Introduction
In locally advanced non-small cell lung cancer
(NSCLC), definitive radiation treatment (RT) provides improved
disease control and survival rates through high-dose radiation and
concurrent administration of systemic drugs. The recent
introduction of sophisticated technology, such as three-dimensional
conformal RT (3DCRT), promises to improve the cost/benefit ratio of
therapy further.
Currently, the issue of precisely identifying the
tumor volume remains open; tumor dose escalation and sparing of
normal tissue requires the extent of the disease to be precisely
identified in each patient. As a result, there is considerable
interest in investigating functional imaging, including positron
emission tomography (PET) particularly with
18F-fluorodeoxyglucose (FDG), that provides improved
tumor staging, delineation of the target volume, identification of
the treatment response and detection of recurrence for a wide range
of solid cancer types (1–3). However, due to a lack of anatomical
information, a careful correlation must be made between FDG-PET and
structural images in order to precisely localize the tumor. A range
of image registration strategies allows FDG-PET images to be
directly incorporated into computed tomography (CT) images. This
allows the complementary strengths of functional (PET) and
structural (CT) imaging to be co-registered in a single image
set.
A review of the use of PET/CT to define the tumor
volume in the RT planning of NSCLC identified 15 previously
published studies (4). Only 7 of
those included >21 patients and only limited studies compared
intraobserver variation. The current study is an important
contribution that brings under discussion two critical issues with
respect to the effect of fused PET/CT on the delineation of gross
tumor volume (GTV) in the RT planning of NSCLC patients. These
issues are with regard to what extent PET/CT changes the target
volume and whether PET/CT reduces interobserver variability in
target volume delineation
Materials and methods
Patient characteristics
Between January 2006 and December 2007, 23
consecutive patients with NSCLC were referred for CRT, planned
using a fully integrated PET/CT device. All patients were selected
for RT, and informed written consent was obtained following the
rules of the Zhejiang Cancer Hospital (Hangzhou, China). The
clinical stage of the disease varied between I and IIIB, and the
median age was 63 years (range, 43–76 years). Patients with a
Karnofsky performance status of <80, evidence of distant
metastases at initial staging or a requirement for surgical
procedures were excluded. The clinical characteristics of the
included patients are summarized in Table I. All patients underwent a routine
workup, including a clinical examination, fiber endoscopy,
contrast-enhanced CT of the chest, liver ultrasound and brain
magnetic resonance imaging. The clinical stage was retrospectively
defined according to the 2009 Union for International Cancer
Control classification (5). No
patients were candidates for curative surgery. However, 15 patients
were candidates for combined RT and platinum-based chemotherapy and
five patients for RT alone. In addition, three patients were
diagnosed with metastatic disease based on FDG-PET/CT and received
palliative chemotherapy. This study was approved by the Ethical
Review Committee, Zhejiang Cancer Hospital (Hangzhou, China).
Recommendations from the Helsinki Declaration for biomedical
research involving human subjects were followed.
 | Table ICharacteristics of study
population. |
Table I
Characteristics of study
population.
| Characteristics | Value |
|---|
| Patients, n | 23 |
| Age, years |
| Median | 63 |
| Range | 43–76 |
| Gender, n |
| Male | 19 |
| Female | 4 |
| Histology, n |
| Squamous cell | 16 |
| Adenocarcinoma | 7 |
| UICC stage, n |
| Ia | 1 |
| Ib | 1 |
| IIa | 1 |
| IIb | 3 |
| IIIa | 8 |
| IIIb | 9 |
Image acquisition and fusion
All patients underwent PET/CT simulation in the
supine position, while immobilized with a customized thermoplastic
mask and using the Biograph 16 HI-REZ PET/CT scanner (Siemens
Healthcare, Hoffman Estates, IL, USA). The PET component was a
high-resolution scanner with a spatial resolution of 4.7 mm and no
septa, thus allowing 3D-only acquisitions. The CT component used
was the Somatom Sensation 16-slice CT (Siemens Healthcare). The CT
scanner was used for attenuation correction of the PET results and
for localization of FDG uptake in the PET images. All patients were
advised to fast for ≥6 h prior to PET/CT examination. Following
injection of ~5 MBq FDG per kg of body weight, the patients were
rested for a period of ~60 min in a comfortable chair. Emission
images ranging from the proximal femur to the base of the skull
were acquired for 2–3 min per bed position. The field of view was
50 cm, with a matrix of 512×512 pixels for CT and 128×128 pixels
for PET. The processed images were exhibited in coronal, transverse
and sagittal planes. Following image acquisition, PET and CT data
sets were sent to the treatment planning system, Pinnacle system
(Philips Medical Systems, Milpitas, CA, USA), through compact
discs. The CT and PET images were subsequently fused by means of a
dedicated RT planning system image fusion tool based on a mutual
information algorithm.
