyzed. The
results indicated that compared with the control, treatment of B371
and PC-3 cells with ADAM10 siRNA resulted in a significant increase
in apoptosis (P<0.05; Fig. 4A and
B), therefore indicating that ADAM10 expression increases the
resistance of the cells to apoptosis.
Discussion
ADAM10 has been shown to exhibit substrate
specificity, which overlaps with MMPs, thus indicating that ADAM10
has potential ECM-remodeling capabilities (23). Furthermore, ADAM10 may be critical
during development and in adult tissues (21). ADAM10 is predominantly a sheddase,
which is known to cleave epidermal growth factor (EGF)-like ligands
from the cell surface and promote EGF receptor family signaling
(24). TNF-α is a cytokine involved
in systemic and acute inflammation (25). TNF-α has been implicated in
inflammation-associated cancer and is produced by tumor cells
and/or infiltrating leukocytes (26). In the present study, the results
indicated that ADAM10 expression was increased following TNF-α
stimulation in a time- and dose-dependent manner. Moreover, the
TNF-α-induced ADAM10 expression was attenuated following the
application of anti-TNF-α.
The p38MAPK signaling pathway is critical in normal
immune and inflammatory responses (27) and is activated by numerous
extracellular mediators of inflammation, including
chemoattractants, cytokines, chemokines and bacterial
lipopolysaccharides (28). TNF-α is
able to activate the p38MAPK signaling pathway (29) and p38MAPK is key in the production
of proinflammatory cytokines, in addition to being able to regulate
cytokine expression by modulating transcription factors, such as
NF-κB (30). Upon stimulation by
TNF-α, TNF receptor (TNF-R)-1 recruits various groups of adaptor
proteins, which are required for the activation of NF-κB inhibitor
kinase (31) and induces the
activation of the NF-κB signaling pathway (32). In the present study, TNF-α-induced
ADAM10 expression, which demonstrated that in the local tumor
microenvironment a variety of cells regulate the expression of
tumor-associated molecules through paracrine and autocrine
pathways, with important implications in the occurrence of tumors
and their development and metastasis. p38MAPK and NF-κB inhibition
resulted in lower ADAM10 expression, which indicate that TNF-α
upregulated ADAM10 expression through the p38MAPK/NF-κB pathway in
patients with PCa.
The cell surface-bound receptor Fas (also termed
APO-1 or CD95) belongs to a subgroup of the TNF-R family, which
contains an intracellular death domain and triggers apoptosis.
Furthermore, FasL is a member of the TNF cytokine family (33). A previous study identified that
cytotoxic T cells, which express FasL in its membrane-bound form
(mFasL) on their surface, are able to kill Fas+ target
cells (34). The Fas/FasL system is
significant in tumorigenesis and a previous investigation has
indicated that the impairment of the Fas/FasL system in cancer
cells may lead to apoptosis resistance and contribute to tumor
progression (35). ADAM10
downregulates the Fas/FasL signaling pathway through Fas shedding
(36), which results in increased
sFasL. In the present study, the results indicated that ADAM10 was
involved in FasL shedding and ADAM10 expression inhibited
FasL-mediated apoptosis. ADAM10, as an active metalloprotease,
influenced the tumor via numerous pathways and may have
participated in apoptosis-related protein hydrolysis. Malignant
tumor development may, therefore, be associated with FasL loss by
escaping the Fas/FasL scavenge system. sFasL is important during
cell apoptosis as it is able to bind to Fas and act as an
antagonist within the Fas/mFasL conjugate, which suppresses cell
apoptosis. In PCa, increased levels of sFasL may compete with mFasL
and bind to Fas, thus, blocking FasL-mediated apoptosis. Therefore,
increased sFasL expression in the tumor cell microenvironment may
be a mechanism of immune evasion. In conclusion, the present study
indicated that ADAM10 hydrolyzed mFasL in patients with PCa, which
increased the local sFasL concentration. Further investigation is
required to establish whether ADAM10 may serve as a target for
chemotherapeutic agents.
Acknowledgements
This study was supported by the Natural Science
Funds of Fujian Province (no. 2010J05079).
References
|
1
|
Fox JJ, Schöder H and Larson SM: Molecular
imaging of prostate cancer. Curr Opin Urol. 22:320–327. 2012.
View Article : Google Scholar
|
|
2
|
Ouyang DY, Xu LH, He XH, et al: Autophagy
is differentially induced in prostate cancer LNCaP, DU145 and PC-3
cells via distinct splicing profiles of ATG5. Autophagy. 9:20–32.
2013. View Article : Google Scholar
|
|
3
|
Hao Y, Zhao Y, Zhao X, et al: Improvement
of prostate cancer detection by integrating the PSA test with miRNA
expression profiling. Cancer Invest. 29:318–324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Damber JE and Aus G: Prostate cancer.
Lancet. 371:1710–1721. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eastham JA, May RA, Whatley T, Crow A,
Venable DD and Sartor O: Clinical characteristics and biopsy
specimen features in African-American and white men without
prostate cancer. J Natl Cancer Instit. 90:756–760. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mononen N and Schleutker J: Polymorphisms
in genes involved in androgen pathways as risk factors for prostate
cancer. J Urol. 181:1541–1549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berasain C, Castillo J, Perugorria MJ,
Latasa MU, Prieto J and Avila MA: Inflammation and liver cancer:
new molecular links. Ann NY Acad Sci. 1155:206–221. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coyle YM: Lifestyle, genes, and cancer.
