Introduction
Oxaliplatin is an effective agent for the adjuvant
or palliative treatment of patients with colorectal cancer (CRC)
(1–3). Oxaliplatin frequently causes
neurotoxicity, which often results in dose reduction or treatment
discontinuation. In total, 12 to 18% of patients suffer from
chronic and cumulative neurotoxicity when the cumulative
oxaliplatin dose reaches 800 mg/m2 (1), whereas other patients receive high
doses of oxaliplatin without developing neurotoxicity. Therefore,
elucidation of the predictive factors and pathogenetic mechanism of
oxaliplatin-induced neurotoxicity is required to optimize the
oxaliplatin dose for CRC patients. The current study reports the
case of a patient with metastatic rectal cancer, who was
administered a cumulative oxaliplatin dose of >5,000
mg/m2 during treatment with a modified 5-fluorouracil
(5-FU), leucovorin and oxaliplatin (mFOLFOX6) regimen without
experiencing neurotoxicity. In addition, the result of an analysis
of the glutathione S-transferase P1 (GSTP1) gene polymorphism,
which has been shown to be associated with oxaliplatin-induced
neurotoxicity, is also reported. Previously published studies on
the association between the GSTP1 polymorphism and
oxaliplatin-induced neurotoxicity are also reviewed.
Case report
Patient
A 57-year-old male who was diagnosed with rectal
cancer accompanied by bladder invasion, underwent resection of the
locally invasive, primary tumor (T4N0M0, stage II). Approximately
three years after surgery, the carcinoembryonic antigen (CEA) level
of the patient increased and a computed tomography (CT) scan
revealed a recurrent tumor in the right lobe of the liver. The
patient then underwent surgery to remove the recurrent liver
metastasis and was treated intravenously (IV) with FOLFOX4 (200
mg/m2 leucovorin, 400 mg/m2 5-FU bolus and
600 mg/m2 5-FU over 22 h on days 1 and 2, and an
additional 85 mg/m2 oxaliplatin on day 1, every two
weeks) as adjuvant chemotherapy for four cycles. Approximately two
years later, the CEA level of the patient increased again and lymph
node metastases were identified along the common hepatic artery
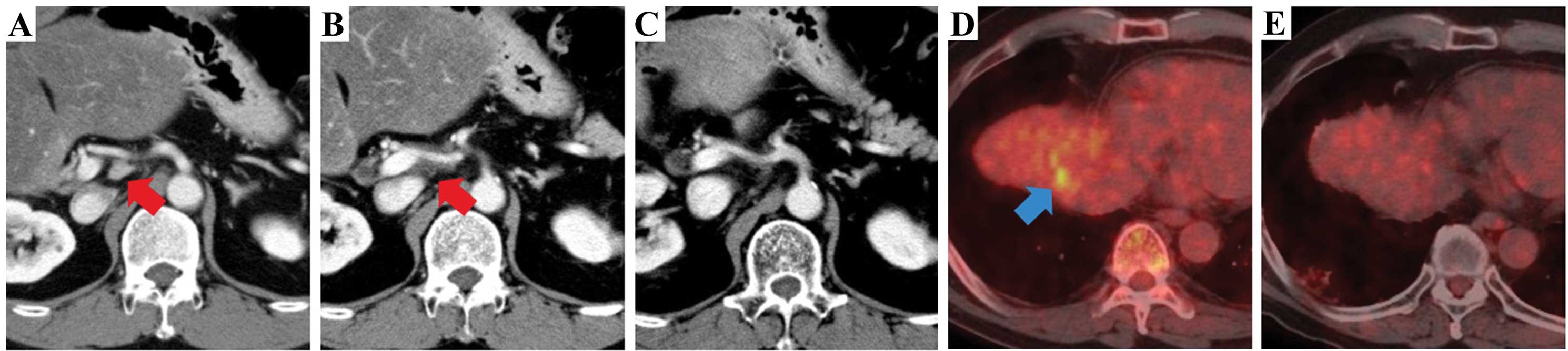
(CHA) on the CT scan (Fig. 1A).
