Introduction
Solitary fibrous tumors (SFTs), also named
hemangiopericytomas, are rare spindle cell tumors first documented
as arising from the pleura by Klemperer and Rabin (1). SFTs are rare entities accounting for
<2% of all soft tissue sarcomas (2). Although the majority of reported
tumors arise in the thoracic cavity, SFTs from a wide range of
anatomic sites have been reported (3–6).
Extrathoracic SFTs (ESFTs), particularly those in the abdominal and
pelvic cavities, are rare among soft tissue tumors. In a more
recent retrospective study, abdominopelvic SFTs accounted for 34%
of all SFTs, illustrating that the abdominopelvic cavity has become
the major primary site of SFTs (7).
Patients with abdominopelvic SFTs may present with abdominal
distention/pain, a palpable mass and neurological or vascular
symptoms. Hypoglycemia may also be observed in certain cases.
However the association between clinical behavior and
histopathological characteristics of abdominopelvic SFTs requires
further clarification. Usually, the tumor follows an indolent
clinical course with no recurrence and metastasis, yet its elusive
clinical behavior makes it impossible to provide an exact
prognostic prediction and between benign and malignant SFTs. In the
present study, 10 cases of abdominopelvic SFTs were retrospectively
analyzed to highlight the clinicopathological profiles of this rare
entity.
Patients and methods
Patient identification
Between January, 2002 and January, 2013, 10
abdominopelvic tumors were histologically identified as SFTs at the
Northern Jiangsu People’s Hospital (Yangzhou, China). Clinical data
were collected from discharge records, operating theater archives
and telephone calls to the patients. Follow-up data were available
for all patients and consisted of clinical examinations, chest
X-rays, abdominal ultrasounds and computed tomography (CT) or
positron emission tomography-computed tomography (PET) of the tumor
site. This retrospective study was approved by the ethics committee
of the Northern Jiangsu People’s Hospital. The patients consented
to the publication of this study.
Pathological review
Fine needle aspiration biopsy specimens were
obtained in one case. The resection specimens were evaluated for
tumor size, primary location, surgical margin and cut surface. A
macroscopic photograph of the cut surface was obtained in selected
cases. Histopathological examination was performed by two
experienced soft tissue tumor pathologists (Xuewen Gu and Qing Xu).
The diagnosis was confirmed by morphological and
immunohistochemical (IHC) findings available for review. The
pathological diagnostic criteria of SFT used were circumscribed
tumors characterized by a haphazard growth pattern (‘patternless
pattern’) of short spindle cells with scant cytoplasm and bland
cytological appearance separated by strands of rope-like collagen.
IHC analysis included the following antibodies, supplied by
Zhongshan Golden Bridge Biotechnology, Inc. (Beijing, China):
Cluster of differentiation (CD)34, Bcl-2, CD99, CD117, cytokeratin
(CK) pan, epithelial membrane antigen (EMA), S-100 protein,
vimentin, smooth muscle actin (SMA), desmin, Ki-67, insulin
receptor [IR; sc-20739, Santa Cruz Biotechnology, Inc. (Santa Cruz,
CA, USA)] and insulin-like growth factor 1 receptor (IGF-1R).
Tumors were scored for Ki-67 labeling index, mitotic activity
[mitotic fields per 10 high-power fields (HPFs)], cellularity,
nuclear pleomorphism and necrosis. The identification of a
malignant component was based on the mitotic count (activity in
≥4/10 high-power fields) and the presence of necrosis and nuclear
pleomorphism.
Results
Clincal features
The present cohort of patients included six males
and four females, with a mean age at presentation of 53.3 years
(range, 21–75 years). The tumors existed 6 months to 10 years prior
to diagnosis. The clinical features of the 10 cases are summarized
in Table I. Tumors remained
painless or became symptomatic by their mass effect, causing
localized pain, distension, or, as in one patient (no. 5),
constipation. Hypoglycemia was observed only in one patient (no.
1). There were no other symptoms. In one patient (no. 2), the mass
remained asymptomatic and was identified incidentally in a routine
physical examination. One patient (no. 1) was admitted to the
Northern Jiangsu People’s Hospital for emergency surgery due to
spontaneous tumor rupture and hypovolemic shock.
 | Table IClinical features of abdominopelvic
SFTs. |
Table I
Clinical features of abdominopelvic
SFTs.