Tumor staging and target volume
delineation
The clinical staging was analyzed by comparing the
PET/CT with the CT observations alone. For clinical purposes, the
PET image was always considered as additional information to the CT
image for tumor staging or target contouring for treatment
planning.
The target volumes were outlined by two radiation
oncologists with specific experience in lung cancer management
according to the guidelines to contour the GTV of the Radiation
Therapy Oncology Group 0515 (6).
The oncologists were not blinded to each other and worked together
to outline the contours, achieving a final consensus. The GTV-CT
was defined per CT result as only the gross tumor and any lymph
nodes with a cross-sectional diameter of ≥1 cm. Lung window
settings were used to contour the primary lesion, and soft tissue
windows were used for contouring the lymph nodes. GTV-PET/CT was
then defined using fully fused PET/CT imaging as the PET-visualized
enhancement of the gross tumor and any lymph node with an average
standard uptake value (SUV) of ≥2.5 (regardless of any deficiency
in adequate nodal size criteria for malignancy as visualized by CT
images alone) or any lymph nodes with a cross-sectional diameter of
≥1 cm on CT. The GTV-PET/CT included PET and CT information.
For the second phase, four independent observers
were asked to contour and record the GTV-CT and GTV-PET/CT.
Results
Tumor staging
PET/CT imaging led to a change in the
tumor-node-metastasis (TNM) stage of 8/23 cases (35%) compared with
