Introduction
The life span of animals has grown longer due to
vaccinations for various infectious diseases, improvements in food
and environment and the development of veterinary medicine. As a
result, the incidence of various illnesses that are associated with
aging have been increasing in pet populations. In particular,
cancer is a significant problem. As in human medicine, there are
three major treatments for cancer in veterinary medicine, surgery,
chemotherapy and radiation. However, it is difficult to treat all
cases with these therapies. Therefore, it is necessary to develop
new treatments.
Indocyanine green (ICG) generates heat in response
to light near a wavelength of 800 nm (hyperthermia) (1–4).
Furthermore, ICG generates active oxygen in response to light at
600–800 nm (photodynamic effect) (5–8). The
principle advantage of ICG is low toxicity (9). ICG has become widely used during
sentinel biopsies (10 12). Based on this, we developed a new
cancer therapy, called photodynamic hyperthermal therapy (PHT),
using ICG and a broadband light source apparatus. We previously
reported that PHT induced morphological cell death and inhibited
the proliferation of the murine B16F10 melanoma cell line (13). Furthermore, it has been demonstrated
that PHT induces apoptosis and cell cycle arrest in vitro
(14). However, there are no in
vivo experimental data with regard to PHT-related tumor growth
and histological changes.
The present study aimed to investigate the effects
of PHT on tumor growth and histological changes using colon 26
tumor-bearing mice in vivo.
Materials and methods
Preparation of the tumor-bearing mouse
model
A total of 23 female five-week-old BALB/c mice were
purchased from CLEA Japan, Inc. (Osaka, Japan). The animals were
maintained under conventional conditions. The use of these animals
and the procedures they underwent were approved by the Animal
Research Committee of Tottori University. Colon 26 tissue, which is
of murine colon cancer origin, was transplanted subcutaneously into
the dorsal regions of the mice.
The mice were bred for nine days with free access to
food and water, following which, the experiments were performed.
The mice whose tumors grew to 5 mm in size were used in this
study.
Study design
The mice (n=23) were divided into four groups that
were subjected to light + ICG (PHT group; n=8), ICG alone (ICG
group; n=5), light alone (light group; n=5) or were untreated
(control group; n=5).
All the treatments were performed at day 0 under
general anesthesia with inhalation of 5% isoflurane. In the PHT
group, 25 mg ICG (Diagnogreen; Daiichi Sankyo, Tokyo, Japan) was
dissolved in 10 ml saline and adjusted to pH 5.0. A total of 0.5 ml
ICG solution was injected into the tumor tissue, following which,
irradiation was performed using a near-infrared light source (Super
Lizer™, Hyper 5000; maximum output, 5.0 W; 600–1600 nm of output
wavelength bands; Tokyo Iken Co., Ltd., Tokyo, Japan). Irradiation
was performed for 10 min using 20% output so that the distance from
the tumor to the light source was 3–5 cm. The temperature of the
tumor tissue and on the tumor surface during irradiation was
measured using a digital temperature indicator (Anritsu Meter Co.,
Ltd., Tokyo, Japan) and maintained in a range of 42.5–45.0°C in the
tumor and <45.0°C on the tumor surface. In the ICG group, the
ICG solution was injected without the administration of
irradiation. In the light group, irradiation was performed under
similar conditions to those that were used in the PHT group.
Following nine days of treatment (day 9), all the
mice were sacrificed by inhalation of 5% isoflurane followed by
cervical dislocation. On days 0 and 9, the volume of tumor tissue
was calculated by measuring the mediastinum and the transverse
length, and the depth of the tumor. Based on volumes of the tumor
on days 0 and 9, the tumor growth rate (mm3/day) was
calculated as follows: (tumor volume on day 9 - tumor volume on day
0)/9. The tumor tissue was removed and fixed in 10% buffered
formalin.
Histological examination
The fixed samples were embedded in paraffin and
sectioned in a routine manner. The sections were stained with
hematoxylin and eosin (HE staining) and examined
immunohistologically for Ki-67 and terminal
deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)
staining.