Local excision was performed for the recurrent lesion, and the
patient began IV treatment with mFOLFOX6 (85 mg/m2
oxaliplatin, 200 mg/m2 leucovorin, 400 mg/m2
5-FU bolus on day 1 and 2,400 mg/m2 5-FU over 46 h every
two weeks) as adjuvant chemotherapy. As the patient requested
long-term adjuvant therapy, mFOLFOX6 was administered for 18 months
without recurrence. Subsequent to this, TS-1 (Taiho Pharmaceutical,
Tokyo, Japan) was initiated at a dosage of 60 mg twice daily on
days 1 to 28 every six weeks following a discussion with the
patient. However, the CEA level of the patient increased and the
recurrent tumor around the CHA showed progression on the CT scan
(Fig. 1B). Next, treatment with
mFOLFOX6 was restarted and continued for 17 months without
progressive disease (Fig. 1C).
However, the CEA level once again increased and the recurrent tumor
was detected in the right subphrenic area on positron emission
tomography/CT; this was treated by local radiation therapy
(Fig. 1D). Panitumumab (6 mg/kg
every two weeks) was added to the mFOLFOX6 regimen, and the patient
continued the therapy (Fig. 1E).
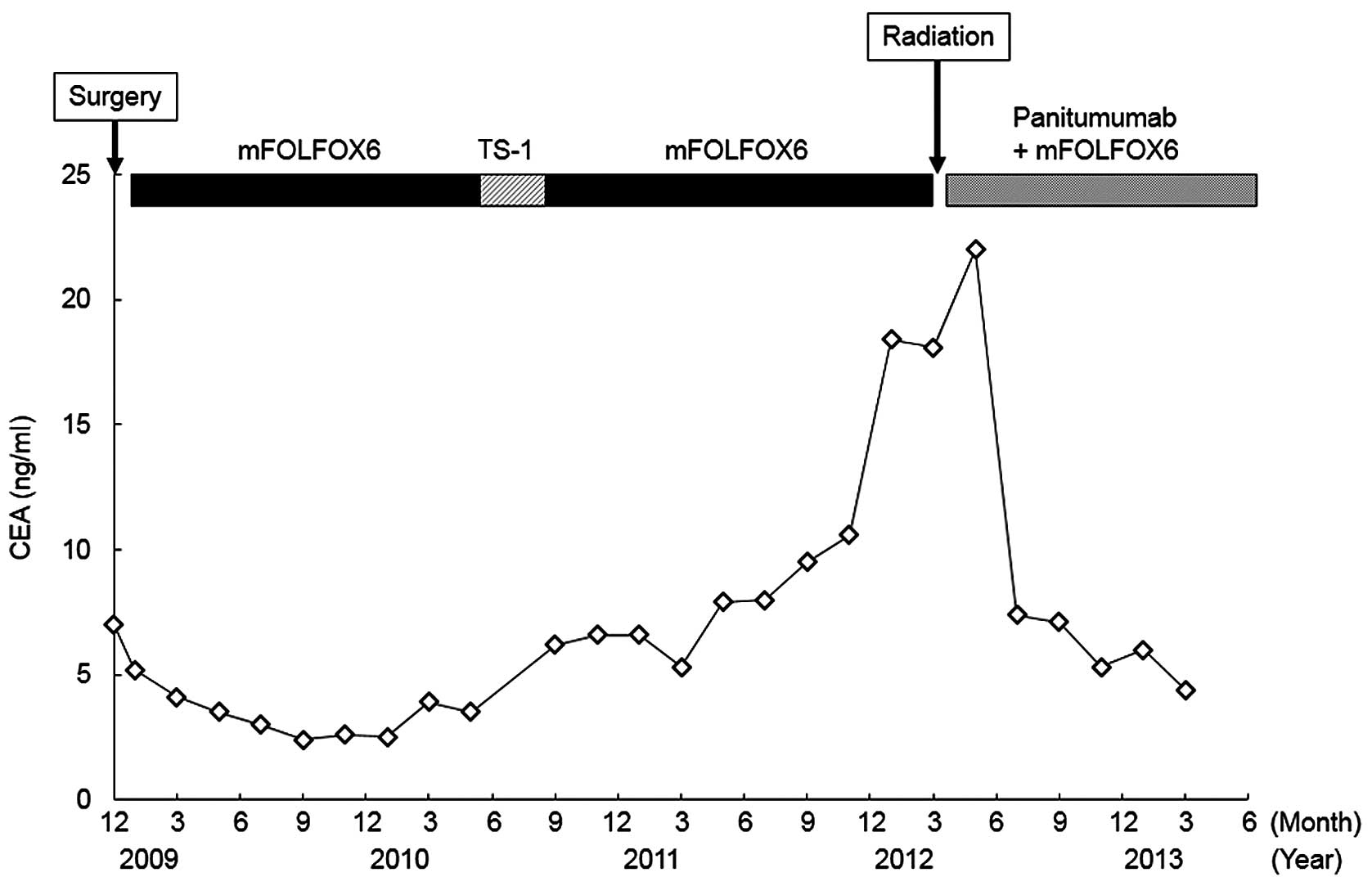
Although the patient was administered ~5,000 mg/m2
oxaliplatin during the clinical course, the treatment was safe and
tolerated without causing neurotoxicity (Fig. 2).