| No. | Gender | Age, years | First
manifestation | Location | Size, cm | Management |
Recurrence/metastasis | Follow-up time,
months | Follow-up status |
|---|
| 1 | M | 49 | Hypoglycemia, tumor
rupture | Sigmoid
mesocolon | 16.9 | R0
resection | No | 13 | Alive |
| 2 | F | 62 | Asymptomatic | Retroperitoneum | 10.5 | Palliative
chemotherapy | No | 32 | Deceased |
| 3 | F | 21 | Painless mass | Retroperitoneum | 10.3 | R0
resection | No | 21 | Alive |
| 4 | M | 29 | Abdominal pain,
distention | Greater omentum | 28.0 | R0
resection | No | 60 | Alive |
| 5 | M | 56 | Constipation | Pelvis | 9.5 | R1 +
adjuvant chemotherapy | Local recurrence | 6 | Alive |
| 6 | M | 72 | Painless mass | Small bowel
mesentery | 17.0 | R0
resection | No | 18 | Alive |
| 7 | M | 40 | Painless mass | Sigmoid
mesocolon | 12.8 | R0
resection | No | 53 | Alive |
| 8 | F | 61 | Abdominal pain | Retroperitoneum | 14.0 | R0
resection | No | 75 | Alive |
| 9 | F | 75 | Abdominal pain | Pelvis | 5.5 | R0
resection | No | 96 | Alive |
| 10 | M | 68 | Abdominal pain | Pelvis | 2.5 | R0
resection | No | 126 | Alive |
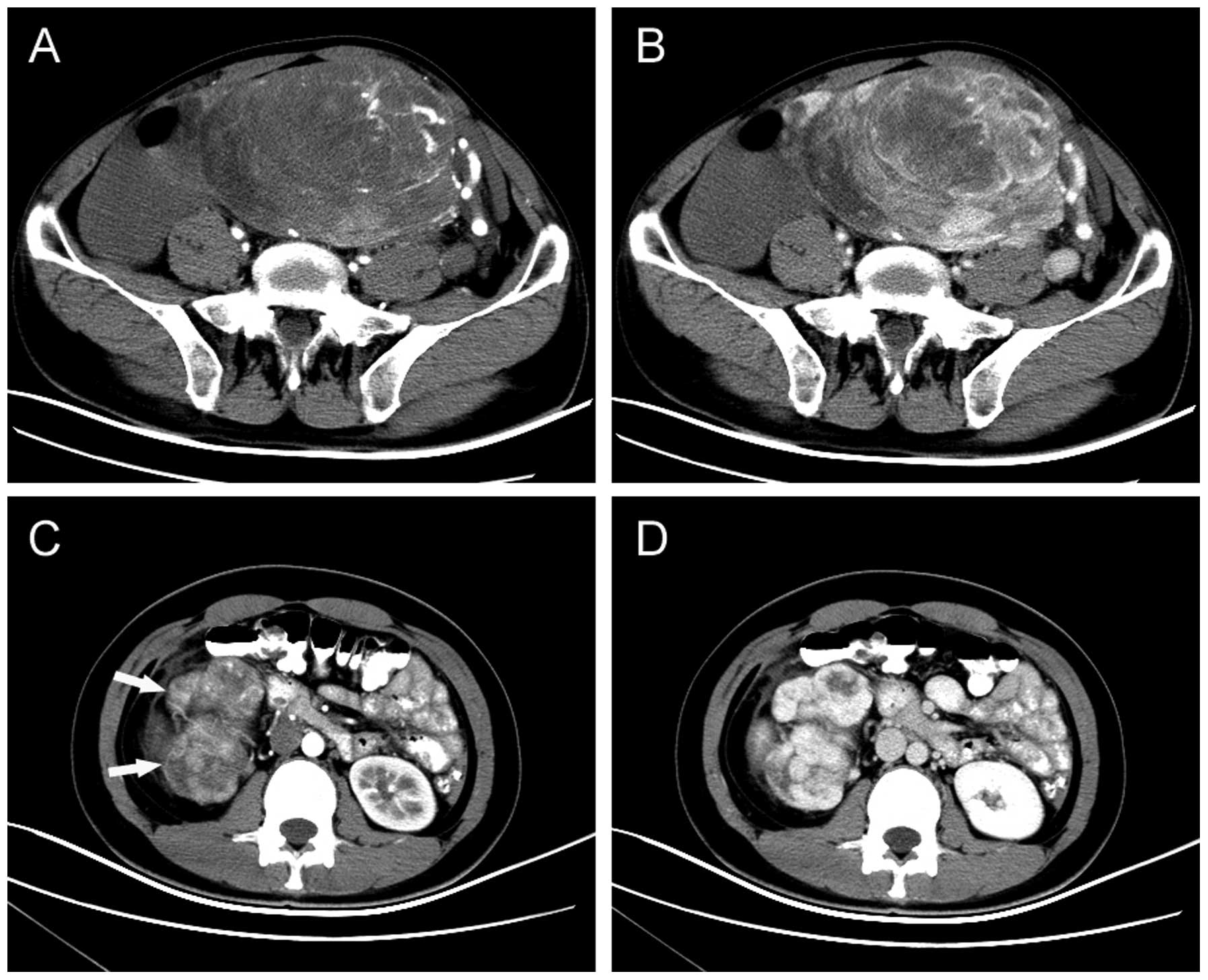
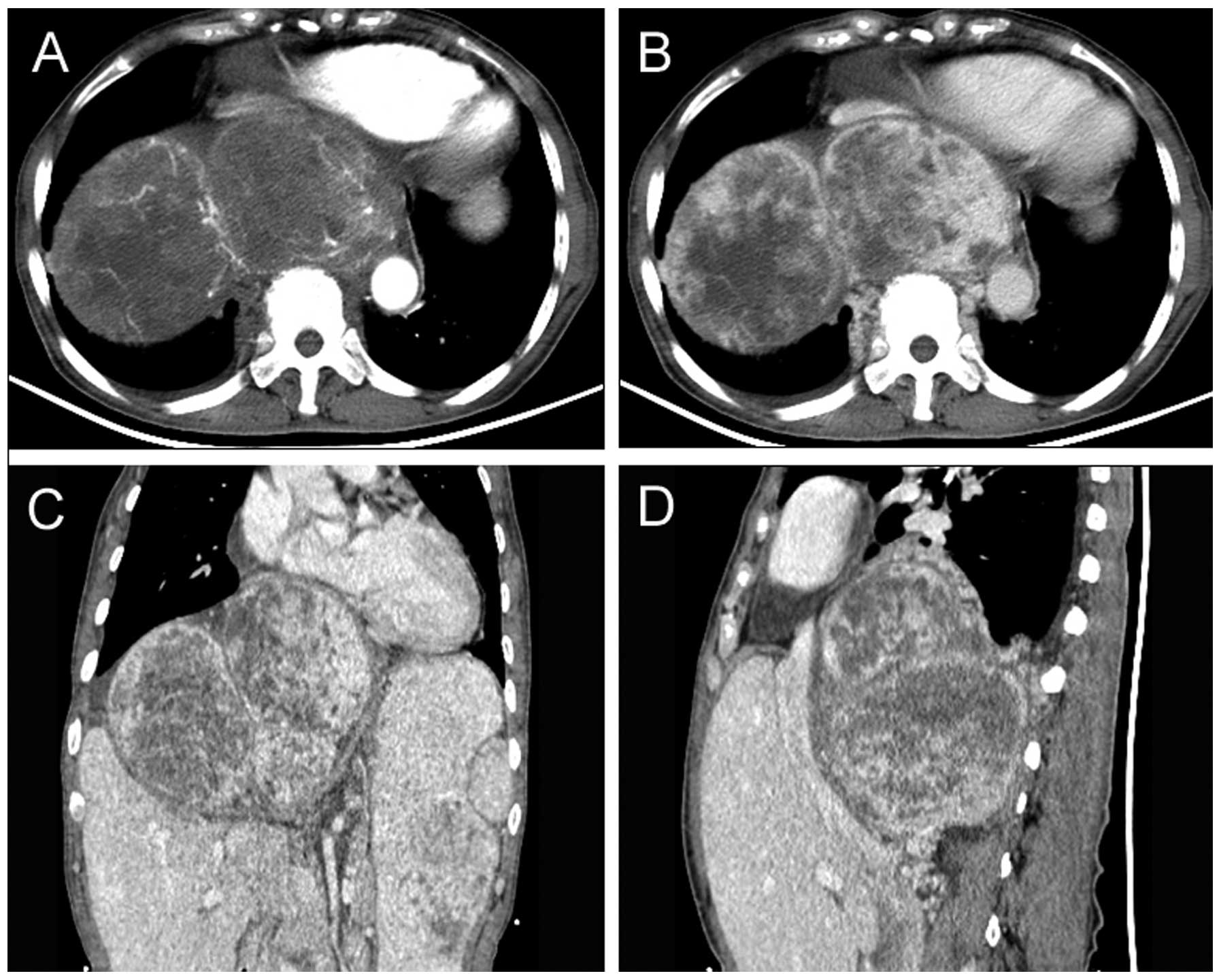
Radiological findings
All patients received CT scans prior to diagnosis.
Of the 10 tumors, three were located in the retroperitoneum, two in
the sigmoid mesocolon, three in the pelvis, and one each in the
greater omentum and the small bowel mesentery. The maximum diameter
of the tumors was 2.5–28 cm (mean, 12.7 cm). SFTs appeared as
well-circumscribed hypervascular masses (two were lobulated and
eight were round) that displaced or exerted pressure effects on
neighboring organs, including the liver, bowel, vessels, kidneys,
bladder and ureter. Central hypoenhancing or nonenhancing areas
could be observed in the tumors, which represent necrosis, cystic
change or hemorrhage (Figs. 1 and
2). PET/CT examination was
performed in one patient who underwent R1 resection and
suffered local relapse. The recurrent mass, located between the
bladder and rectum, showed heterogeneous uptake of
fluorodeoxyglucose, and the initial standardized uptake value,
normalized to lean body mass, was 5.64.
Management
None of the patients had a history of benign or
malignant tumors. In eight patients, primary resection was
performed with negative surgical margins. One out of the 10
patients (no. 5) received R1 resection and adjuvant
chemotherapy, as the tumor was located between the bladder and the
rectum and had adhered to the right seminal vesicle. One patient
(no. 2) with a giant, inoperable tumor located between the liver
and diaphragm received palliative chemotherapy.