CT alone (Table II). T-stage
changed in 4/23 cases (17%) and N-stage in 7/23 cases (30%).
 | Table IIChange of clinical stage associated
with PET/CT in 8/23 patients (35%). |
Table II
Change of clinical stage associated
with PET/CT in 8/23 patients (35%).
| Case, n | CT stage | PET/CT stage |
|---|
| 1 | T1 N0 M0 | T2 N1 M0 |
| 2 | T4 N2 M0 | T3 N3 M0 |
| 3 | T1 N0 M0 | T1 N1 M0 |
| 4 | T2 N0 M0 | T2 N1 M0 |
| 5 | T2 N0 M0 | T2 N1 M0 |
| 6 | T4N2 M0 | T3 N3 M1 |
| 7 | T4 N2 M0 | T2 N2 M1 |
| 8 | T3 N1 M0 | T3 N2 M1 |
Target volumes
Of the 20 patients who were not diagnosed with
metastatic disease based on FDG-PET/CT planned with 3DCRT, PET/CT
clearly altered the radiation therapy volume (>10%) in 12/20
patients (60%) in comparison with CT targeting, which were outlined
together by two radiation oncologists. The analyzed volumes for all
patients are reported in Table
III. PET aided in the ability to distinguish tumors from
atelectasis in all five patients with atelectasis. Atelectasis
patients 3, 12, 19 and 20 were cases where the addition of FDG
significantly reduced GTV. In atelectasis patient 16, the primary
lesion was reduced, but positive mediastinal lymph nodes were
detected by PET/CT. Overall, no change in GTV was identified.
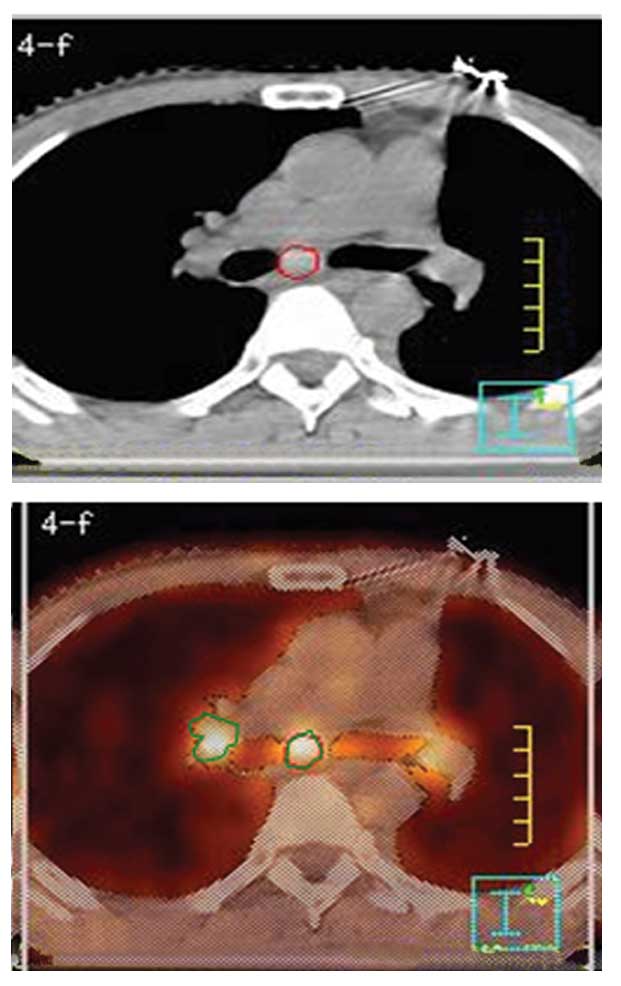
Unsuspected nodal disease was detected by PET/CT in all 10 patients
(two patients with atelectasis of the lung). Patient 10 exhibited a
separate tumor focus detected within the same lobe of the lung, and
