Introduction
Colorectal adenocarcinoma is one of the most common
malignancies and its prevalence has recently been increasing. With
the second highest incidence among all types of cancer, it is also
the second most common cause of cancer-related mortality worldwide
(1,2). The carcinogenesis of colorectal
adenocarcinoma is a multi-step process that involves a number of
genomic alterations. Investigating the molecular mechanisms of this
process may aid in cancer prevention, early diagnosis and the
development of effective treatments.
Employing a cDNA subtractive library and cDNA
microarray technology, our previous study identified 86 cDNA
sequences differentially expressed between human colorectal
adenocarcinoma and paratumor normal colorectal tissues (3). In particular, the present study
focused on one differentially expressed sequence (GenBank accession
number, ES274071), identified its full-length cDNA and determined
it to be the SET gene. The SET gene is a largely
nuclear protein, located on chromosome 9q34 centromeric to c-abl
and Nup214, which is fused to Nup214 (also termed CAN), apparently
as a result of translocation (4,5). It is
predicted to encode a 27-amino acid protein with a molecular weight
of 39 kDa. SET is overexpressed and has been found to play a role
in acute myeloid leukemia oral carcinoma and ovarian cancer
(6–9), but its involvement in the development
of colorectal adenocarcinoma remains unknown. In order to determine
whether SET is involved in the carcinogenesis of colorectal
adenocarcinoma and the Wnt signaling pathway, the mRNA expression
of SET, protein phosphatase 2 (PP2A) and
β-catenin in human colorectal adenocarcinoma and paratumor
normal tissues was determined by quantitative real-time polymerase
chain reaction (qPCR) analysis. In addition, SET expression
was knocked down by transient transfection of small interfering RNA
(siRNA) targeting SET, and the intracellular changes were
investigated at the mRNA and protein level.
Materials and methods
Tissue specimens
Colorectal adenocarcinoma and paratumor normal
tissues were obtained from 31 patients at the West China Hospital
of Sichuan University (Chengdu, China). The sample acquisition was
approved by the Medical Research Ethics Committee of Sichuan
University (Chengdu, China) and written informed consent was
obtained from all patients. The absence of cancer cells in the
collected normal tissues was confirmed through pathological
examination, and these tissues were snap-frozen and stored in
liquid nitrogen prior to subsequent analysis.
Antibodies
The antibodies used were as follows: Anti-SET (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), -PP2A, -β-catenin,
-c-Myc and -β-actin (Signalway Antibody Co., Ltd., College Park,
MD, USA); and anti-rabbit or -mouse horseradish
peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc.). Detection was performed using a
Chemiluminescent Western detection kit (Thermo Fisher Scientific,
Waltham, MA, USA).
qPCR of tissue RNA extracts
Total RNA from tumor and paratumor normal tissues
was isolated using the TRIzol RNA isolation reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. cDNA was synthesized using the M-MuLV
reverse transcriptase kit (Fermentas International Inc.,
Burlington, ON, Canada) and qPCR was performed using SYBR Premix Ex
Taq (Takara Bio, Inc., Shiga, Japan). The amplification reaction
was conducted according to the manufacturer’s instructions and
associated international standards (10,11).
qPCR primers used to amplify the SET, PP2A and
β-catenin genes are shown in Table I.
 | Table IQuantitative real-time polymerase
chain reaction primers. |
Table I
Quantitative real-time polymerase
chain reaction primers.
| Expression | Primer sequence | Product length,
bp |
|---|
| SET | Forward:
5′-GCTCAACTCCAACCACGAC-3′
Reverse: 5′-TCCTCACTGGCTTGTTCATTA-3′ | 120 |
| PP2A | Forward:
5′-AGTTGGCCAAATGTGTCTCC-3′
Reverse: 5′-GAGTTGCGGTACAAGGAAGG-3′ | 145 |
| β-catenin | Forward:
5′-GCTGGTGACAGGGAAGACATC-3′
Reverse: 5′-GGTAGTCCATAGTGAAGGCGAAC-3′ | 116 |
| E-cadherin | Forward:
5′-TTGCTACTGGAACAGGGACACT-3′
Reverse: 5′-GGAGATGTATTGGGAGGAAGGTC-3′ | 154 |
| c-Myc | Forward:
5′-TCAAGAGGCGAACACACAAC-3′
Reverse: 5′-GGCCTTTTCATTGTTTTCCA-3′ | 110 |
| p53 | Forward:
5′-TAGTGTGGTGGTGCCCTATGAG-3′
Reverse: 5′-AGTGTGATGATGGTGAGGATGG-3′ | 129 |
| GAPDH | Forward:
5′-GGAAGGTGAAGGTCGGAGT-3′
Reverse: 5′-TGAGGTCAATGAAAGGGGTC-3′ | 117 |
Cell culture
Human colon adenocarcinoma cell lines, LS174T and
SW480 (American Type Culture Collection, Manassas, VA, USA), were
cultured in DMEM, with 10% fetal bovine serum, penicillin (100
U/ml) and streptomycin (0.1 mg/ml) in a humidity controlled 37°C
incubator with 5% CO2 atmosphere.