Methods Mol Biol. 472:25–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu H, Haag D, Muley T, et al:
Tumor-microenvironment interactions studied by zonal
transcriptional profiling of squamous cell lung carcinoma. Genes
Chromosomes Cancer. 52:250–264. 2013. View Article : Google Scholar
|
|
10
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
López-Otín C and Matrisian LM: Emerging
roles of proteases in tumour suppression. Nat Rev Cancer.
7:800–808. 2007.PubMed/NCBI
|
|
12
|
Hua H, Li M, Luo T, Yin Y and Jiang Y:
Matrix metalloproteinases in tumorigenesis: an evolving paradigm.
Cell Mol Life Sci. 68:3853–3868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Orlichenko LS and Radisky DC: Matrix
metalloproteinases stimulate epithelial-mesenchymal transition
during tumor development. Clin Exp Metastasis. 25:593–600. 2008.
View Article : Google Scholar
|
|
14
|
Mochizuki S and Okada Y: ADAMs in cancer
cell proliferation and progression. Cancer Sci. 98:621–628. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rocks N, Paulissen G, El Hour M, et al:
Emerging roles of ADAM and ADAMTS metalloproteinases in cancer.
Biochimie. 90:369–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edwards DR, Handsley MM and Pennington CJ:
The ADAM metalloproteinases. Mol Aspects Med. 29:258–289. 2008.
View Article : Google Scholar
|
|
17
|
Seals DF and Courtneidge SA: The ADAMs
family of metalloproteases: multidomain proteins with multiple
functions. Genes Dev. 17:7–30. 2003. View Article : Google Scholar
|
|
18
|
Grabowska MM, Sandhu B and Day ML: EGF
promotes the shedding of soluble E-cadherin in an ADAM10-dependent
manner in prostate epithelial cells. Cell Signal. 24:532–538. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maretzky T, Scholz F, Köten B, Proksch E,
Saftig P and Reiss K: ADAM10-mediated E-cadherin release is
regulated by proinflammatory cytokines and modulates keratinocyte
cohesion in eczematous dermatitis. J Invest Dermatol.
128:1737–1746. 2008. View Article : Google Scholar
|
|
20
|
Schirrmeister W, Gnad T, Wex T, et al:
Ectodomain shedding of E-cadherin and c-Met is induced by
Helicobacter pylori infection. Exp Cell Res. 315:3500–3508.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arima T, Enokida H, Kubo H, et al: Nuclear
translocation of ADAM-10 contributes to the pathogenesis and
progression of human prostate cancer. Cancer Science. 98:1720–1726.
2007. View Article : Google Scholar
|
|
22
|
Liu WH and Chang LS: Piceatannol induces
Fas and FasL up-regulation in human leukemia U937 cells via
Ca2+/p38alpha MAPK-mediated activation of c-Jun and
ATF-2 pathways. Int J Biochem Cell Biol. 42:1498–1506. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McCulloch DR, Akl P, Samaratunga H,
Herington AC and Odorico DM: Expression of the disintegrin
metalloprotease, ADAM-10, in prostate cancer and its regulation by
dihydrotestosterone, insulin-like growth factor I, and epidermal
growth factor in the prostate cancer cell model LNCaP. Clin Cancer
Res. 10:314–323. 2004. View Article : Google Scholar
|
|
24
|
Blobel CP: ADAMs: key components in EGFR
signalling and development. Nat Rev Mol Cell Biol. 6:32–43. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lamour NF, Wijesinghe DS, Mietla JA, Ward
KE, Stahelin RV and Chalfant CE: Ceramide kinase regulates the
production of tumor necrosis factor alpha (TNFalpha) via inhibition
of TNFα-converting enzyme. J Biol Chem. 286:42808–42817. 2011.
|
|
26
|
Szlosarek PW and Balkwill FR: Tumour
necrosis factor alpha: a potential target for the therapy of solid
tumours. Lancet Oncol. 4:565–573. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chung KF: p38 mitogen-activated protein
kinase pathways in asthma and COPD. Chest. 139:1470–1479. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bhoj VG and Chen ZJ: Ubiquitylation in
innate and adaptive immunity. Nature. 458:430–437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karin M and Greten FR: NF-kappaB: linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Strasser A, Jost PJ and Nagata S: The many
roles of FAS receptor signaling in the immune system. Immunity.
30:180–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krammer PH: CD95’s deadly mission in the
immune system. Nature. 407:789–795. 2000.
|
|
35
|
Villa-Morales M and Fernández-Piqueras J:
Targeting the Fas/FasL signaling pathway in cancer therapy. Expert
Opin Ther Targets. 16:85–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu WH and Chang LS: Fas/FasL-dependent
and -independent activation of caspase-8 in doxorubicin-treated
human breast cancer MCF-7 cells: ADAM10 down-regulation activates
Fas/FasL signaling pathway. Int J Biochem Cell Biol. 43:1708–1719.
2011. View Article : Google Scholar
|