GTV was also increased. The alteration of GTV by PET/CT scan is
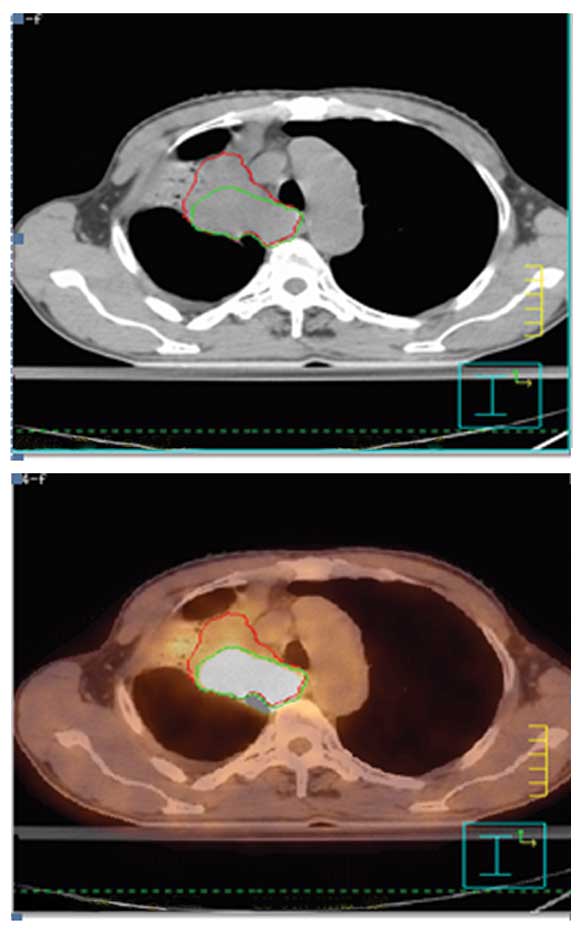
demonstrated in Fig. 1 in one
atelectasis patient and in Fig. 2
in one unsuspected nodal disease patient.
 | Table IIIGTV identified by CT and PET/CT in
each case. |
Table III
GTV identified by CT and PET/CT in
each case.
| Patients | GTV-CT,
cm3 | GTV-PET/CT,
cm3 | Ratio |
|---|
| 1 | 19.9 | 24.3 | 1.22 |
| 2 | 142.2 | 158.4 | 1.11 |
| 3 | 305.2 | 240.3 | 1.27 |
| 4 | 30.5 | 39.7 | 1.30 |
| 5 | 1.5 | 1.5 | 1.00 |
| 6 | 16.1 | 15.1 | 1.07 |
| 7 | 40.0 | 48.3 | 1.21 |
| 8 | 11.4 | 12.3 | 1.08 |
| 9 | 78.0 | 76.3 | 1.02 |
| 10 | 31.8 | 33.4 | 1.05 |
| 11 | 36.3 | 59.9 | 1.65 |
| 12 | 143.7 | 55.3 | 2.60 |
| 13 | 155.0 | 183.9 | 1.19 |
| 14 | 54.5 | 62.1 | 1.14 |
| 15 | 60.1 | 60.9 | 1.01 |
| 16 | 79.5 | 78.7 | 1.01 |
| 17 | 145.9 | 167.0 | 1.14 |
| 18 | 94.8 | 94.2 | 1.01 |
| 19 | 122.1 | 52.6 | 2.32 |
| 20 | 185.5 | 62.9 | 2.95 |
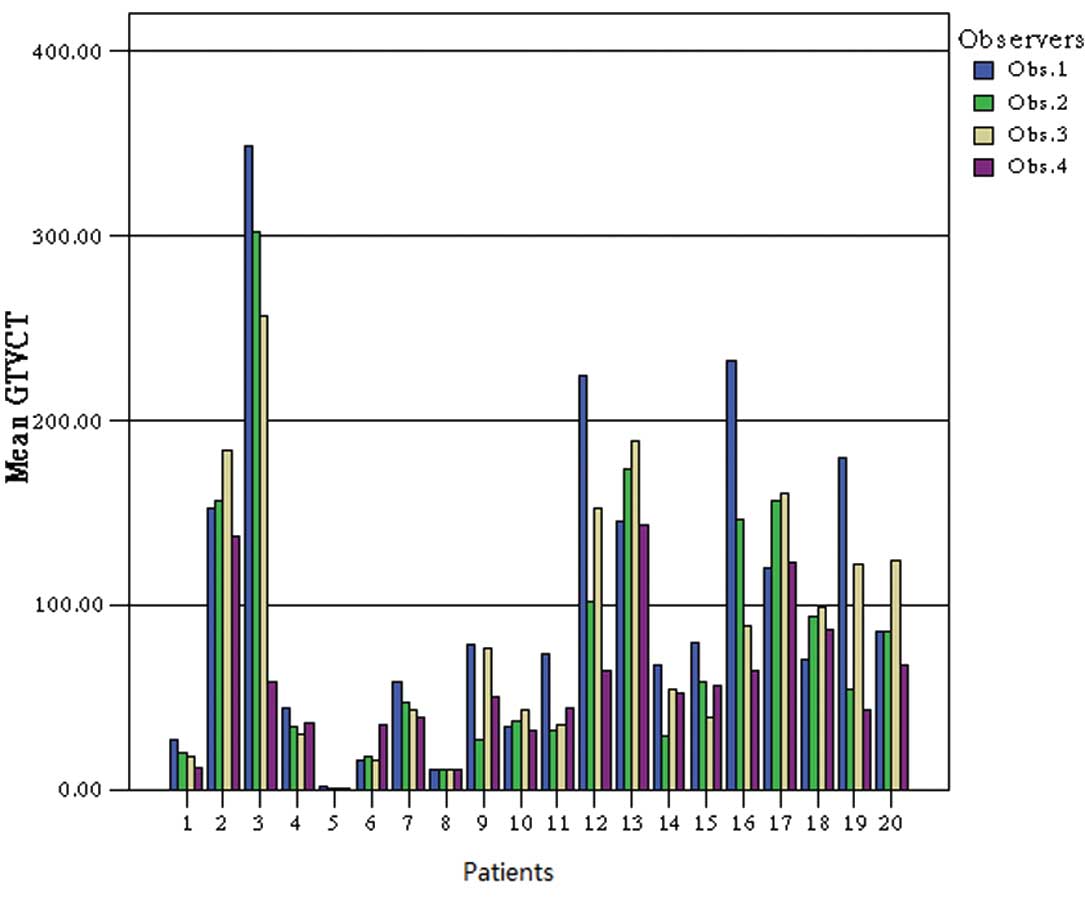
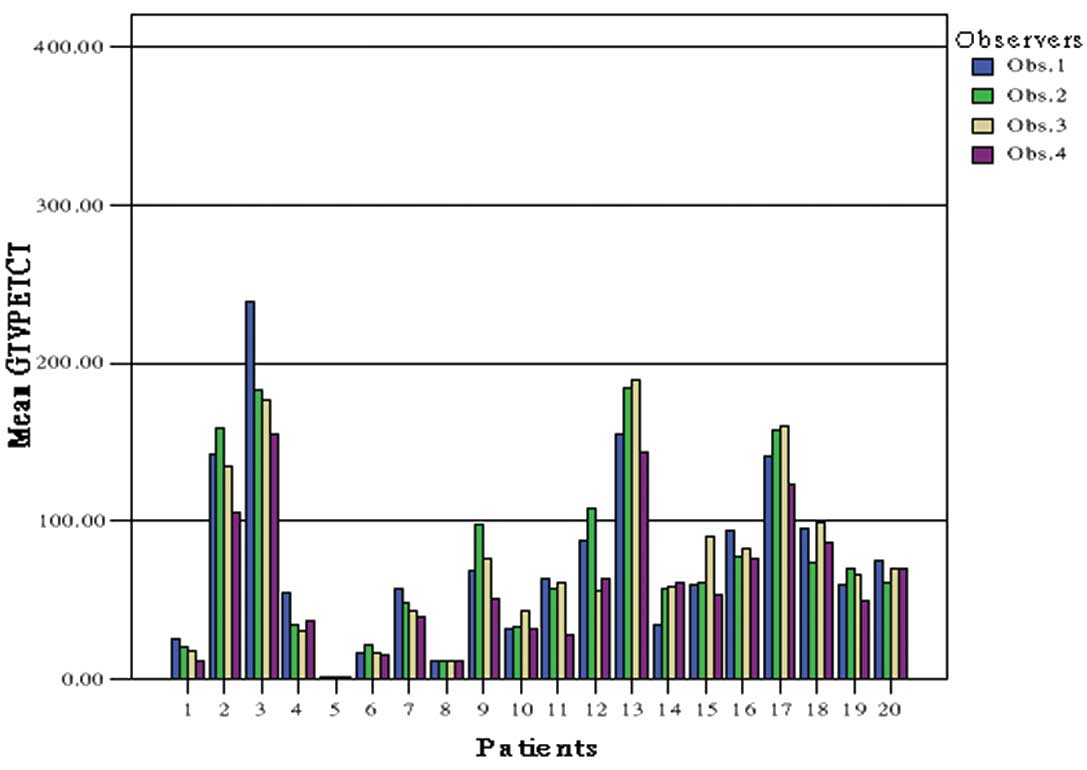
Magnitude of interobserver
variability
For the second phase, the intraobserver variation in
delineating tumor volumes was assessed by four observers. The
concordance in GTV of the four observers was increased by the use
of PET/CT. The mean ratio of largest to smallest CT-based GTV was
2.31 (range, 1.01–5.96). The addition of the PET results reduced
the mean ratio to 1.46 (range, 1.02–2.27). The comparison of GTV-CT
and GTV-PET/CT for each patient and observer is reported in
Table IV.