Knockdown of SET mRNA expression and
transient transfection
The knockdown of SET expression was conducted
via the transfection of siRNA at a concentration of 100 nM. Cells
were transiently transfected with a pool of six siRNA duplexes
targeting SET (cat. no. 10621164446; Guangzhou RiboBio Co.,
Ltd., Guangzhou, China). LS174T and SW480 cells were seeded at a
density of 2.3×106 cells/well in 100-mm dishes.
Transient transfection of siRNA targeting SET or control
oligonucleotides into LS174T and SW480 cells was performed using
Lipofectamine 2000 (Invitrogen Life Technologies).
qPCR analysis of siRNA-transfected
cells
The knockdown cells lines of SET mRNA were
assessed by qPCR. According to the manufacturer’s instructions,
total RNA from the tumor and matched normal tissues were isolated
using the TRIzol RNA isolation reagent (Invitrogen Life
Technologies). The total RNA was reverse-transcribed using the
M-MuLV Reverse Transcriptase kit (Fermentas International Inc.) and
qPCR was performed using SYBR Premix ExTaq (Takara). qPCR was
performed on the the tumor and matched normal tissues of patients
with colorectal cancer. The mean threshold cycle (Ct) and standard
error were calculated from individual Ct values obtained from three
replicates per specimen. The normalized mean Ct was calculated as
ΔCt by subtracting the mean Ct of GAPDH from the target genes. ΔΔCt
was calculated as the difference between the control ΔCt and the
values obtained for each sample. The n-fold change in gene
expression, relative to the untreated controls, was calculated
using the 2−ΔΔCt method (12). qPCR primers used for the
amplification of these genes are shown in Table I.
Western blot analysis
Protein extracts of the siRNA-transfected cells were
