Introduction
Lymphadenoma, which is classified as sebaceous and
non-sebaceous lymphadenoma based on the presence or absence of
sebaceous differentiation, is a rare type of tumor of the salivary
gland. In the majority of cases, this type of tumor presents as a
painless mass of long duration, and the epithelial component
comprises benign basal and squamous cells. Since the first report
in 1960 by McGavran et al (1), to the best of our knowledge, <110
cases of the salivary gland have been reported in the English
language literature. However, this may be due to diagnostic
difficulty as this type of tumor partially resembles numerous other
types of salivary gland neoplasm, including cystadenoma, Warthin’s
tumor and pleomorphic adenoma; even mucoepidermoid carcinoma or
metastatic adenocarcinoma may enter into the differential diagnosis
(1–3). The present study reports a large
series of cases of lymphadenoma of the salivary gland in the
Chinese population, with a complete analysis of the clinical and
pathological data to enable the discussion of the features of the
clinical diagnosis and histogenesis in these cases.
Patients and methods
Clinical data
Ten consecutive patients with lymphadenoma in the
parotid gland who were treated at the Department of Oral and
Maxillofacial-Head and Neck Oncology, Ninth People’s Hospital,
Shanghai Jiao Tong University School of Medicine (Shanghai, China)
between 1996 and 2012 were retrospectively reviewed by their
clinical data (including age, gender and tumor location, process of
tumor development, imaging data and surgical treatment) and
pathological features.
Surgery
Following the signing of informed consent forms for
the surgery, all patients received surgical resection of the masses
with preservation of important neighboring structures, including
the facial nerve, great auricular nerve, sternocleidomastoideus
muscle, internal jugular vein and carotid arteries. All patients
provided written informed consent for their participation in this
study.
Histological and immunohistochemical
examination
A specimen from each patient was submitted for
histological examination and, following fixation in formalin
solution and inclusion in paraffin, 3–5-μm sections were stained
with hematoxylin and eosin for conventional evaluation. The
histopathological diagnoses of all patients following the surgery
were lymphadenoma. Immunohistochemical examination was performed in
all patients, including the detection of cytokeratin 8 (CK8), CK19,
Ki-67, CKpan, S-100, smooth muscle actin and vimentin. All patients
were followed up by a return visit with a follow-up period of 3–36
months. When the patients returned, routine physical examination
was performed, and if any suspicious mass was present in the
parotid gland and neck region, image examination was suggested.
Fine needle aspiration biopsy was also suggested if necessary.
Results
Demographic data
As shown in Tables I
and II, among the total 10 cases,
five were male and five were female (ratio of the tumor sites, six
left parotid gland to four right parotid gland). Three cases (two
male and one female) were diagnosed with sebaceous lymphadenoma and
seven (four female and three male) with non-sebaceous lymphadenoma.
The ratio of the tumor sites was two left parotid gland to one
right parotid gland for sebaceous lymphadenoma and four left
parotid gland to three right parotid gland for non-sebaceous
lymphadenoma. The mean age of all patients was 50.2 years, with a
range of 10–75 years. Patients >50 years old accounted for 50%
of the 10 patients and the ratio of sebaceous to non-sebaceous
lymphadenoma in these cases was 3:2. Only one patient was a child;
this was a 10-year-old male who was diagnosed with non-sebaceous
lymphadenoma.
 | Table ISebaceous and non-sebaceous
lymphadenomas: Clinical information. |
Table I
Sebaceous and non-sebaceous
lymphadenomas: Clinical information.
| Case | Age (years) | Gender | Site | Presentation | Size (cm) | Treatment | Pre-surgery
diagnosis | Follow-up
(months) | Recurrence |
|---|
| S 1 | 73 | M | L parotid gland | Mass for 6
months | 2.3×1.8 | Surgical
resection | Pleomorphic
adenoma | 24 | No |
| S 2 | 60 | M | R parotid gland | Mass for 3
months | 4.0×3.0 | Surgical
resection | Pleomorphic
adenoma | 36 | No |
| S 3 | 72 | F | L parotid gland | Mass for 20
years | 3.0×2.0 | Surgical
resection | Lymphadenoma | 36 | No |
| NS 1 | 20 | M | R parotid gland | Mass for 6 years | 2.0×3.0 | Surgical
resection | Pleomorphic
adenoma | 36 | No |
| NS 2 | 39 | F | R parotid gland | Mass for 5 years | 2.0×2.0 | Surgical
resection | Pleomorphic
adenoma | 36 | No |
| NS 3 | 75 | F | L parotid gland | Mass for 20
years | 5.0×6.0 | Surgical
resection | Pleomorphic
adenoma | 28 | No |
| NS 4 | 70 | F | L parotid gland | Unknown | 2.5×2.0 | Surgical
resection | Pleomorphic
adenoma | 36 | No |
| NS 5 | 33 | F | L parotid gland | Mass for 3 years | 2.0×1.5 | Surgical
resection | Pleomorphic
adenoma | 36 | No |
| NS 6 | 48 | M | L parotid gland | Mass for 1 month | 2.0×3.0 | Surgical
resection | Pleomorphic
adenoma | 36 | No |
| NS 7 | 10 | M | R parotid gland | Mass for 8
months | 1.0×1.0 | Surgical
resection | Pleomorphic
adenoma | 36 | No |
 | Table IIAll lymphadenoma cases. |
Table II
All lymphadenoma cases.