 | Table IVComparison between CT-GTV and
PET/CT-GTV for each patient and observer. |
Table IV
Comparison between CT-GTV and
PET/CT-GTV for each patient and observer.
| CT-GTV,
cm3 | PET/CT-GTV,
cm3 |
|---|
|
|
|
|---|
| Patient | Obs. 1 | Obs. 2 | Obs. 3 | Obs. 4 | Ratio | Obs. 1 | Obs. 2 | Obs. 3 | Obs. 4 | Ratio |
|---|
| 1 | 27.3 | 20.3 | 18.4 | 12.3 | 2.22 | 25.9 | 20.3 | 17.4 | 21.7 | 1.49 |
| 2 | 152.6 | 156.4 | 184.5 | 137.8 | 1.34 | 142.2 | 158.4 | 134.5 | 118.1 | 1.34 |
| 3 | 349.3 | 302.2 | 257.3 | 58.6 | 5.96 | 239.0 | 242.7 | 226.4 | 255.2 | 1.13 |
| 4 | 44.7 | 34.1 | 30.4 | 36.9 | 1.47 | 45.1 | 34.1 | 30.4 | 36.9 | 1.48 |
| 5 | 1.7 | 1.4 | 1.5 | 1.0 | 1.70 | 1.7 | 1.3 | 1.5 | 1.0 | 1.70 |
| 6 | 16.2 | 18.1 | 16.3 | 35.5 | 2.19 | 16.1 | 21.1 | 16.3 | 15.5 | 1.36 |
| 7 | 59.0 | 47.3 | 43.3 | 39.4 | 1.50 | 57.0 | 48.3 | 43.3 | 39.4 | 1.45 |
| 8 | 11.3 | 11.3 | 11.4 | 11.4 | 1.01 | 11.4 | 11.3 | 11.5 | 11.5 | 1.02 |
| 9 | 78.6 | 27.3 | 76.8 | 50.4 | 2.88 | 68.0 | 98.3 | 76.8 | 50.4 | 1.95 |
| 10 | 34.8 | 37.4 | 43.3 | 32.2 | 1.34 | 31.8 | 33.4 | 43.3 | 32.2 | 1.36 |
| 11 | 75.5 | 32.5 | 35.4 | 44.6 | 2.32 | 64.0 | 57.5 | 61.5 | 28.2 | 2.27 |
| 12 | 224.7 | 102.3 | 153.2 | 65.3 | 3.44 | 87.5 | 107.7 | 56.1 | 63.3 | 1.92 |
| 13 | 145.8 | 173.9 | 189.5 | 143.5 | 1.32 | 155.0 | 183.9 | 189.5 | 143.5 | 1.32 |
| 14 | 67.5 | 29.2 | 54.6 | 52.3 | 2.31 | 34.5 | 56.6 | 58.6 | 61.1 | 1.77 |
| 15 | 80.1 | 58.9 | 39.9 | 56.7 | 2.01 | 60.1 | 60.9 | 64.9 | 59.5 | 1.09 |
| 16 | 232.6 | 147.0 | 88.7 | 65.3 | 3.56 | 93.5 | 78.0 | 82.4 | 76.6 | 1.22 |
| 17 | 120.9 | 157.0 | 160.8 | 123.4 | 1.33 | 140.9 | 157.0 | 160.8 | 123.4 | 1.30 |
| 18 | 71.0 | 94.2 | 98.7 | 86.7 | 1.39 | 94.8 | 73.6 | 98.7 | 86.7 | 1.34 |
| 19 | 180.5 | 55.2 | 122.7 | 43.4 | 4.16 | 59.1 | 69.4 | 66.7 | 48.9 | 1.42 |
| 20 | 186.1 | 85.9 | 124.7 | 68.2 | 2.73 | 75.1 | 60.6 | 69.4 | 70.4 | 1.24 |
The variation in GTV between observers using CT
alone is illustrated in Fig. 3,
whereas the concordance in delineating GTV and PTV by PET/CT is
demonstrated in Fig. 4.