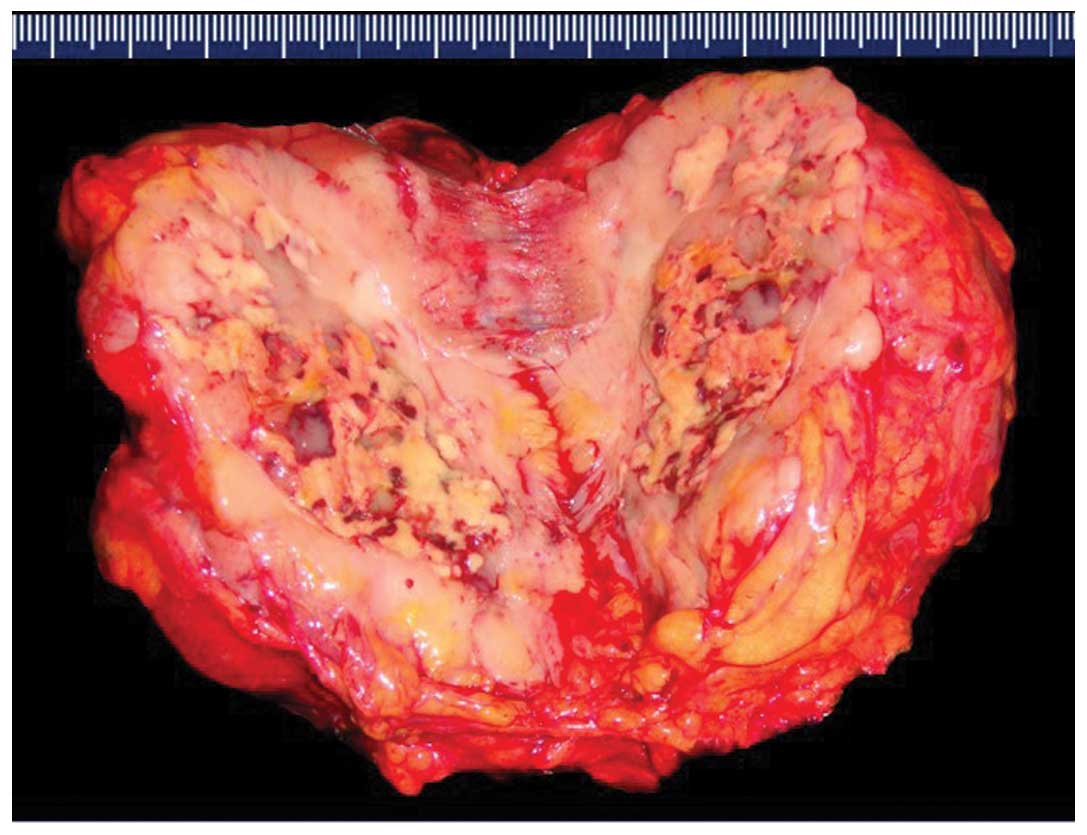
Histological features
A CT-guided fine needle aspiration biopsy specimen
was obtained in one patient (no. 2), while resected specimens were
obtained in the remaining nine patients. The tumors appeared as
solid, well-encapsulated and smooth to firm or soft tissue masses,
and had a gray-white to red-brown color on the cut surface
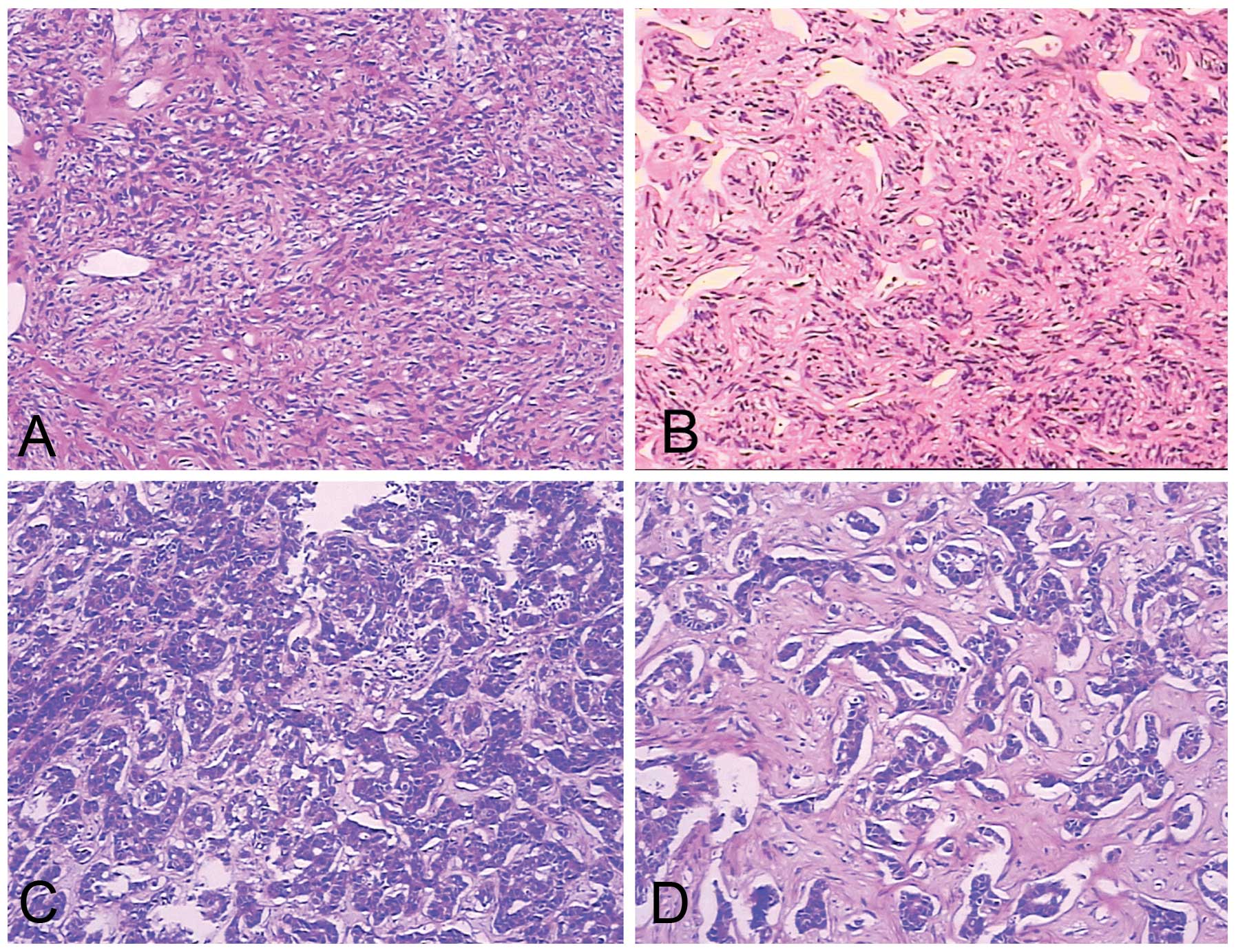
(Fig. 3). The tumors consisted
primarily of spindle cells. The arrangement of the cells varied in
different areas of the tumors. In certain areas the cells were
arranged in short, ill-defined fascicles, whereas in other areas,
cells were arranged at random in a ‘patternless pattern’. The tumor
matrix included variable amounts of partly hyalinzed collagen
bundles and hyalinization was observed in certain areas.
Artifactual ‘cracks’ between the cells and collagen were observed
(Fig. 4). The mitotic rate in
morphologically benign SFTs was <4 mitotic fields/10 HPFs. Two
lesions were diagnosed as atypical or malignant variants of ESFTs
due to markedly increased cellularity, cellular atypia (nuclear
pleomorphism, nuclear hyperchromasia), increased mitotic index and
tumor necrosis (patient nos. 1 and 5).
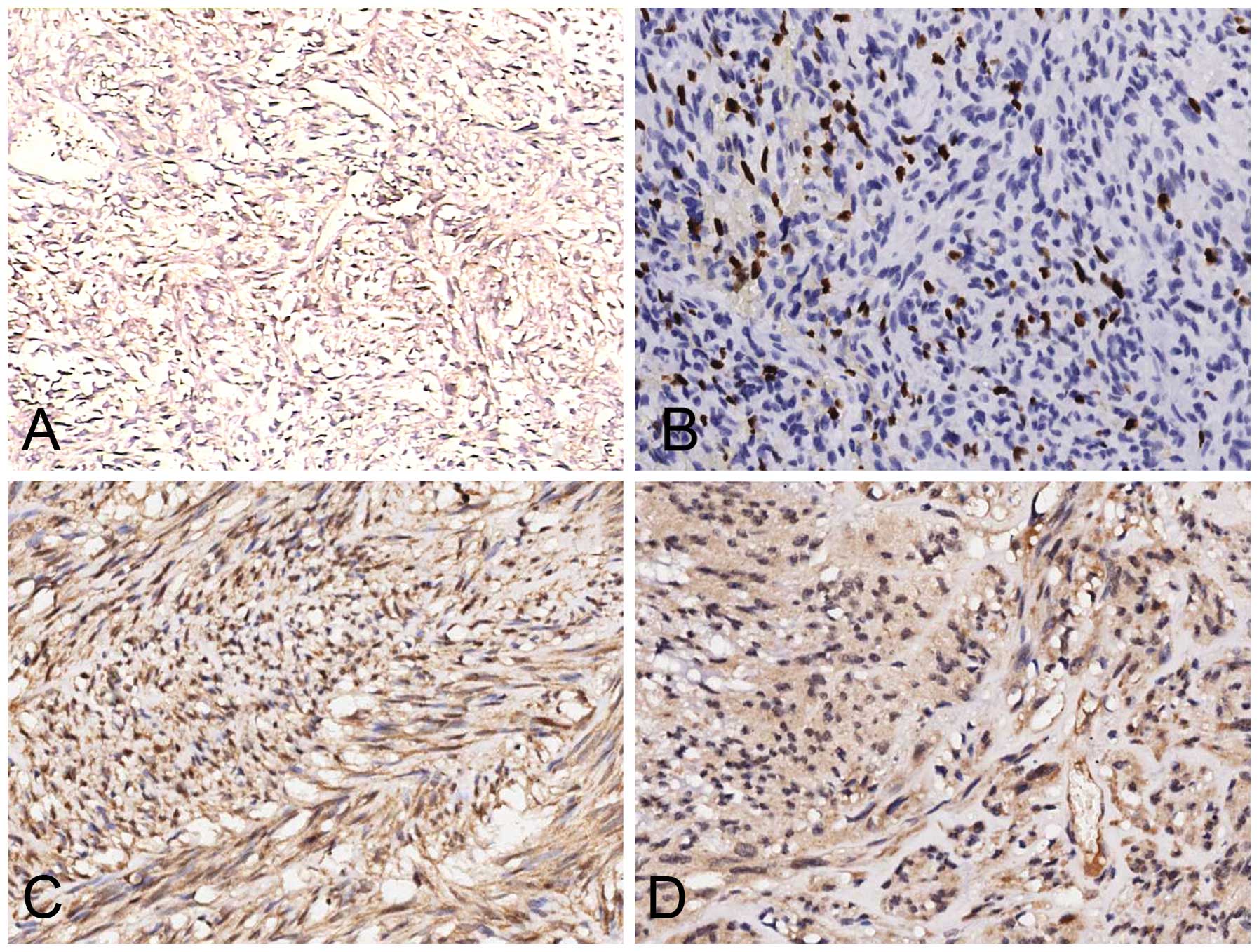
IHC analysis
ESFTs of abdominopelvic origin commonly expressed
CD34 (90%), vimentin (70%), CD99 (60%) and Bcl-2 (50%), and less
commonly expressed SMA (40%), EMA (20%) and S-100 (10%). CD117, CK
pan and desmin were absent. In addition, special attention was paid