| Lymphadenoma
type | Mean age (range;
years) | Gender ratio |
|---|
| Sebaceous (n=3) | 68.3 (60–73) | 1 F:2 M |
| Non-sebaceous
(n=7) | 42.4 (10–75) | 4 F:3 M |
| All (n=10) | 50.2 (10–75) | 5 F:5 M |
Clinical study
All tumors occurred in the parotid gland and
presented as painless masses, which were slowly enlarging. The
duration of the symptoms ranged from a few months to 20 years.
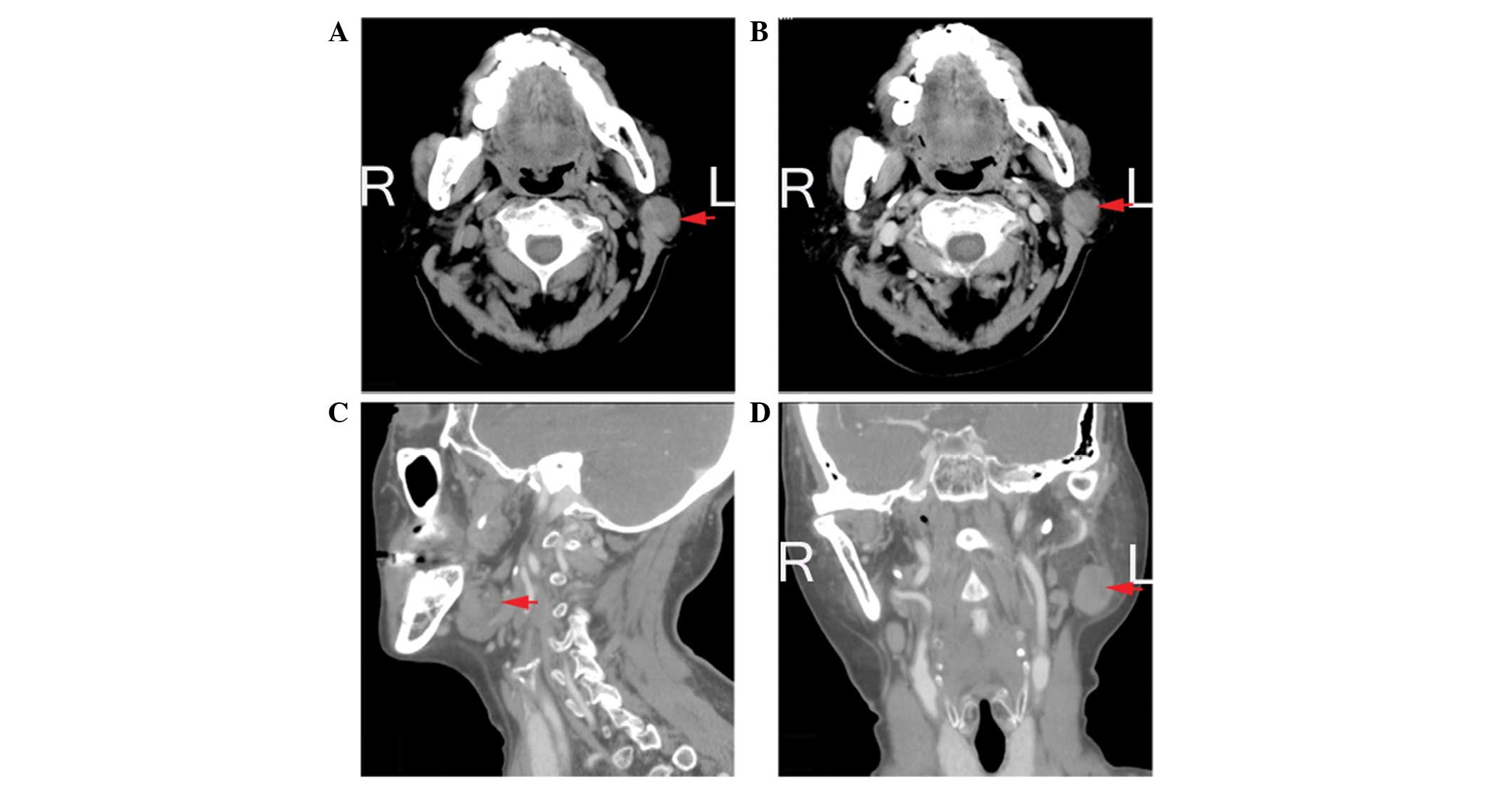
Fig. 1 shows the non-sebaceous
lymphadenoma computed tomography data of the fourth patient.