Discussion
The use of FDG-PET/CT prior to treatment has gained
interest in the radiation oncology community in association with a
potential improvement in tumor staging, the optimization of
treatment strategy and an improved delineation of target volume
(7). Preliminary results for
numerous tumor locations, including the head and neck, lung,
esophagus, rectum, anal canal and pancreas, have been reported in a
number of previous literature studies (8–12).
Certain studies concerning the advantage of PET/CT fusion have been
previously reported for radiation planning of patients with NSCLC
(13–15).
In the current study, based on PET/CT, changes in
TNM stage occurred in 8/23 cases (35%). Similar results were
reported by Bradley et al (16), where 26 patients were enrolled and
PET/CT fusion was found to alter the TNM categories in 31% of
patients (8/26). In the present study, three patients were
diagnosed with metastatic disease based on FDG-PET and received
palliative radiation therapy. To date, it appears that the best
benefit from FDG-PET in NSCLC is the evaluation of the cancer
extent. FDG-PET/CT identifies patients with tumor spread not
detected by standard methods in ~20% of the cases (17).
In the current study, the effect of PET/CT on the
delineation of GTV was evaluated considering the PET information in
addition to that of CT. PET aided the ability to distinguish tumors
from atelectasis in all five patients with atelectasis. Unsuspected
nodal disease was detected by PET in 10 patients, and one patient
exhibited a separate tumor focus detected within the same lobe of
the lung. Previous studies have shown that fused PET and CT alters
~50% of the GTV delineation compared with CT targeting alone, as
observed by visual evaluation or using specific mathematical
algorithms, such as a fixed SUV or threshold (18,19).
The presence of atelectasis, which appears easier to differentiate
from tumors with the use of combined PET/CT information, often
leads to a significant decrease in GTV (20–24).
Since routine elective nodal irradiation is often omitted in NSCLC,
accurate identification of the involved nodal areas is pivotal in
modern treatment planning with curative intent. A number of
previous studies have shown significant changes in GTV volume due
to the inclusion or exclusion of nodal areas compared with CT-alone
GTV (25–27). In a prospective clinical study, De
Ruysscher et al (28)
evaluated the patterns of recurrence following FDG-PET-based
selective mediastinal node irradiation in 44 patients with NSCLC.
The rationale of the study was the higher diagnostic accuracy of
FDG-PET on mediastinal lymph node areas. Only one case of nodal
recurrence was reported from the 44 patients selectively irradiated
on the FDG-avid areas of the mediastinum.
In the second part of the present study, the results
showed that FDG-PET reduced the ratio of largest to smallest GTV in
the majority of cases (14/20). The concordance in treatment
planning of the four observers was increased by the use of PET/CT.
The mean ratio of largest to smallest CT-based GTV was 2.31 (range,
1.01–5.96). The addition of the PET results reduced the mean ratio
to 1.46 (range, 1.12–2.27). Atelectasis patients 3, 12, 16, 19 and
20 were cases where the addition of FDG evidently reduced
interobserver variability. For patient 19 (Fig. 1), it was difficult to distinguish
atelectasis of the lung from the tumor using CT alone; FDG
clarified the location of the tumor and aided the determination of
a consistent boundary. For patient 15, FDG aided the ability of the
four observers to achieve a more consistent conclusion concerning
the presence of nodal disease. In the majority of cases where the
addition of FDG appeared to increase interobserver variability, the
increase was minor. However, in patient 11, the addition of FDG did
not significantly reduce variability. The SUV of FDG uptake was
<2.5 in the lymph nodes with a cross-sectional diameter of 1 cm
on CT, which three of the four observers interpreted as being
positive lymph nodes. However, the fourth observer interpreted the
FDG uptake in the lymph nodes as not indicating tumor
metastasis.
The majority of previous studies, as well as the
present study, may promote certain criticism, as there is an
uncertain correlation between the PET/CT observations and the real
tumor extension, which may only be precisely assessed on the
surgical specimen. In the current study, only one case exhibited a
cytological correlation with lymph nodes; PET/CT overestimated the
lymph node tumor extension. An additional case exhibited a
cytological correlation with the primary lesion, however, it was
difficult to identify tumor necrosis. FDG uptake was identified in
the area of the apparent tumor necrosis, which three of the four
observers interpreted as being indicative of tumor. However, the
fourth observer interpreted the borderline FDG uptake in the area
as not indicating tumor. This difference in interpretation of FDG
uptake led to a large degree of interobserver variability in the
PET/CT-GTV. The correlation between tumor delineation on PET
compared with pathology was investigated by Faria et al
(29), who compared FDG-PET/CT
image fusion with the histopathology of 32 patients. The
CT-determined stage was altered by the pathological examination in
22/32 patients (69%), while the PET-determined stage was altered in
16/32 patients (50%). The N-stage was associated with the most
significant alterations. The TNM stage was altered by PET in 15/32
patients (44%) compared with CT alone, however, only seven of these
alterations were confirmed by the pathological observations.