to the expression pattern of IR and IGF-1R (Fig. 5). A detailed summary of the
histopathological findings is provided in Table II.
 | Table IIHistopathological findings from the 10
patients. |
Table II
Histopathological findings from the 10
patients.
| Marker | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|
| CD34 | + | + | + | + | + | + | + | − | + | + |
| Bcl-2 | − | + | − | + | − | + | − | + | − | + |
| CD99 | + | + | + | − | + | − | − | + | + | − |
| Vimentin | + | − | − | + | + | + | + | − | + | + |
| Smooth muscle
actin | − | − | − | + | − | + | − | + | + | − |
| CD117 | − | − | − | − | − | − | − | − | − | − |
| Cytokeratin
pan | − | − | − | − | − | − | − | − | − | − |
| Desmin | − | − | − | − | − | − | − | − | − | − |
| Epithelial membrane
antigen | − | + | + | − | − | − | − | − | − | − |
| S-100 | − | − | + | − | − | − | − | − | − | − |
| Insulin
receptor | + | + | − | + | + | + | + | + | − | − |
| IGF-1R | + | − | − | + | − | − | − | − | − | − |
| Ki-67 labeling
index, % | >5 | 2 | 1 | 1 | 5 | 0 | 1 | 2 | 0 | 1 |
| Mitotic fields/10
HPFs | 10 | 1 | 0 | 1 | 3 | 0 | 1 | 2 | 0 | 0 |
| Cellularity | + | − | − | − | + | − | + | − | − | − |
| Pleomorphism | + | − | − | − | + | + | + | + | − | − |
| Necrosis | + | + | − | − | + | − | − | − | − | − |
| Risk scorea | 5 | 4 | 2 | 4 | 3 | 4 | 4 | 4 | 2 | 1 |
Follow-up
Follow-up data were available for all patients and
consisted of clinical examination including chest X-ray, abdominal
ultrasonographic examination and CT or PET/CT of the tumor site.
Follow-up time ranged between 6 and 126 months (mean, 50 months).