acquired by lysing cells in ice-cold radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Haimen, China)
according to the manufacturer’s instructions. The total protein
concentration was determined using an Enhanced BCA Protein assay
kit (Beyotime Institute of Biotechnology). Equal protein samples
were then denatured and separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (Millipore, Billerica,
MA, USA), transferred to nitrocellulose membranes (Millipore) and
immunoblotted with anti-SET, -PP2A, -β-catenin, -c-Myc and -β-actin
antibody.
Statistical analysis
Data are presented as the means ± standard deviation
of three or more replicate experiments. Statistical analysis was
performed by Student’s t-test, Fisher’s exact probability test and
analysis of variance (ANOVA) using SPSS 19.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
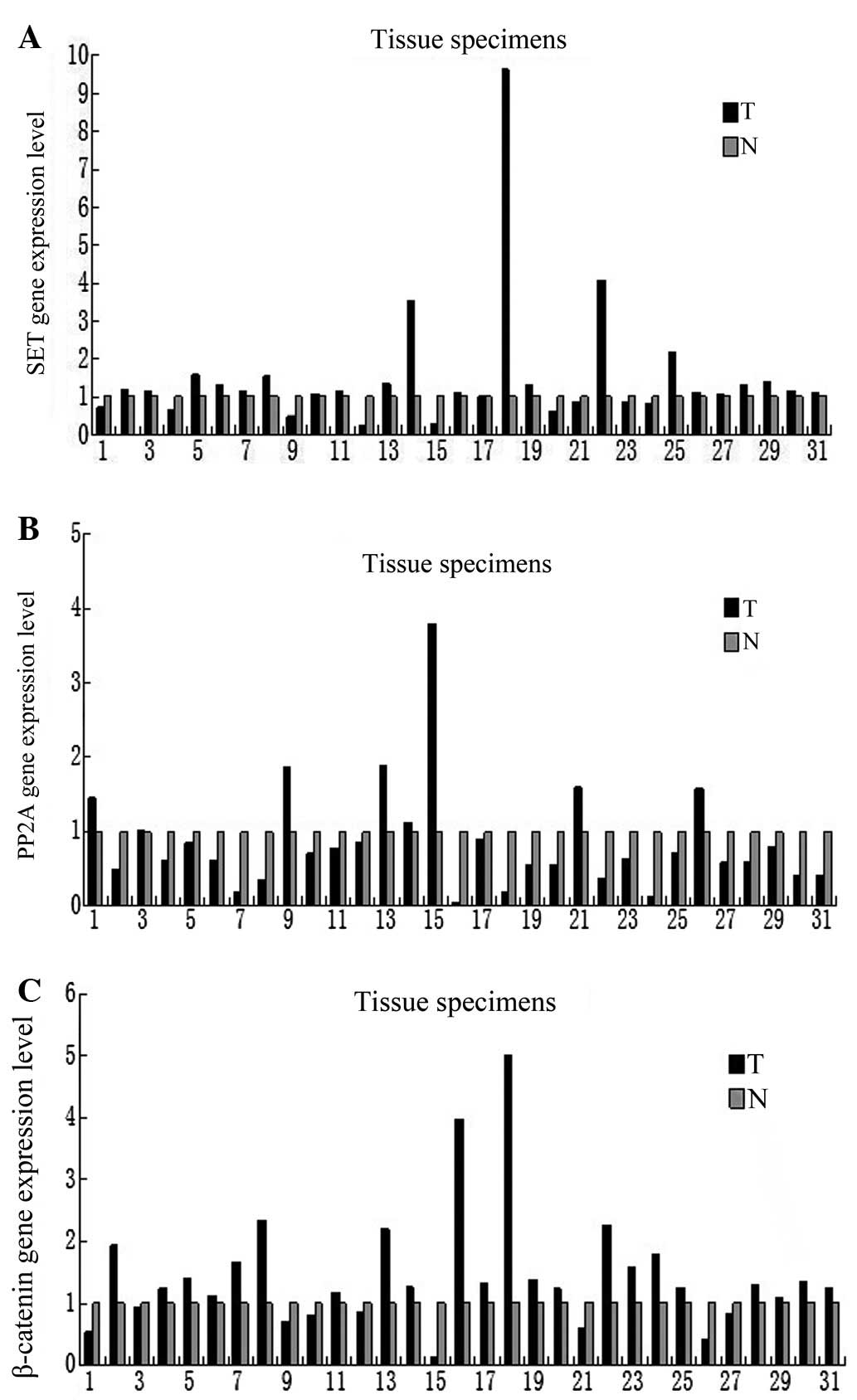
mRNA expression levels of SET, PP2A and
β-catenin in human colorectal adenocarcinoma tissues
The expression of SET, PP2A and
β-catenin at the mRNA level was tested in
non-malignant and malignant tissues, and SET was found to be
markedly elevated in 70.9% of the tumor samples (22 out of 31
samples; Fig. 1A). PP2A was
upregulated in 25.8% of the tumor samples (eight out of 31;
Fig. 1B), while β-catenin
was upregulated in 70.9% of the tumor samples (22 out of 31;
Fig. 1C) compared with the
paratumor normal tissues. No significant correlation was found
between the mRNA expression levels of SET, PP2A and
β-catenin and gender, age, Dukes’ stage and
differentiation degree by Fisher’s exact probabilities test
(Table II).
 | Table IISET, PP2A and β-catenin expression
and patient characteristics. |
Table II
SET, PP2A and β-catenin expression
and patient characteristics.