All patients underwent surgical therapy for the
tumors. The parotid lesions were excised by superficial or complete
parotidectomy with dissection and preservation of the facial
nerve.
During the follow-up period, which ranged between 3
and 36 months with a mean of 30 months, no recurrence of the lesion
occurred and the patient’s quality of life was good.
Histological analysis
All tumors were observed to be well circumscribed
and 8 of the 10 tumors (80%) were encapsulated. The contents in the
cystic tumors were gelatinous and yellow sebum-like.
Microscopically, in the cases of sebaceous
lymphadenoma the epithelial component comprised solid nests,
tubular structures, glands, cords, and tubules of basal, glandular
cells. There were always two cell layers, namely an outer basal
cell layer and an inner luminal glandular cell layer that was
typically composed of cuboidal or low columnar cells. This finding
is similar to those of a previous study (2). In the cases of non-sebaceous
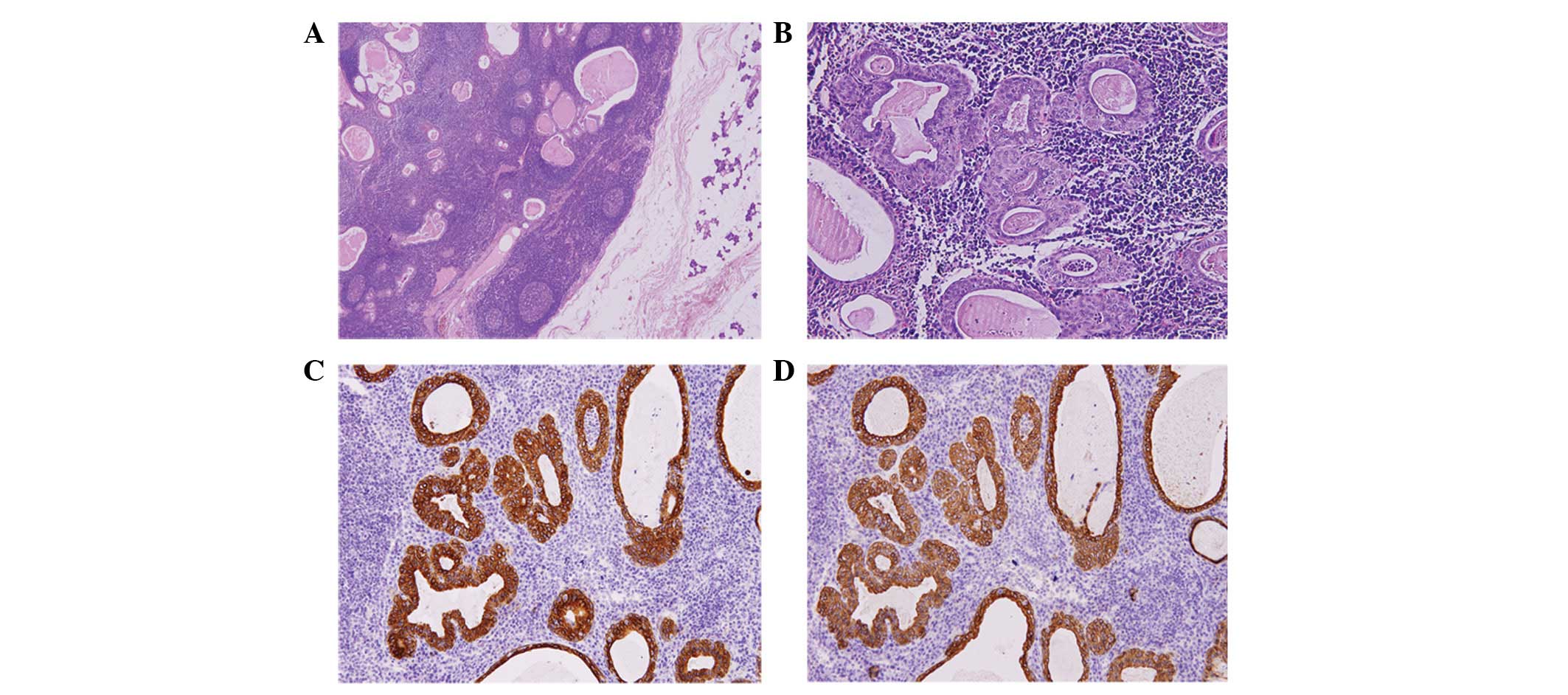
lymphadenoma, significant lymphoid stroma and an epithelial
component (Fig. 2A), which together
formed solid islands, tubular structures and also lumens of
different sizes, were visible. Similarly, two layers were detected
in the cases of non-sebaceous lymphadenoma. The outer cells were
flat, cubic or cylindrical. The chambers inside the outer layer, in
which the formation of lymphoid stroma follicles was usually
observed, contained eosinophilic red amorphous material, without
sebaceous secretion (Fig. 2B).