The consistency of target delineation on PET images
is an issue that remains unclear. The literature has shown GTV
contouring with FDG-PET to be varied, but these data have typically
been based on the SUV. In the present study, an average SUV of ≥2.5
was adopted, consistent with the SUV proposed for lung cancer by
another study (30).
The present study shows that FDG-PET/CT images for
primary NSCLC exhibit a potential impact on disease staging and
treatment planning. A clinical stage variation was observed in 35%
of cases (8/23). Based on the results from the present study and
from previous literature, the future scenario of the imaging for RT
of NSCLC may include the use of functional imaging, such as
FDG-PET/CT, with the aim of characterizing the biological features
of the tumor and optimizing the use of highly conformal and
biologically effective RT.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (No.
81272505/H1610).
References
|
1
|
Abramyuk A, Appold S, Zöphel K, et al:
Quantitative modifications of TNM staging, clinical staging and
therapeutic intent by FDG-PET/CT in patients with non small cell
lung cancer scheduled for radiotherapy - a retrospective study.
Lung Cancer. 78:148–152. 2012. View Article : Google Scholar
|
|
2
|
Vaidya M, Creach KM, Frye J, et al:
Combined PET/CT image characteristics for radiotherapy tumor
response in lung cancer. Radiother Oncol. 102:239–245. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Satoh Y, Nambu A, Onishi H, et al: Value
of dual time point F-18 FDG-PET/CT imaging for the evaluation of
prognosis and risk factors for recurrence in patients with stage I
non-small cell lung cancer treated with stereotactic body radiation
therapy. Eur J Radiol. 81:3530–3534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greco C, Rosenzweig K, Cascini GL and
Tamburrini O: Current status of PET/CT for tumour volume definition
in radiotherapy treatment planning for non-small cell lung cancer
(NSCLC). Lung Cancer. 57:125–134. 2007.
|
|
5
|
Edge SB, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual. 7th ed. Springer Verlag; New York, NY:
2009
|
|
6
|
Bradley J, Bae K, Choi N, et al: A phase
II comparative study of gross tumor volume definition with or
without PET/CT fusion in dosimetric planning for non-small-cell
lung cancer (NSCLC): primary analysis of Radiation Therapy Oncology
Group (RTOG) 0515. Int J Radiat Oncol Biol Phys. 82:435–441.
2012.
|
|
7
|
Paulino AC, Thorstad WL and Fox T: Role of
fusion in radiotherapy treatment planning. Semin Nucl Med.
33:2338–2343. 2003. View Article : Google Scholar
|
|
8
|
Abramyuk A, Appold S, Zöphel K, et al:
Modification of staging and treatment of head and neck cancer by
FDG-PET/CT prior to radiotherapy. Strahlenther Onkol. 189:197–201.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mac Manus MP and Hicks RJ: The role of
positron emission tomography/computed tomography in radiation
therapy planning for patients with lung cancer. Semin Nucl Med.
42:308–319. 2012.PubMed/NCBI
|
|
10
|
Buijsen J, van den Bogaard J, van der
Weide H, et al: FDG-PET-CT reduces the interobserver variability in
rectal tumor delineation. Radiother Oncol. 102:371–376. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Baardwijk A, Baumert B, Bosmans G, et
al: The current status of FDG-PET in tumour volume definition in
radiotherapy treatment planning. Cancer Treat Rev. 32:245–260.
2006.PubMed/NCBI
|
|
12
|
Pakzad F, Groves A and Ell PJ: The role of
positron emission tomography in the management of pancreatic
cancer. Semin Nucl Med. 36:248–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fleckenstein J, Hellwig D, Kremp S, et al:
F-18-FDG-PET confined radiotherapy of locally advanced NSCLC with
concomitant chemotherapy: results of the PET-PLAN pilot trial. Int
J Radiat Oncol Biol Phys. 81:e283–e289. 2011. View Article : Google Scholar
|
|
14
|
Rodríguez N, Sanz X, Trampal C, et al:
18F-FDG PET definition of gross tumor volume for radiotherapy of
lung cancer: is the tumor uptake value-based approach appropriate
for lymph node delineation? Int J Radiat Oncol Biol Phys.