| Gender | Age, years | Dukes’ stage | Differentiation
degree |
|---|
|
|
|
|
|
|---|
| Expression | Male, n | Female, n | ≥50 | <50 | A | B | C | D | Well | Moderate | Poor |
|---|
| Upregulation |
| SET | 13 | 9 | 15 | 7 | 1 | 8 | 10 | 3 | 2 | 19 | 1 |
| PP2A | 5 | 3 | 4 | 4 | 0 | 6 | 1 | 1 | 1 | 6 | 1 |
| β-catenin | 14 | 8 | 15 | 7 | 2 | 9 | 9 | 2 | 2 | 19 | 1 |
| Downrugulation |
| SET | 5 | 4 | 6 | 3 | 1 | 6 | 2 | 0 | 2 | 6 | 1 |
| PP2A | 13 | 10 | 16 | 7 | 2 | 8 | 11 | 2 | 3 | 19 | 1 |
| β-catenin | 13 | 10 | 16 | 7 | 2 | 8 | 11 | 2 | 3 | 19 | 1 |
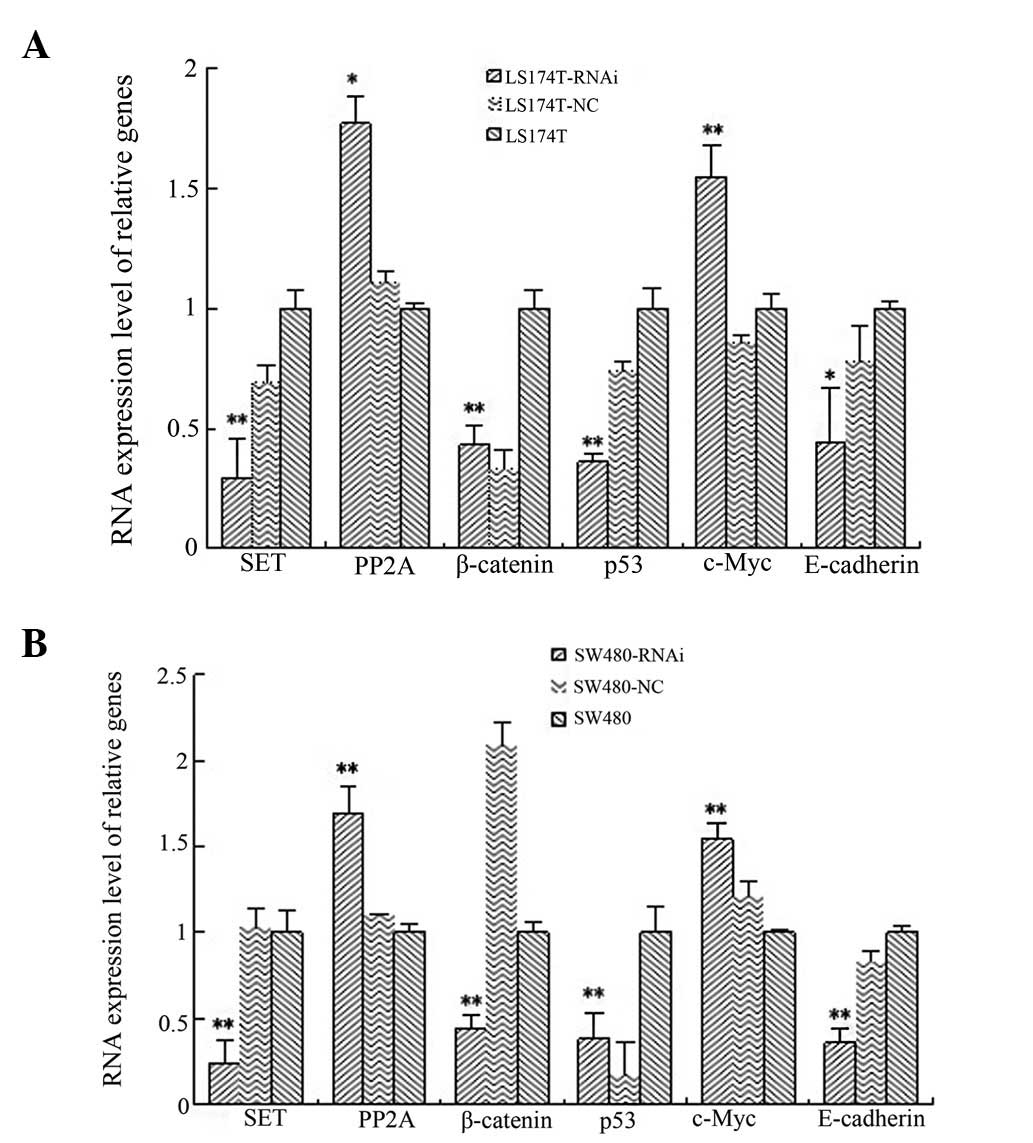
mRNA expression levels of SET, PP2A,
β-catenin, p53, c-Myc and E-cadherin in LS174T and SW480 cell lines
following SET knockdown
The mRNA expression levels of SET were
analyzed in the LS174T SET-knockdown cell line and found to
be significantly lower than those in the LS174T cell line. The
reduction rate was 69.43%, which was statistically significant as
shown by the ANOVA test (P=0.002). In addition, the SET
expression levels in the SW480 SET-knockdown cell line were
significantly lower than those in the SW480 cell line. The
reduction rate was 75.68%, which was statistically significant as
shown by the ANOVA test (P<0.001). Compared with the control
cells, the mRNA expression levels of p53, β-catenin and
E-cadherin were reduced in the LS174T and SW480
SET-knockdown cell lines. The percentage reduction in gene
expression in the LS174T cells was 35.38, 62.89 and 26.29%,
respectively, and all these values were found to be statistically
significant by ANOVA when compared with the blank control group and
the negative control groups (P=0.001, P=0.001 and P=0.048,
respectively). The respective decrease rates in the SW480 cells
were 23.16, 63.14 and 47.88%, and all these values were found to be
statistically significant by ANOVA when compared with the blank
control and the negative control groups (P=0.001, P<0.001 and
P=0.001, respectively).