Notably, no specific immunohistochemical indicators
had been reported in previous studies, despite the salivary gland
lymphadenoma having characteristic pathological features. However,
in the present study, the majority of the tumors exhibited positive
immunohistochemical staining of CK8 and CKpan, as shown in Fig. 2C and D.
Discussion
Lymphadenoma, including sebaceous and non-sebaceous
lymphadenoma, is a rare type of salivary gland tumor. In the
present study, this type of tumor was observed to be well
circumscribed and exhibited typically benign behaviors, with the
majority of the cases affecting adults aged >30 years (80%). Of
the 10 cases, approximately one-third exhibited sebaceous
differentiation to an extent, which was the opposite to the
findings of a previous study (3).
The underlying specific reasons for this phenomenon are not yet
clear.
Dardick and Thomas (4) previously described the following
criteria for the diagnosis of non-sebaceous lymphadenoma: No
sebaceous differentiation; no oncocytic epithelium; a predominant
lymphocytic component; solid, glandular or cystic epithelial nests;
and lack of nodal capsule or subcapsular sinusoids. Although
lymphadenoma has been considered as a form of basal cell adenoma
accompanied by a heavy lymphoid infiltrate (5), according to this perspective,
lymphadenoma would be identified as a variant of other types of
adenomatous epithelial tumors with a prominent lymphoid component,
not a specific type of tumor. However, the majority of studies
strongly support the view that lymphadenoma is an entity comprising
a heterogeneous spectrum of epithelial differentiation and
including a variable degree of cystic transformation (7,8,9).
Numerous types of salivary gland tumor, including Warthin’s tumor,
acinic cell carcinoma, acquired immunodeficiency syndrome-related
lymphoepithelial cysts, lymphoepithelial carcinoma or
mucoepidermoid carcinoma, often have large amounts of lymphoid
stroma and epithelial neoplasms (6). Furthermore, the epithelium in
non-sebaceous lymphadenoma is morphologically bland and does not
infiltrate nearby tissue. Additionally, no mitotic activity and the
lack of invasive tumor features support the diagnosis of
non-sebaceous lymphadenoma (4).
The pathogenetic or histological origin of
non-sebaceous lymphadenoma has been proposed to be embryonic
salivary gland inclusions in the intraparotid or periparotid lymph
nodes. A strong argument for this hypothesis are that studies have
identified an unequivocal hilus structure with embryonic
parenchymal inclusions, in conjunction with frequent secondary
follicles and lymph vessels within the marginal sinus structures
(2,3,7,10).
However, this theory is in conflict with the majority of studies,
which instead regard the lymphoid component as reactive
tumor-associated lymphoid tissue (6,8,11–13).
According to the present study, only lymphoid component accompanied
with epithelial tissue was identified, which provides positive
evidence for the aforementioned second view. However, this is
uncertain due to the insufficient number of samples in the present
study.
Thus far, to the best of our knowledge, only 37
cases of non-sebaceous lymphadenoma have been reported in the
English language literature (2,4,6–8,10–16);
thus, the seven cases described in the present study increases the
total number of reported cases to 44. Although the present study
shared numerous equivalent findings with those of the previous
studies, there are also noteworthy differences. For example, one
case in the present study was in a 10-year-old male, which, to the
best of our knowledge, is the youngest case of non-sebaceous
lymphadenoma to be reported (9,10,16–18).
Lymphadenoma may be rare in children since sebaceous
differentiation in the salivary glands develops later in life and
is not present in infants and children (19).
Overall, the present study reported 10 cases of
lymphadenoma, in which the patients typically presented with a
painless mass for which complete surgical excision appeared to be
curative, and attempted to discuss an exact method of diagnosis for
this type of tumor, particularly for non-sebaceous lymphadenoma.