78:659–666. 2010.
|
|
15
|
Biehl KJ, Kong FM, Dehdashti F, et al:
18F-FDG PET definition of gross tumor volume for radiotherapy of
non-small cell lung cancer: is a single standardized uptake value
threshold approach appropriate? J Nucl Med. 47:1808–1812. 2006.
|
|
16
|
Bradley J, Thorstad WL, Mutic S, et al:
Impact of FDG-PET on radiation therapy volume delineation in
non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 59:78–86.
2004.PubMed/NCBI
|
|
17
|
MacManus MP, Hicks RJ, Matthews JP, et al:
High rate of detection of unsuspected distant metastases by PET in
apparent stage III non-small-cell lung cancer: Implications for
radical radiation therapy. Int J Radiat Oncol Biol Phys.
50:287–293. 2001. View Article : Google Scholar
|
|
18
|
Hong R, Halama J, Bova D, et al:
Correlation of PET standard uptake value and CT window-level
thresholds for target delineation in CT-based radiation treatment
planning. Int J Radiat Oncol Biol Phys. 67:720–726. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Daisne JF, Sibomana M, Bol A, et al:
Tri-dimensional automatic segmentation of PET volumes based on
measured source-to-background rations: influence of reconstruction
algorithms. Radiother Oncol. 69:247–250. 2003.
|
|
20
|
Giraud P, Grahek D, Montravers F, et al:
CT and (18 F-deoxyglucose (FDG) image fusion for optimization of
conformal radiotherapy of lung cancers. Int J Radiat Oncol Biol
Phys. 49:1249–1257. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nestle U, Walter K, Schmidt S, et al:
18F-deoxyglucose positron emission tomography (FDGPET) for the
planning of radiotherapy in lung cancer: high impact in patients
with atelectasis. Int J Radiat Oncol Biol Phys. 44:593–597. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deniaud-Alexandre E, Touboul E, Lerouge D,
et al: Impact of computed tomography and 18F-deoxyglucose
coincidence detection emission tomography image fusion for
optimization of conformal radiotherapy in non-small-cell lung
cancer. Int J Radiat Oncol Biol Phys. 63:1432–1441. 2005.
View Article : Google Scholar
|
|
23
|
Ciernik IF, Dizendorf E, Baumert BG, et
al: Radiation treatment planning with an integrated positron
emission and computer tomography (PET/CT): a feasibility study. Int
J Radiat Oncol Biol Phys. 57:853–863. 2003. View Article : Google Scholar
|
|
24
|
Ceresoli GL, Cattaneo GM, Castellone P, et
al: Role of computed tomography and [18F]
fluorodeoxyglucose positron emission tomography image fusion in
conformal radiotherapy of non-small cell lung cancer: a comparison
with standard techniques with and without elective nodal
irradiation. Tumori. 93:88–96. 2007.
|
|
25
|
Vanuytsel LJ, Vansteenkiste JF, Stroobants
SG, et al: The impact of (18)F-fluoro-2-deoxy-D-glucose1432
positron emission tomography (FDG-PET) lymph node staging on the
radiation treatment volumes in patients with non-small cell lung
cancer. Radiother Oncol. 55:317–324. 2000.
|
|
26
|
van Der Wel A, Nijsten S, Hochstenbag M,
et al: Increased therapeutic ratio by 18FDG-PET CT planning in
patients with clinical CT stage N2–N3M0 non-small cell lung cancer:
a modeling study. Int J Radiat Oncol Biol Phys. 61:649–655.
2005.PubMed/NCBI
|
|
27
|
Nestle U, Hellwig D, Schmidt S, et al:
2-Deoxy-2-[18F]fluoro-D-glucose positron emission tomography in
target volume definition for radiotherapy of patients with
non-small-cell lung cancer. Mol Imaging Biol. 4:257–263. 2002.
|
|
28
|
De Ruysscher D, Wanders S, van Haren E, et
al: Selective mediastinal node irradiation based on FDG-PET scan
data in patients with non-small-cell lung cancer: a prospective
clinical study. Int J Radiat Oncol Biol Phys. 62:988–994.
2005.PubMed/NCBI
|
|
29
|
Faria SL, Menard S, Devic S, Sirois C, et
al: Impact of FDG-PET/CT on radiotherapy volume delineation in
non-small-cell lung cancer and correlation of imaging stage with
pathologic findings. Int J Radiat Oncol Biol Phys. 70:1035–1038.
2008.PubMed/NCBI
|
|
30
|
Ashamalla H, Rafla S, Parikh K, et al: The
contribution of integrated PET/CT to the evolving definition of
treatment volumes in radiation treatment planning in lung cancer.
Int J Radiat Oncol Biol Phys. 63:1016–1023. 2005.PubMed/NCBI
|