Written informed consent was obtained from the
patient, and the analysis was approved by the Institutional Ethics
Committee of Houju Memorial Hospital (Nomi, Japan).
Genomic analysis
All genomic analyses were carried out by Falco
Biosystems Ltd. (Kyoto, Japan). Genomic DNA was extracted from
whole blood using a QIAamp DNA Blood Mini kit (Qiagen, Valencia,
CA, USA). The DNA sample was analyzed using polymerase chain
reaction (PCR). The PCR reaction contained 50 ng genomic DNA, 25 μl
2X PCR Master Mix (Promega, Madison, WI, USA) and 100 μM of each
primer. The PCR conditions were 94°C for 2 min, followed by 35
cycles of 94°C for 1 min, 55°C for 30 sec, 72°C for 1 min and then
72°C for 10 min. The PCR products were purified using the Agencourt
AMPure XP kit (Beckman Coulter, Inc., Miami, FL, USA), and the
sequence reactions of the purified products were then performed
using the BigDye Terminators v1.1 Cycle Sequencing kit (Applied
Biosystems, Foster City, CA, USA). Cycle conditions were 96°C for 1
min, followed by 25 cycles of 96°C for 10 sec, 50°C for 5 sec and
60°C for 4 min. The reaction products were purified using the
Wizard Magnesil Sequencing Reaction Clean-Up System (Promega) for
the elimination of Dye Terminator. The purified products underwent
capillary electrophoresis using Genetic Analyzer 3130xl (Applied
Biosystems). Data were analyzed using Sequencing Analysis Software
(Applied Biosystems).
Results
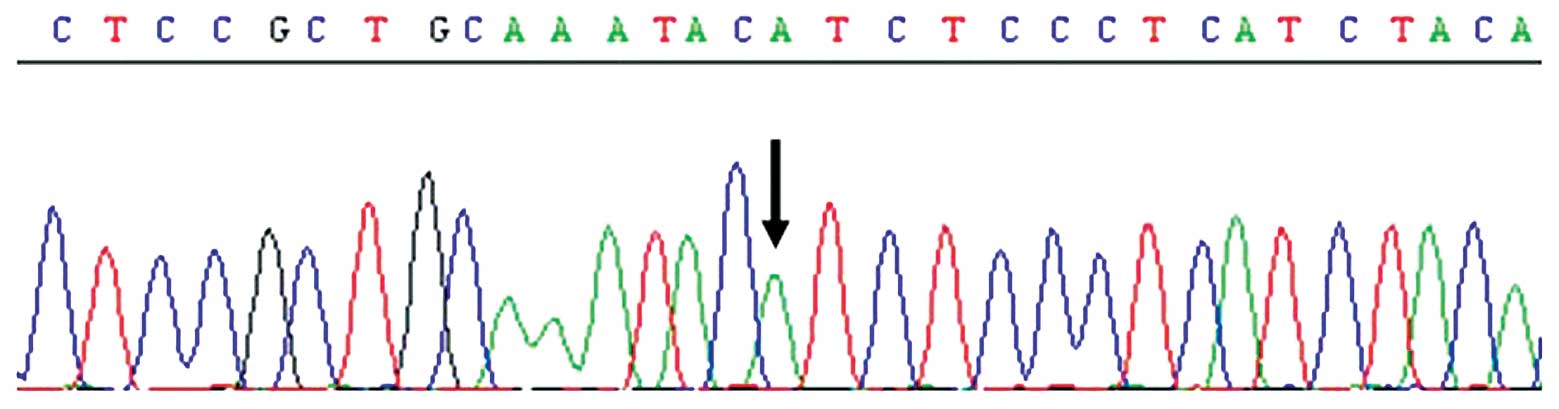
A single nucleotide polymorphism at position 313 of
exon 5 in the GSTP1 gene leads to isoleucine/valine substitution
(4). GSTP1 genotype analysis
demonstrated that the patient was found to possess the wild type
(Ile/Ile) genotype, as the GSTP1 polymorphism was homozygous for
A/A at position 313 of exon 5 in amino acid codon 105 (Fig. 3).