For the Ki-67 staining, 3-μm tissue sections were
placed on glass slides and deparaffinized, then washed with ethanol
and water and soaked in phosphate-buffered saline (PBS). The
sections were autoclaved using 0.01 M citrate buffer (pH 6.0) for
15 min at 121°C, washed with PBS and incubated with rabbit
polyclonal anti-Ki-67 antibodies (1:50; code no. E0468; Dako,
Glostrup, Denmark) for 30 min at room temperature. Subsequent to
being washed with PBS, the sections were incubated with rat
anti-immunoglobulin G antibodies (1:100; sc-372; Vector
Laboratories, Inc., Burlingame, CA, USA) for 30 min at room
temperature. The slides were washed with PBS and avidin/biotin
complex methods were performed (PK-4000; Vector Laboratories, Inc.)
for 30 min. The tissue sections were counterstained with histogreen
and then stained with nuclear fast red.
For the TUNEL staining, 3-μm tissue sections were
placed on glass slides and deparaffinized, then washed with ethanol
and water and soaked in diluted water. The TUNEL staining was
performed using an In situ Apoptosis Detection kit (Takara
Bio, Inc., Shiga, Japan) according to the manufacturer’s
instructions. The tissue sections were counterstained with
histogreen and then stained with nuclear fast red. A total of 10
random high-power fields were selected and the number of positive