Patient statuses at last follow-up are summarized in Table I.
Discussion
SFTs, first reported by Klemperer and Rabin in 1931
(1), are rare mesenchymal neoplasms
that account for <2% of all soft tissue tumors (2). Although SFTs were previously thought
to exclusively involve the pleura, it is now established that SFTs
can originate in almost any part of the body, with ESFTs being more
common than pleural SFTs (3–6). In
2012, Demicco et al (7)
conducted a retrospective study of 110 cases of thoracic and
extrathoracic SFTs, and found that the majority of cases were
located in the abdominopelvic cavity (34%). The pleura and
extremities were less common primary sites (28% and 16%,
respectively) and ~22% of cases arose in the soft tissue of the
head and neck (11%) or trunk (11%). In the present study, 10 cases
of SFTs in the abdomen and pelvis were analyzed
retrospectively.
SFTs in the abdomen and pelvis are primarily tumors
of adult life which affect both genders equally. Clinically, SFTs
manifest as slow-growing, often asymptomatic masses. Common
symptoms include abdominal pain, a palpable mass, and neurological
or vascular symptoms. Symptoms due to mass effect, including
urinary retention, bowel obstruction or constipation, and abdominal
distention, may be observed with tumors in the abdomen or pelvis
(8). In the present study, tumors
remained asymptomatic for 6 months to 10 years, until mass effects
became apparent. The tumor size of abdominopelvic SFTs is usually
large (commonly >10 cm).