Discussion
Oxaliplatin-based chemotherapy has become a standard
regimen for CRC, as an adjuvant treatment and as a treatment for
advanced disease (1–3). Neurotoxicity is the major
dose-limiting toxicity of oxaliplatin, and it greatly affects the
continuation of therapy and the patients’ quality of life. Although
there have been several studies on the prevention of
oxaliplatin-related neurotoxicity, including the use of ‘Stop and
Go’ therapy, Ca/Mg infusions and goshajinkigan, no recommendation
has been made for the prophylaxis of oxaliplatin-induced
neurotoxicity (5–7). However, it is noteworthy that there
was no neurotoxicity despite the efficacious long-term, high-dose
administration of oxaliplatin in the present case.
It has been indicated that oxaliplatin-induced
neurotoxicity is caused by alteration of the voltage-gated sodium
channels by its metabolite, oxalate (8,9). In
addition, pharmacogenetics is becoming extremely important in the
prediction of toxicity and response (10). The GSTs are a multigene family of
enzymes that catalyze the conjugation of glutathione to
electrophilic xenobiotics and facilitate their excretion from the
body (4). The enzymes have at least
five major classes. Among them, GSTP1, which is highly expressed in
CRC, participates in the detoxification of platinum drugs and may
be involved in the resistance to platinum-based chemotherapy
(11,12). A single nucleotide polymorphism
(A>G) at position 313 of exon 5 causes an isoleucine to valine
substitution in amino acid codon 105 (Ile105Val) in the GSTP1 gene
(4). Several studies have shown an
association between the GSTP1 polymorphism and oxaliplatin efficacy
and toxicity (Table I). Certain
studies have reported longer progression-free survival (PFS) or
overall survival (OS) times in patients with a homozygous (Val/Val)
or heterozygous (Ile/Val) genotype when treated with oxaliplatin
(13–15). While it has been found that
cumulative neurotoxicity is more common in patients with the
Ile/Ile genotype (16–18), certain studies have shown that
neurotoxicity is more frequent in patients with the Val/Val or
Ile/Val genotypes (14,15,19–21).
However, other studies found no correlation between PFS, OS or
neurotoxicity and the GSTP1 genotype (18,20,22–24).
As shown in Fig. 3, the present
patient possessed the Ile/Ile genotype. It is thought that the
GSTP1-105Ile protein could enhance oxaliplatin-induced
neurotoxicity through inhibition of c-Jun NH2-terminal kinase
(JNK), whereas the Val variant shows higher JNK activity, thereby
increasing the expression of genes involved in cellular defense,
and thus possibly protecting the cells against platinum-induced
toxicity (25). The association
between the GSTP1 polymorphism and oxaliplatin efficacy and
toxicity remains controversial.
 | Table IOverview of published studies on GSTP1
polymorphism and efficacy or neurotoxicity of oxaliplatin among
patients with gastrointestinal cancers. |
Table I
Overview of published studies on GSTP1
polymorphism and efficacy or neurotoxicity of oxaliplatin among
patients with gastrointestinal cancers.