cells was counted.
Image analysis of HE and Ki-67
staining
An analysis of the necrotic regions was performed
using the bio-imaging analysis system (Lumina Vision; Mitani
Corporation, Tokyo, Japan). The necrotic regions were assessed
based on the inhibition of cytoplasm, denaturation and nuclear
fragmentation. In brief, the images of 10 randomly chosen
high-power fields (magnification, ×200) in each cross section were
captured using a digital camera attached to an Olympus microscope
system (Olympus Corporation, Tokyo, Japan). The proportion of the
necrotic areas among the total area was calculated. All the tumor
tissues were analyzed. The mean proportion of the necrotic areas
was calculated.
With regard to the Ki-67 staining, a quantitative
digital morphometric analysis of the Ki-67-positive areas was
performed. In brief, the images of 10 randomly chosen high-power
fields (magnification, ×200) in each cross section were captured
using a digital camera attached to an Olympus microscope system
(Olympus Corporation). The color wavelengths of the copied image
were transformed into digital readings using the Lumina Vision
software program (Mitani Corporation), allowing for the
quantification of the various color wavelengths, with pixels as the
unit of measurement. The proportion of the positive areas in the
tumor tissues was calculated by dividing the total pixel area of
the positive areas by the total pixel area that corresponded with
the total tumor tissue in the field of view. The tumor tissues of
three mice in each group were analyzed. The mean proportion of the
positive areas in 30 fields was calculated in each group.
Statistical analysis
The data are expressed as the mean ± SE. The
statistical analyses were performed using one-way ANOVA, followed
by Tukey-Kramer’s test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of PHT on tumor growth
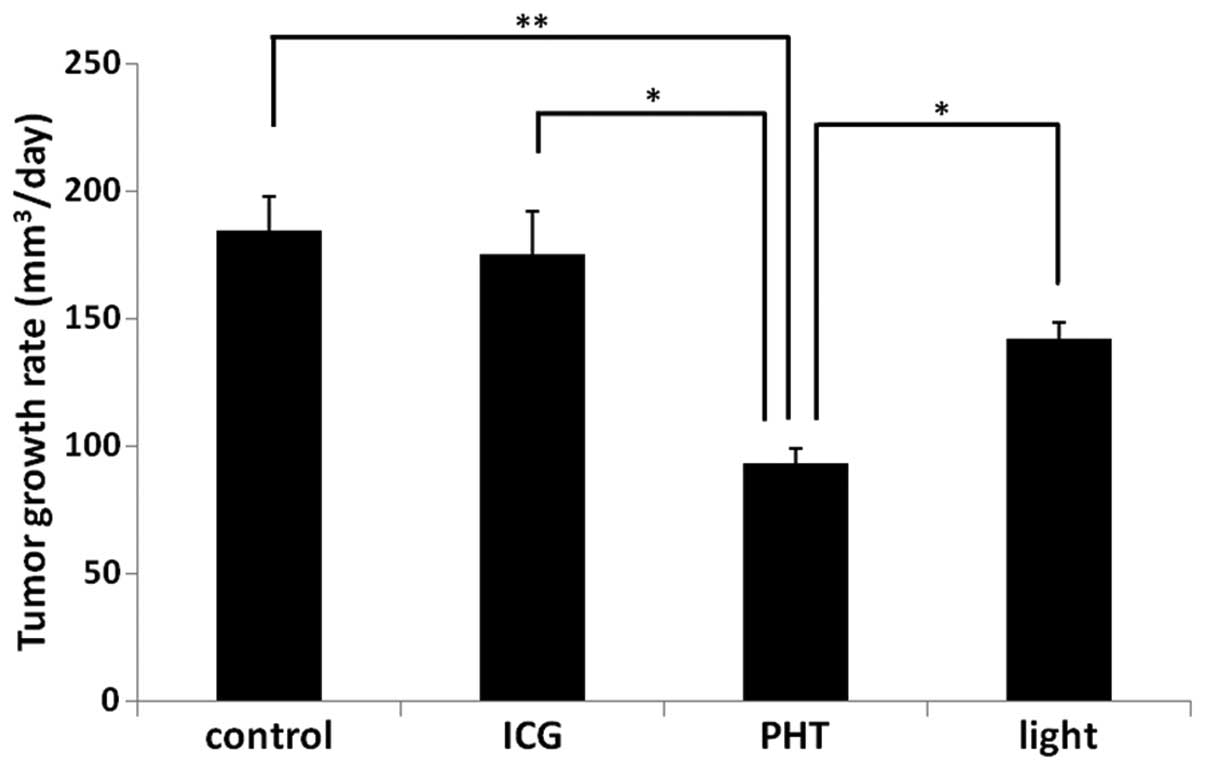
The tumor growth rates are shown in Fig. 1. In the PHT group (93.6±5.7
mm3/day), the growth rates of the tumor tissues were
significantly decreased compared with those observed in the ICG
(175.4±16.5 mm3/day), light (142.0±6.3
mm3/day) and control (184.8±13.0 mm3/day)
groups (P<0.05).
Histological observations
In the PHT group, uniform, large necrotic regions
were observed at the tumor margin on the skin side. By contrast,
numerous foci of necrosis were observed in the light group,
primarily at the tumor margin on the skin side. Among the necrotic
areas, no immunocytes, including lymphocytes or neutrophils, were
observed in any of the groups.