Hypoglycemia has been reported in ~5% of SFTs,
particularly in malignant SFTs, cases of Doege-Potter syndrome and,
most frequently, in tumors located in the pelvis and
retroperitoneum. It is mediated through production of IGFs by the
tumor. IGFs and IGF-R mRNA can be identified in tumor cells even in
the absence of clinical hypoglycemia (9,10). An
IHC study on three SFTs with hypoglycemia revealed marked staining
for IGF-1R (11). By contrast, Li
et al (12) demonstrated the
uniform activation of the IR pathway in SFTs, but failed to
identify expression of IGF-1R in these tumors. Thus, the authors
suggested that IGF-2-mediated downstream signaling occurs through
IR rather than IGF-1R. Similarly, Hajdu et al (13) reported that IGF-2 expression was
consistently upregulated in SFTs of all anatomic sites, and that
overexpression of IR paralleled that of IGF-2 in the majority of
SFTs while IGF-1R expression was consistently negligible. By using
an IHC analysis, the present study demonstrated that IR is commonly
expressed (60%) in SFT tissues that were identified as malignant or
moderate-risk cases, whereas IGF-1R was only expressed in two
cases. The current study validates the published results (12,13)
and supports the suggestion that IGF-2/IR autocrine loop activation
plays an oncogenic role in SFTs.
Macroscopically, the majority of SFTs appear as
rounded (occasionally lobulated), encapsulated masses of homogenous
density with a yellow-brown to white whorled appearance of the cut
surface. Areas of necrosis and hemorrhage can be observed in tumors
of large size. Under microscopic analysis, SFTs are typically
composed of juxtaposed hyper- and hypocellular spindle cell
proliferation, a dense collagenous matrix, and numerous thin-walled
blood vessels with an antler-like configuration (a histological
hallmark of SFT) (14).
Immunohistochemically, ESFTs primarily express CD34 (80–90%), CD99
(70%), Bcl-2 (30%), EMA (30%) and SMA (20%) (15–21).
Desmin, CK and S-100 protein are usually absent (15). The IHC analysis results of the
current study validate these findings.
The majority of SFTs are benign (~78–88%) and 12–22%
are malignant (22,23). The criteria proposed by England
et al (24) for malignant
SFTs are large size (>5 cm), increased mitotic rate (≥4 mitotic
fields/10 HPFs), high cellularity, pleomorphism, presence of
hemorrhage and necrosis. In the present study, two out of 10 cases
were identified as malignant SFT (no. 1 and no. 5). By contrast,
Demicco et al (7) suggested
using a risk stratification model (low, moderate and high risk)
based on age (<55 or ≥55 years), tumor size (<5, 5–10, 10–15
and ≥15 cm) and mitotic index (0, 1–3 and ≥4 mitotic fields/10
HPFs) to predict SFT behavior (metastasis and mortality), rather
than simply classifying tumors as either benign or malignant.
Surgical excision remains the treatment of choice
for SFTs. All patients undergoing complete surgical excision were
alive at five years following treatment (25). Surgical resectability is the most
important prognostic factor (24).
In the present study, the only patient to suffer a local relapse
was primarily resected incompletely. Although adjuvant treatment
has been introduced into cases of incompletely resected or
inoperable SFTs, no significant benefits of adjuvant radiation
therapy or chemotherapy have been reported. In the current study,
the patient who received palliative chemotherapy due to an
inoperable SFT succumbed to the disease 32 months after diagnosis,
and the patient who received adjuvant chemotherapy for an
incomplete resection suffered local relapse 6 months later.
SFTs may develop late recurrences or metastases even
in cases which have been identified as benign. Thus, long follow-up
periods (≥15 years) should be maintained with closer follow-up
during the first two years (26).
In conclusion, the majority of abdominopelvic SFTs
follow a benign clinical course following surgical resection with
free margins. Closer surveillance is warranted for those tumors
that are >10 cm or have a component of histological
malignancy.
References
|
1
|
Klemperer P and Rabin CB: Primary
neoplasmas of the pleura. A report of five cases. Am J Ind Med.
22:4–31. 1992. View Article : Google Scholar
|
|
2
|
Gold JS, Antonescu CR, Hajdu C, et al:
Clinicopathologic correlates of solitary fibrous tumors. Cancer.
94:1057–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brunnemann RB, Ro JY, Ordonez NG, Mooney
J, El-Naggar AK and Ayala AG: Extrapleural solitary fibrous tumor:
a clinicopathologic study of 24 cases. Mod Pathol. 12:1034–1042.
1999.
|
|
4
|
Fukunaga M, Naganuma H, Nikaido T, Harada
T and Ushigome S: Extrapleural solitary fibrous tumor: a report of
seven cases. Mod Pathol. 10:443–450. 1997.PubMed/NCBI
|
|
5
|
Hasegawa T, Matsuno Y, Shimoda T, Hasegawa
F, Sano T and Hirohashi S: Extrathoracic solitary fibrous tumors:
their histological variability and potentially aggressive behavior.