| First author
(ref.) | Year | Sample size, n | Cancer type | Regimen | Genotype associated
with longer PFS time | Genotype associated
with longer OS time | Genotype associated
with frequent neurotoxicity |
|---|
| Stoehlmacher J et
al (13) | 2002 | 107 | CRC | 5-FU/L-OHP | - | A/G or G/G | - |
| Grothey A et
al (19) | 2005 | 299 | CRC | FOLFOX4 | - | - | A/G or G/G |
| Lecomte T et
al (16) | 2006 | 64 | CRC, GC, PC | FOLFOX4 (72%) | - | - | A/A |
| Gamelin L et
al (22) | 2007 | 122 | CRC | FOLFOX | - | - | Not significant |
| Ruzzo A et al
(20) | 2007 | 166 | CRC | FOLFOX4 | Not significant | - | A/G or G/G |
| Paré L et al
(17) | 2008 | 126 | CRC | 5-FU/L-OHP | - | - | A/A |
| Kweekel DM et
al (23) | 2009 | 56 | CRC | XELOX | Not significant | Not significant | Not significant |
| Goekkurt E et
al (18) | 2009 | 134 | GC | FLO or FLP | Not significant | Not significant | A/A |
| Chen YC et al
(14) | 2010 | 166 | CRC | FOLFOX4 | A/G or G/G | A/G or G/G | A/G or G/G |
| Kanai M et al
(21) | 2010 | 82 | CRC | mFOLFOX6 | - | - | A/G or G/G |
| Inada M et al
(24) | 2010 | 51 | CRC | mFOLFOX6 | - | - | Not significant |
| Hong J et al
(15) | 2011 | 52 | CRC | SOX | A/G or G/G | - | A/G or G/G |
In conclusion, the current study describes the case
of a patient with rectal cancer, who safely received long-term
treatment with oxaliplatin. It has been reported that
administration of the three active agents, 5-FU/leucovorin,
irinotecan and oxaliplatin, is associated with prolonged OS in
advanced CRC (26). When specific
anti-tumor agents show efficacy in patients, it is important that
adverse events are prevented properly in order to continue the
therapy for long periods. Further development of individualized
chemotherapy with an analysis of genomic polymorphisms in the drug
target genes, including GSTP1, is required to prevent
oxaliplatin-induced cumulative neurotoxicity.
References
|
1
|
de Gramont A, Figer A, Seymour M, et al:
Leucovorin and fluorouracil with or without oxaliplatin as
first-line treatment in advanced colorectal cancer. J Clin Oncol.
18:2938–2947. 2000.
|
|
2
|
Goldberg RM, Sargent DJ, Morton RF, et al:
A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004. View Article : Google Scholar
|
|
3
|
André T, Boni C, Mounedji-Boudiaf L, et
al: Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment
for colon cancer. N Engl J Med. 350:2343–2351. 2004.
|
|
4
|
Watson MA, Stewart RK, Smith GB, Massey TE
and Bell DA: Human glutathione S-transferase P1 polymorphisms:
relationship to lung tissue enzyme activity and population
frequency distribution. Carcinogenesis. 19:275–280. 1998.
View Article : Google Scholar
|
|
5
|
Tournigand C, Cervantes A, Figer A, et al:
OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin
in a stop-and-Go fashion in advanced colorectal cancer - a GERCOR
study. J Clin Oncol. 24:394–400. 2006. View Article : Google Scholar
|
|
6
|
Hosokawa A, Ogawa K, Ando T, et al:
Preventive effect of traditional Japanese medicine on neurotoxicity
of FOLFOX for metastatic colorectal cancer: a multicenter
retrospective study. Anticancer Res. 32:2545–2550. 2012.
|
|
7
|
Grothey A, Nikcevich DA, Sloan JA, et al:
Intravenous calcium and magnesium for oxaliplatin-induced sensory
neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J Clin Oncol.
29:421–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakurai M, Egashira N, Kawashiri T, Yano
T, Ikesue H and Oishi R: Oxaliplatin-induced neuropathy in the rat:
involvement of oxalate in cold hyperalgesia but not mechanical
allodynia. Pain. 147:165–174. 2009. View Article : Google Scholar
|
|
9
|
Grolleau F, Gamelin L, Boisdron-Celle M,
Lapied B, Pelhate M and Gamelin E: A possible explanation for a
neurotoxic effect of the anticancer agent oxaliplatin on neuronal
voltage-gated sodium channels. J Neurophysiol. 85:2293–2297.
2001.
|
|
10
|
Wang L, McLeod HL and Weinshilboum RM:
Genomics and drug response. N Engl J Med. 364:1144–1153. 2011.
View Article : Google Scholar
|
|
11
|
Moscow JA, Fairchild CR, Madden MJ, et al:
Expression of anionic glutathione-S-transferase and P-glycoprotein
genes in human tissues and tumors. Cancer Res. 49:1422–1428.