The expression of PP2A and c-Myc were
found to be elevated in the SET-knockdown cells. The
increase rates in gene expression were 70.53 and 46.40%,
respectively, in LS174T cells (P=0.015 and P=0.002, respectively),
whereas the increase rates were 61.33 and 37.55%, respectively, in
SW480 cells (P=0.001 and P<0.001, respectively) (Fig. 2).
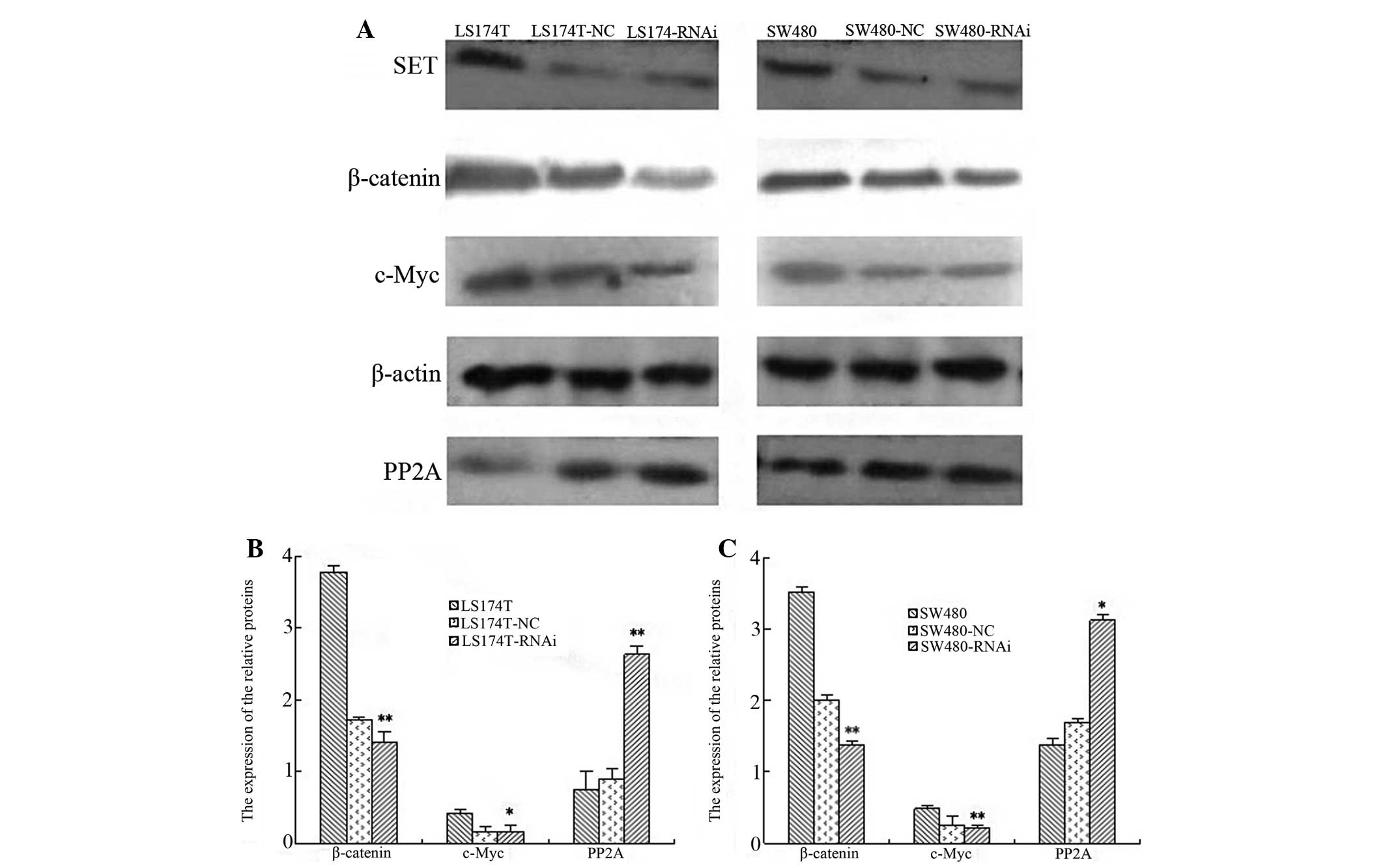
Knockdown of SET alters the protein
expression of β-catenin, c-Myc, SET and PP2A in LS174T and SW480
cell lines
The knockdown of SET led to the reduced
protein expression of c-Myc and β-catenin in the LS174T and SW480
cell lines. The inhibition rates were 45.14 and 62.06%,
respectively, in the LS174T cells (P=0.029 and P=0.003), whereas in
the SW480 cells, the rates were 42.32 and 51.48%, accordingly (both
P<0.001). Additionally, the protein expression of PP2A was
upregulated in the LS174T and SW480 cell lines following SET
knockdown. The increase rate was 37.55% in the LS174T cells
(P=0.012), whereas in theSW480 cells, the rate was 28.39% (P=0.025)
(Fig. 3).
Discussion
SET is a multifunctional protein that is
overexpressed in human neoplasms (9,13). The
SET protein is a potent PP2A inhibitor that is overexpressed in
various human malignancies. Previously, it has been demonstrated
that SET upregulation, which leads to PP2A inhibition, is critical
for BCR/ABL-positive cells to fulfill their tumorigenic potential
(14). Recently, Jiang et al
(6) reported that SET was
overexpressed at the mRNA level in 21 tumor samples (70.0%)
compared with the corresponding normal tissues. The results of the
present study also identified that SET expression (at the
mRNA level) in 31 patients was markedly increased in 70.9% of tumor
specimens compared with adjacent normal tissues. Therefore, these