Hum Pathol. 30:1464–1473. 1999. View Article : Google Scholar
|
|
6
|
Young RH, Clement PB and McCaughey WT:
Solitary fibrous tumors (‘fibrous mesotheliomas’) of the
peritoneum. A report of three cases and a review of the literature.
Arch Pathol Lab Med. 114:493–495. 1990.
|
|
7
|
Demicco EG, Park MS, Araujo DM, et al:
Solitary fibrous tumor: a clinicopathological study of 110 cases
and proposed risk assessment model. Modern Pathology. 25:1298–1306.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yi B, Bewtra C, Yussef K and Silva E:
Giant pelvic solitary fibrous tumor obstructing intestinal and
urinary tract: a case report and literature review. Am Surg.
73:478–480. 2007.PubMed/NCBI
|
|
9
|
Pavelić K, Spaventi S, Gluncić V, et al:
The expression and role of insulin-like growth factor II in
malignant hemangiopericytomas. J Mol Med (Berl). 77:865–869.
1999.
|
|
10
|
Höög A, Sandberg Nordqvist AC, Hulting AL
and Falkmer UG: High molecular weight IGF-2 expression in a
hemangiopericytoma associated with hypoglycemia. APMIS.
105:469–482. 1997.PubMed/NCBI
|
|
11
|
Chang ED, Lee EH, Won YS, Kim JM, Suh KS
and Kim BK: Malignant solitary fibrous tumor of the pleura causing
recurrent hypoglycemia; immunohistochemical stain of insulinlike
growth factor I receptor in three cases. J Korean Med Sci.
16:220–224. 2001. View Article : Google Scholar
|
|
12
|
Li Y, Chang Q, Rubin BP, et al: Insulin
receptor activation in solitary fibrous tumours. J Pathol.
211:550–554. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hajdu M, Singer S, Maki RG, Schwartz GK,
Keohan ML and Antonescu CR: IGF2 over-expression in solitary
fibrous tumours is independent of anatomical location and is
related to loss of imprinting. J Pathol. 221:300–307. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ide F, Obara K, Mishima K, Saito I and
Kusama K: Ultrastructural spectrum of solitary fibrous tumor: a
unique perivascular tumor with alternative lines of
differentiation. Virchows Arch. 446:646–652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gengler C and Guillou L: Solitary fibrous
tumour and haemangiopericytoma: evolution of a concept.
Histopathology. 48:63–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vallat-Decouvelaere AV, Dry SM and
Fletcher CD: Atypical and malignant solitary fibrous tumors in
extrathoracic locations: evidence of their comparability to
intrathoracic tumors. Am J Surg Pathol. 22:1501–1511. 1998.
View Article : Google Scholar
|
|
17
|
Suster S, Nascimento AG, Miettinen M,
Sickel JZ and Moran CA: Solitary fibrous tumors of soft tissues: a
clinicopathologic and immunohistochemical study of 12 cases. Am J
Surg Pathol. 19:1257–1266. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van de Rijn M, Lombard CM and Rouse RV:
Expression of CD34 by solitary fibrous tumors of the pleura,
mediastinum and lung. Am J Surg Pathol. 18:814–820. 1994.PubMed/NCBI
|
|
19
|
Chilosi M, Facchetti F, Dei Tos AP, et al:
Bcl-2 expression in pleural and extrapleural solitary fibrous
tumours. J Pathol. 181:362–367. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanau CA and Miettinen M: Solitary fibrous
tumor: histological and immunohistochemical spectrum of benign and
malignant variants presenting at different sites. Hum Pathol.
26:440–449. 1995. View Article : Google Scholar
|
|
21
|
Hasegawa T, Hirose T, Seki K, Yang P and
Sano T: Solitary fibrous tumor of the soft tissue: an
immunohistochemical and ultrastructural study. Am J Clin Pathol.
106:325–331. 1996.PubMed/NCBI
|
|
22
|
Robinson LA: Solitary fibrous tumor of the
pleura. Cancer Control. 13:264–269. 2006.PubMed/NCBI
|
|
23
|
de Perrot M, Fischer S, Bründler MA,
Sekine Y and Keshavjee S: Solitary fibrous tumors of the pleura.
Ann Thorac Surg. 74:285–293. 2002.
|
|
24
|
England DM, Hochholzer L and McCarthy MJ:
Localized benign and malignant fibrous tumors of the pleura: a
clinicopathologic review of 223 cases. Am J Surg Pathol.
13:640–658. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Espat NJ, Lewis JJ, Leung D, et al:
Conventional hemangiopericytoma: modern analysis of outcome.
Cancer. 95:1746–1751. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Daigeler A, Lehnhardt M, Langer S,
Steinstraesser L, Steinau HU, Mentzel T and Kuhnen C:
Clinicopathological findings in a case series of extrathoracic
solitary fibrous tumors of soft tissues. BMC Surg. 6:102006.
View Article : Google Scholar : PubMed/NCBI
|