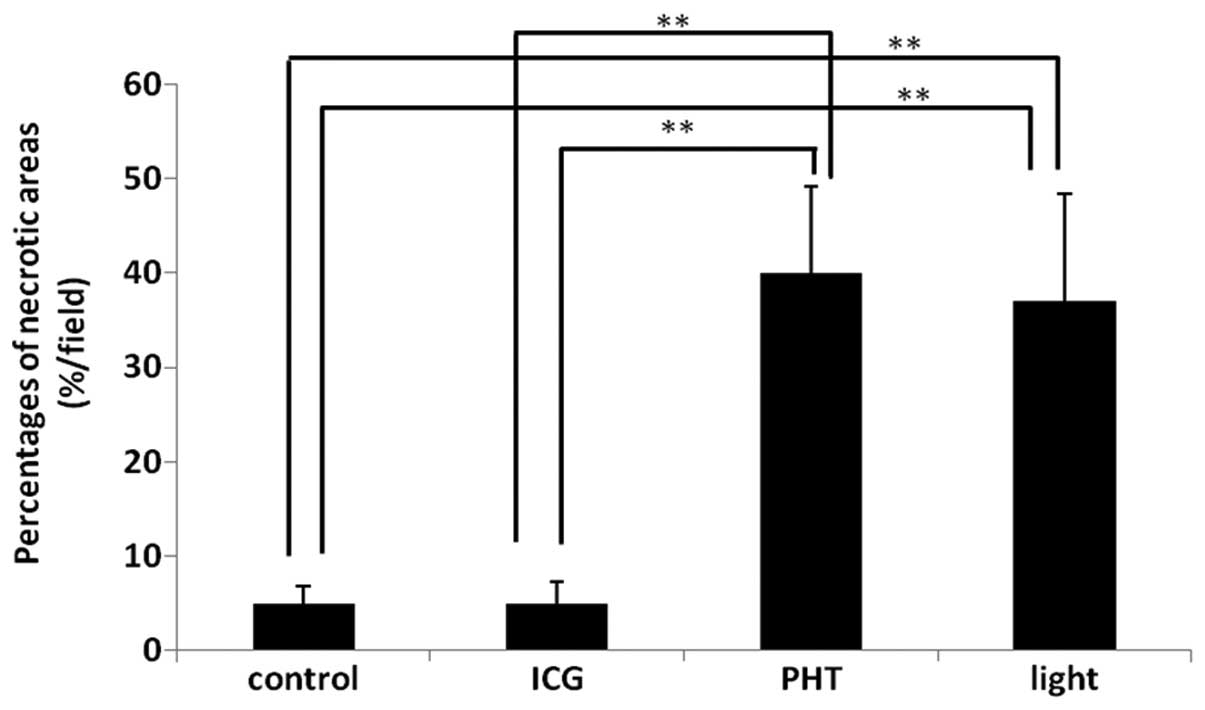
The proportions of the necrotic areas are shown in
Fig. 2. The values in the PHT
(40.0±9.1%) and light (37.0±11.4%) groups were increased
significantly compared with those observed in the ICG (5.0±2.3%)
and control (5.0±1.8%) groups. However, there were no significant
differences between the PHT and light groups.
Immunohistological analysis
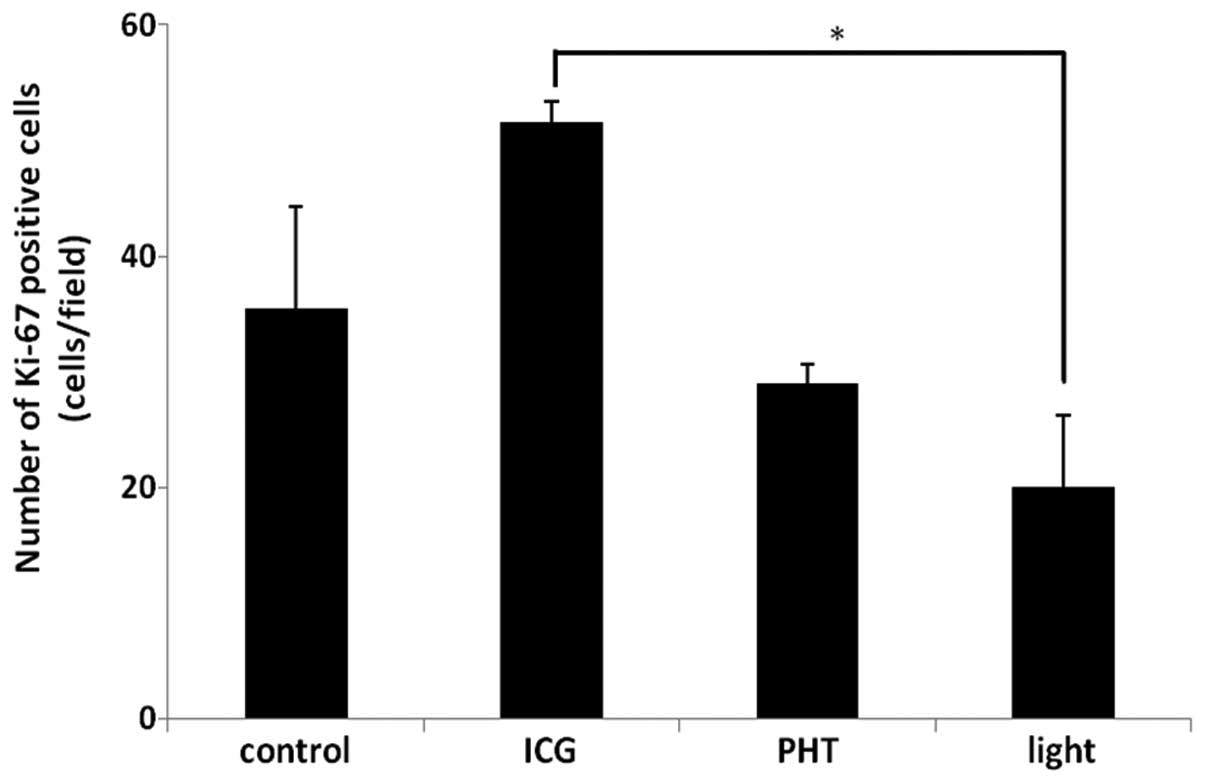
The results of the Ki-67 immunohistochemistry are
shown in Fig. 3. The proportions of
the Ki-67-positive areas in the PHT (29.0±1.6%/field)
and light (20.1±6.1%/field) groups were less than those observed in
the ICG (51.6±1.7%/field) and control (35.4±8.9%/field)
groups. In the light group, the proportion of the Ki-67-positive
areas was significantly decreased compared with that observed in
the ICG group (P<0.05).
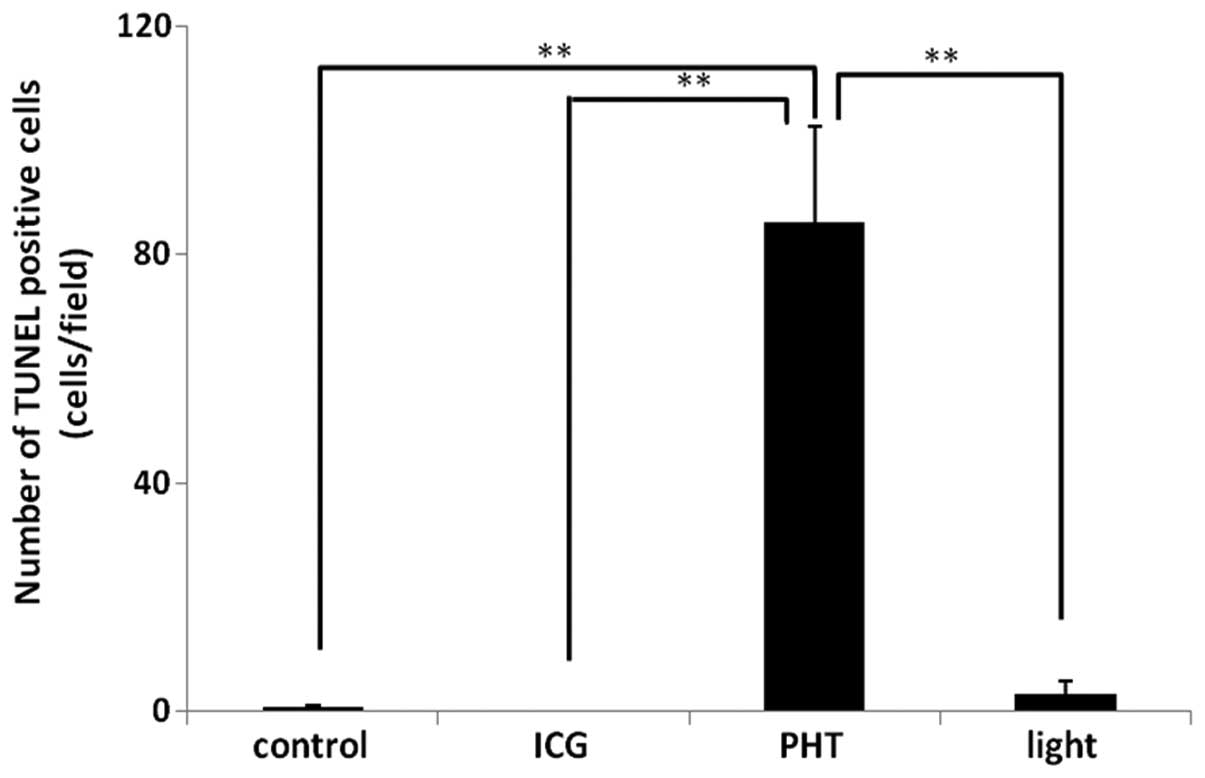
The results of the TUNEL immunohistochemistry are
shown in Fig. 4. The number of
TUNEL-positive cells (85.5±16.9 cells/field) in the PHT group was
increased significantly compared with that observed in the other
groups. In the ICG and control groups, almost no TUNEL-positive
cells were observed in the tumor tissues. In the light group,
TUNEL-positive cells were observed in the tumor tissues (3.0±2.3
cells/field).