results indicated that SET overexpression correlates with
colorectal carcinoma progression and that it may play a vital role
in the pathogenesis of colorectal cancer. In addition, PP2A was
upregulated in 25.8% of samples. PP2A is a widely conserved protein
serine/threonine phosphatase that functions as a trimeric protein
complex consisting of a catalytic subunit (C or PP2Ac), a scaffold
subunit (A or PR65) and one of the alternative regulatory B
subunits (15). PP2A plays a
crucial role in regulating the cell cycle, signal transduction,
cell differentiation, DNA replication and malignant transformation.
Previous studies have shown that in target molecules, for which
dephosphorylation is critical for the tumor suppressor (16,17),
dephosphorylation of the oncogenic transcription factor, c-Myc, is
critical for PP2A tumor suppressor activity. Inhibition of PP2A
activity induces c-Myc serine 62 (S62) phosphorylation and c-Myc
protein stabilization. In addition, it has been reported that c-Myc
S62 dephosphorylation inhibits cellular transformation and
PP2A-mediated c-Myc dephosphorylation. The c-Myc dephosphorylation
is suffice for SV40 small t antigen in human transformation assay
activity of PP2A (18). The
Wnt/β-catenin signaling pathway often correlates with the
overexpression or amplification of the c-Myc oncogene. Paradoxical
to the cellular transformation potential of c-Myc is its ability to
also induce apoptosis. Notably, c-Myc has been identified as a
transcriptional target of the adenomatous polyposis coli
(APC)/β-catenin/T-cell factor pathway in colorectal cancer cells
(19), suggesting that a method of
Wnt signaling function in oncogenesis is through the growth
promoting activity of c-Myc (20–22).