1989.PubMed/NCBI
|
|
12
|
Goto S, Iida T, Cho S, Oka M, Kohno S and
Kondo T: Overexpression of glutathione S-transferase pi enhances
the adduct formation of cisplatin with glutathione in human cancer
cells. Free Radic Res. 31:549–558. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stoehlmacher J, Park DJ, Zhang W, et al:
Association between glutathione S-transferase P1, T1, and M1
genetic polymorphism and survival of patients with metastatic
colorectal cancer. J Natl Cancer Inst. 94:936–942. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YC, Tzeng CH, Chen PM, et al:
Influence of GSTP1 I105V polymorphism on cumulative neuropathy and
outcome of FOLFOX-4 treatment in Asian patients with colorectal
carcinoma. Cancer Sci. 101:530–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong J, Han SW, Ham HS, et al: Phase II
study of biweekly S-1 and oxaliplatin combination chemotherapy in
metastatic colorectal cancer and pharmacogenetic analysis. Cancer
Chemother Pharmacol. 67:1323–1331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lecomte T, Landi B, Beaune P, Laurent-Puig
P and Loriot MA: Glutathione S-transferase P1 polymorphism
(Ile105Val) predicts cumulative neuropathy in patients receiving
oxaliplatin-based chemotherapy. Clin Cancer Res. 12:3050–3056.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paré L, Marcuello E, Altés A, et al:
Pharmacogenetic prediction of clinical outcome in advanced
colorectal cancer patients receiving oxaliplatin/5-fluorouracil as
first-line chemotherapy. Br J Cancer. 99:1050–1055. 2008.
|
|
18
|
Goekkurt E, Al-Batran SE, Hartmann JT, et
al: Pharmacogenetic analyses of a phase III trial in metastatic
gastroesophageal adenocarcinoma with fluorouracil and leucovorin
plus either oxaliplatin or cisplatin: a study of the
arbeitsgemeinschaft internistische onkologie. J Clin Oncol.
27:2863–2873. 2009. View Article : Google Scholar
|
|
19
|
Grothey A, McLeod HL, Green EM, et al:
Glutathione S-transferase P1 I105V (GSTP1 I105V) polymorphism is
associated with early onset of oxaliplatin-induced neurotoxicity. J
Clin Oncol. 23(Suppl 1): abstr 3509. 2005.
|
|
20
|
Ruzzo A, Graziano F, Loupakis F, et al:
Pharmacogenetic profiling in patients with advanced colorectal
cancer treated with first-line FOLFOX-4 chemotherapy. J Clin Oncol.
25:1247–1254. 2007. View Article : Google Scholar
|
|
21
|
Kanai M, Yoshioka A, Tanaka S, et al:
Associations between glutathione S-transferase pi Ile105Val and
glyoxylate aminotransferase Pro11Leu and Ile340Met polymorphisms
and early-onset oxaliplatin-induced neuropathy. Cancer Epidemiol.
34:189–193. 2010. View Article : Google Scholar
|
|
22
|
Gamelin L, Capitain O, Morel A, et al:
Predictive factors of oxaliplatin neurotoxicity: the involvement of
the oxalate outcome pathway. Clin Cancer Res. 13:6359–6368. 2007.
View Article : Google Scholar
|
|
23
|
Kweekel DM, Gelderblom H, Antonini NF, et
al: Glutathione-S-transferase pi (GSTP1) codon 105 polymorphism is
not associated with oxaliplatin efficacy or toxicity in advanced
colorectal cancer patients. Eur J Cancer. 45:572–578. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inada M, Sato M, Morita S, et al:
Associations between oxaliplatin-induced peripheral neuropathy and
polymorphisms of the ERCC1 and GSTP1 genes. Int J Clin Pharmacol
Ther. 48:729–734. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elsby R, Kitteringham NR, Goldring CE, et
al: Increased constitutive c-Jun N-terminal kinase signaling in
mice lacking glutathione S-transferase Pi. J Biol Chem.
278:22243–22249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grothey A and Sargent D: Overall survival
of patients with advanced colorectal cancer correlates with
availability of fluorouracil, irinotecan, and oxaliplatin
regardless of whether doublet or single-agent therapy is used first
line. J Clin Oncol. 23:9441–9442. 2005. View Article : Google Scholar
|