Discussion
In the present study, PHT was observed to be
effective in vivo and in vitro. The tumor growth rate
in the PHT group was decreased significantly compared with that
observed in the other groups. It has been reported that the
combination of ICG and a 805-nm diode laser exhibits anticancer
efficacy in vivo and in vitro (1–3,12). In
these studies, it was speculated that ICG generated heat in
response to light near the 800-nm wavelength, which indicated
hyperthermia. In the present study, a broadband light source was
used instead of a diode laser in the PHT group, as ICG generates
active oxygen in response to light at 600–800 nm (photodynamic
effect) in addition to hyperthermia (5–8). The
present results revealed that PHT is more effective in suppressing
tumor growth compared with hyperthermia alone.
Histologically, the proportion of the necrotic areas
was similar between the PHT and light groups. This indicates that
hyperthermia alone induces tumor necrosis. With regard to the tumor
growth rates, the rate that was observed in the PHT group was
significantly decreased compared with the rate that was measured in
the light group, indicating that the tumor cells in the areas
without necrosis in the PHT group proliferated slowly compared with
those in the light group.
The slow proliferation of tumor cells implies that
numerous tumor cells do not proliferate due to causes such as cell
cycle arrest and apoptosis. The present study investigated cell
cycle arrest and apoptosis using immunohistochemical methods. TUNEL
staining is one method that is used to detect apoptosis (15,16).
The present results revealed that the number of TUNEL-positive
cells was greater in the PHT group than in the light group. This
result indicates that PHT strongly induces apoptosis compared with
hyperthermia. Ki-67 is a cell population marker that is detected
during all the active phases of the cell cycle, but is absent in
resting cells (17). With regard to
Ki-67 staining, the proportions of the Ki-67-positive areas in the
PHT and light groups were less than those observed in the ICG and
control groups, which indicates that PHT and hyperthermia induce
cell cycle arrest. However, there were no significant differences
between the PHT and light groups. Our previous data has shown that
PHT induces cell cycle arrest at an early time following the
treatment (14). The present
results do not support our previous data. One reason for this
discrepancy may be differences in the sampling time following the
treatment. In the present study, the samples were obtained at nine
days post-treatment. Periodical sampling (days 1, 3, 5 and 7)
following treatment is therefore required in future studies.
Tumor cells are more sensitive to heat under acidic
conditions (18). Therefore, saline
that was adjusted to pH 5.0 using acetic acid was used as a solvent
for ICG. In a preliminary experiment, saline that was adjusted to a
pH of 4.0 was observed to induce inflammation in the tissue.
In the present study, single treatments were
performed, following which, tumor growth was observed.
Consequently, it was identified that the single treatments were not
adequate to induce complete tumor remission. In order to achieve
complete remission, several rounds of treatment are necessary.
Further studies are therefore required.
In conclusion, the growth rates of the tumor tissues
were significantly decreased in the PHT group. Necrosis and
apoptosis were induced by the PHT treatment. The Ki-67-positive
areas were significantly decreased by the PHT treatment. The data
indicate the PHT has the potential to be a novel cancer treatment.
Further studies using clinical patients are required.