Our previous study showed that PP2A gene expression was
increased following SET knockdown. Although depletion of
SET effectively reduced c-Myc S62 protein steady-state
levels, c-Myc mRNA expression was not significantly
decreased by SET depletion, implying that SET
regulates c-Myc S62 protein levels post-transcriptionally through
inhibition of PP2A-mediated c-Myc dephosphorylation.
A criticial function of the Wnt pathway is to
activate β-catenin-dependent transcription via
phosphorylation-regulation. Wnt signaling is transduced through
β-catenin, which is regulated by the APC/Axin/glycogen synthase
kinase (GSK) 3β complex (23–25).
In the absence of Wnt stimulation, GSK-3β constitutively
phosphorylates β-catenin at the serine and threonine residues of
the NH2-terminal region (known as the GSK-3β consensus
site), which is well conserved within the catenin family of
proteins (26). Phosphorylated
β-catenin is subsequently ubiquitinated and degraded through the
proteasome pathway. The results of the current study showed that
β-catenin expression (at the mRNA level) was upregulated in
70.9% of the tumor samples. In addition, the expression (at mRNA
and protein level) was significantly decreased following SET
knockdown. Overall, these results suggested that β-catenin is
degraded through phosphorylation via the inhibition of the SET.
Previously, Tian et al (27)
reported that the aberrant expression of the E-cadherin/β-catenin
complex is associated with a wide variety of human malignancies and
fibrotic disorders. In the present study, E-cadherin expression was
significantly downregulated in the LS174TRNAi and
SW480RNAi cell lines, suggesting that the suppression of
the Wnt signaling pathway in these cells may have resulted from
E-cadherin downregulation, which then led to the downregulation of
c-Myc expression through the inhibition of the nuclear
translocation of β-catenin.
SET is critical for colorectal adenocarcinoma cell
growth, since SET knockdown by specific siRNA results in
significantly promoting apoptosis in vivo (6). Previously, Koldobskiy et
al(28) showed that p53 is a
tumor suppressor of which numerous mutations have been found in
>50% of malignancies. It has also been found that p53 not only
inhibits cell growth, but induces apoptosis. However, in the
present study, p53 expression levels were found to significantly
decrease in the LS174TRNAi and SW480RNAi cell
lines, indicating that this increased cell apoptosis was not
directly induced by p53.
In the current study, SET expression at the mRNA and
protein level was found to be markedly reduced in LS174T and SW480
SET-knockdown cell lines. Furthermore, the expression of
PP2A increased and c-Myc levels decreased following SET
knockdown. In conclusion, these results clearly suggested that
SET silencing decreases Wnt signaling, indicating that SET
plays a crucial role in the Wnt signaling pathway. We hypothesize
that SET is a diagnostic marker for prognosis, particularly,
neoplasm invasiveness in colorectal cancer. However, future studies
are required to determine in detail this correlation and to
elucidate the underlying mechanism.
Acknowledgements
The current study was supported by a grant from the
National Natural Science Foundation of China (no. 81072023).
References
|
1
|
Bosetti C, Levi F, Rosato V, Bertuccio P,
Lucchini F, Negri E and La Vecchia C: Recent trends in colorectal
cancer mortality in Europe. Int J Cancer. 129:180–191. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shu Z and Shanrong C: Colorectal cancer
epidemiology and prevention study in China. Chin Ger J Clin Oncol.
2:72–75. 2003. View Article : Google Scholar
|
|
3
|
Chen Y, Zhang YZ, Zhou ZG, et al:
Identification of differentially expressed genes in human
colorectal adenocarcinoma. World J Gastroenterol. 12:1025–1032.
2006.
|
|
4
|
von Lindern M, van Baal S, Wiegant J, Raap
A, Hagemeijer A and Grosveld G: Can, a putative oncogene associated
with myeloid leukemogenesis, may be activated by fusion of its 3′
half to different genes: characterization of the set gene. Mol Cell
Biol. 12:3346–3355. 1992.PubMed/NCBI
|
|
5
|
Li M, Makkinje A and Damuni Z: The myeloid
leukemia-associated protein SET is a potent inhibitor of protein
phosphatase 2A. J Biol Chem. 271:11059–11062. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang Q, Zhang C, Zhu J, Chen Q and Chen
Y: The SET gene is a potential oncogene in human colorectal
adenocarcinoma and oral squamous cell carcinoma. Mol Med Rep.