Due to the similarity of the clinical features of lymphadenoma with
those of other types of parotid tumor, cases of lymphadenoma are
often misdiagnosed and there are no remarkable clinical features
that completely distinguish lymphadenoma from other types of
parotid tumor. The most effective approach of identification is by
the pathology of the tumor. However, the exact mechanisms of the
tumorigenesis of non-sebaceous lymphadenoma from salivary gland
inclusions remain obscure. As there are few studies concerning
cases of lymphadenoma, there is a lack of knowledge of this type of
tumor. An improved understanding and a more in-depth investigation
of lymphadenoma in the salivary gland requires further studies
including larger numbers of cases.
Acknowledgements
This study was supported by grants of the National
Natural Science Foundation of China (NSFC81271112), and ‘Shu Guang’
project (10SG19) supported by Shanghai Municipal Education
Commission.
References
|
1
|
McGavran MH, Bauer WC and Ackerman LV:
Sebaceous lymphadenoma of the parotid salivary gland. Cancer.
13:1185–1187. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seethala RR, Thompson LD, Gnepp DR, et al:
Lymphadenoma of the salivary gland: clinicopathological and
immunohistochemical analysis of 33 tumors. Mod Pathol. 25:26–35.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weiler C, Agaimy A, Zengel P, Zenk J,
Kirchner T and Ihrler S: Nonsebaceous lymphadenoma of salivary
glands: proposed development from intraparotid lymph nodes and risk
of misdiagnosis. Virchows Arch. 460:467–472. 2012. View Article : Google Scholar
|
|
4
|
Dardick I and Thomas MJ: Lymphadenoma of
parotid gland: Two additional cases and a literature review. Oral
Surg Oral Med Oral Pathol Oral Radiol Endod. 105:491–494. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheuk W and Chan JK: Advances in salivary
gland pathology. Histopathology. 51:1–20. 2007. View Article : Google Scholar
|
|
6
|
Yang S, Chen X, Wang L and Zhang J:
Non-sebaceous lymphadenoma of the salivary gland: case report with
immunohistochemical investigation. Virchows Arch. 450:595–599.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castelino-Prabhu S, Li QK and Ali SZ:
Nonsebaceous lymphadenoma of the parotid gland: cytopathologic
findings and differential diagnosis. Diagn Cytopathol. 38:137–140.
2010.
|
|
8
|
Gallego L, Junquera L and Fresno MF:
Non-sebaceous lymphadenoma of the parotid gland:
immunohistochemical study and DNA ploidy analysis. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 107:555–558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang KT, Chadha NK, Leung R, Shago M,
Phillips MJ and Thorner PS: Lymphadenoma: case report of a rare
salivary gland tumor in childhood. Pediatr Dev Pathol. 13:331–337.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwon GY, Kim EJ and Go JH: Lymphadenoma
arising in the parotid gland: a case report. Yonsei Med J.
43:536–538. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bos I, Meyer S and Merz H: Lymphadenoma of
the parotid gland without sebaceous differentiation.
Immunohistochemical investigations. Pathologe. 25:73–78. 2004.(In
German).
|
|
12
|
Ma J, Chan JK, Chow CW and Orell SR:
Lymphadenoma: a report of three cases of an uncommon salivary gland
neoplasm. Histopathology. 41:342–350. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Musthyala NB, Low SE and Seneviratne RH:
Lymphadenoma of the salivary gland: a rare tumour. J Clin Pathol.
57:10072004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gnepp DR and Brannon R: Sebaceous
neoplasms of salivary gland origin. Report of 21 cases. Cancer.
53:2155–2170. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Auclair PL: Tumor-associated lymphoid
proliferation in the parotid gland. A potential diagnostic pitfall.
Oral Surg Oral Med Oral Pathol. 77:19–26. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang JY and Hsiao CH: Lymphadenoma
lacking sebaceous differentiation in the parotid gland. J Formos
Med Assoc. 103:459–462. 2004.PubMed/NCBI
|
|
17
|
Rawlinson NJ, Almarzooqi S and Nicol K:
Sebaceous lymphadenoma of the parotid gland in a 13-year-old girl:
a case report. Head Neck Pathol. 4:144–147. 2010.PubMed/NCBI
|
|
18
|
Sun G, Hu Q, Huang X and Tang E: Sebaceous
lymphadenoma of parotid gland in a child. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 107:253–255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Batsakis JG and el-Naggar AK: Sebaceous
lesions of salivary glands and oral cavity. Ann Otol Rhinol
Laryngol. 99:416–418. 1990. View Article : Google Scholar : PubMed/NCBI
|