References
|
1
|
Chen WR, Adams RL, Higgins AK, Bartels KE
and Nordquist RE: Photothermal effects on murine mammary tumors
using indocyanine green and an 808-nm diode laser: an in vivo
efficacy study. Cancer Lett. 98:169–173. 1996. View Article : Google Scholar
|
|
2
|
Chen WR, Adams RL, Bartels KE and
Nordquist RE: Chromophore-enhanced in vivo tumor cell destruction
using an 808-nm diode laser. Cancer Lett. 94:125–131. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen WR, Adams RL, Heaton S, Dickey DT,
Bartels KE and Nordquist RE: Chromophore-enhanced laser-tumor
tissue photothermal interaction using an 808-nm diode laser. Cancer
Lett. 88:15–19. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu VG, Cowan TM, Jeong SW, Jacques SL,
Lemley EC and Chen WR: Selective photothermal interaction using an
805-nm diode laser and indocyanine green in gel phantom and chicken
breast tissue. Lasers Med Sci. 17:272–279. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abels C, Karrer S, Bäumler W, Goetz AE,
Landthaler M and Szeimies RM: Indocyanine green and laser light for
the treatment of AIDS-associated cutaneous Kaposi’s sarcoma. Br J
Cancer. 77:1021–1024. 1998.
|
|
6
|
Bäumler W, Abels C, Karrer S, Weiss T,
Messmann H, Landthaler M and Szeimies RM: Photo-oxidative killing
of human colonic cancer cells using indocyanine green and infrared
light. Br J Cancer. 80:360–363. 1999.PubMed/NCBI
|
|
7
|
Hirano T, Kohno E, Gohto Y and Obama A:
Singlet oxygen generation due to ICG irradiation. Photomed
Photobiol. 28:15–16. 2006.
|
|
8
|
Bozkulak O, Yamaci RF, Tabakoglu O and
Gulsoy M: Photo-toxic effects of 809-nm diode laser and indocyanine
green on MDA-MB231 breast cancer cells. Photodiagnosis Photodyn
Ther. 6:117–121. 2009. View Article : Google Scholar
|
|
9
|
Hope-Ross M, Yannuzzi LA, Gragoudas ES,
Guyer DR, Slakter JS, Sorenson JA, Krupsky S, Orlock DA and
Puliafito CA: Adverse reactions due to indocyanine green.
Ophthalmology. 101:529–533. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirche C, Murawa D, Mohr Z, Kneif S and
Hünerbein M: ICG fluorescence-guided sentinel node biopsy for
axillary nodal staging in breast cancer. Breast Cancer Res Treat.
121:373–378. 2010. View Article : Google Scholar
|
|
11
|
Hojo T, Nagao T, Kikuyama M, Akashi S and
Kinoshita T: Evaluation of sentinel node biopsy by combined
fluorescent and dye method and lymph flow for breast cancer.
Breast. 19:210–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito N, Fukuta M, Tokushima T, Nakai K and
Ohgi S: Sentinel node navigation surgery using indocyanine green in
patients with lung cancer. Surg Today. 34:581–585. 2004.
|
|
13
|
Radzi R, Osaki T, Tsuka T, Imagawa T,
Minami S and Okamoto Y: Morphological study in B16F10 murine
melanoma cells after photodynamic hyperthermal therapy with
indocyanine green (ICG). J Vet Med Sci. 74:465–472. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Radzi R, Osaki T, Tsuka T, Imagawa T,
Minami S, Nakayama Y and Okamoto Y: Photodynamic hyperthermal
therapy with indocyanine green (ICG) induces apoptosis and cell
cycle arrest in B16F10 murine melanoma cells. J Vet Med Sci.
74:545–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Allen RT, Hunter WJ 3rd and Agrawal DK:
Morphological and biochemical characterization and analysis of
apoptosis. J Pharmacol Toxicol Methods. 37:215–228. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Otsuki Y, Li Z and Shibata MA: Apoptotic
detection methods - from morphology to gene. Prog Histochem
Cytochem. 38:275–339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brown DC and Gatter KC: Ki67 protein: the
immaculate deception? Histopathology. 40:2–11. 2002. View Article : Google Scholar
|
|
18
|
Gerweck LE: Modification of cell lethality
at elevated temperatures. The pH effect. Radiat Res. 70:224–235.
1977. View
Article : Google Scholar
|