4:993–999. 2011.PubMed/NCBI
|
|
7
|
Van Vlierberghe P, van Grotel M, Tchinda
J, et al: The recurrent SET-NUP214 fusion as a new HOXA activation
mechanism in pediatric T-cell acute lymphoblastic leukemia. Blood.
111:4668–4680. 2008.PubMed/NCBI
|
|
8
|
Quentmeier H, Schneider B, Röhrs S, Romani
J, Zaborski M, Macleod RA and Drexler HG: SET-NUP214 fusion in
acute myeloid leukemia and T-cell acute lymphoblastic
leukemia-derived cell lines. J Hematol Oncol. 2:32009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ouellet V, Le Page C, Guyot MC, LuSSier C,
Tonin PN, Proveneher DM and Mes-Masson AM: SET complex in serous
epithelial ovarian cancer. Int J Cancer. 119:2119–2126. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bustin SA, Benes V, Garson JA, et al: The
MIQE guidelines: minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nolan T, Hands RE and Bustin SA:
Quantification of mRNA using real-time RT-PCR. Nat Protoc.
1:1560–1581. 2006. View Article : Google Scholar
|
|
12
|
Kim DW, Kim KB, Kim JY, Lee KS and Seo SB:
Negative regulation of neuronal cell differentiation by INHAT
subunit SET/TAF-Iβ. Biochem Biophys Res Commun. 400:419–425.
2010.PubMed/NCBI
|
|
13
|
Cristóbal I, Garcia-Orti L, Cirauqui C,
Cortes-Lavaud X, García-Sánchez MA, Calasanz MJ and Odero MD:
Overexpression of SET is a recurrent event associated with poor
outcome and contributes to protein phosphatase 2A inhibition in
acute myeloid leukemia. Haematologica. 97:543–550. 2012.
|
|
14
|
Neviani P, Santhanam R, Trotta R, et al:
The tumor suppressor PP2A is functionally inactivated in blast
crisis CML through the inhibitory activity of the BCR/ABL-regulated
SET protein. Cancer Cell. 8:355–368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Janssens V and Goris J: Protein
phosphatase 2A: a highly regulated family of serine/threonine
phosphatases implicated in cell growth and signalling. Biochem J.
353:417–439. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arroyo JD and Hahn WC: Involvement of PP2A
in viral and cellular transformation. Oncogene. 24:7746–7755. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Janssens V, Goris J and Van Hoof C: PP2A:
the expected tumor suppressor. Curr Opin Genet. 15:34–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arnold HK and Sears RC: Protein
phosphatase 2A regulatory subunit B56alpha associates with c-myc
and negatively regulates c-myc accumulation. Mol Cell Biol.
26:2832–2844. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Polakis P: Wnt signaling and cancer.
Genes. 14:1837–1851. 2000.
|
|
20
|
He TC, Sparks AB, Rago C, et al:
Identification of c-myc as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de La Coste A, Romagnolo B, Billuart P, et
al: Somatic mutations of the beta-catenin gene are frequent in
mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA.
95:8847–8851. 1998.PubMed/NCBI
|
|
22
|
Yeh E, Cunningham M, Arnold H, et al: A
signalling pathway controlling c-Myc degradation that impacts
oncogenic transformation of human cells. Nat Cell Biol. 6:308–318.
2004. View
Article : Google Scholar
|
|
23
|
Willert K, Shibamoto S and Nusse R:
Wnt-induced dephosphorylation of axin releases beta-catenin from
the axin complex. Genes. 13:1768–1773. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Behrens J, Jerchow BA, Würtele M, et al:
Functional interaction of an axin homologconductin, with β-catenin,
APC and GSK3β. Science. 280:596–599. 1998.
|
|
25
|
Huang H and He X: Wnt/β-catenin signaling:
new (and old) players and new insights. Curr Opin Cell Biol.
20:119–125. 2008.
|
|
26
|
Bienz M and Clevers H: Linking colorectal
cancer to Wnt signaling. Cell. 103:311–320. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tian X, Liu Z, Niu B, et al:
E-cadherin/β-catenin complex and the epithelial barrier. J Biomed
Biotechnol. 2011:5673052011.
|
|
28
|
Koldobskiy MA, Chakraborty A, Werner JK
Jr, et al: p53-mediated apoptosis requires inositol
hexakisphosphate kinase-2. Proc Natl Acad Sci USA. 107:20947–20951.
2010. View Article : Google Scholar
|