Introduction
Colorectal cancer is one of the most common types of
cancer and the second greatest cause of cancer-associated mortality
in the United States (1).
Epidemiological investigation has revealed the highest incidence of
colorectal cancer in North America and Australia, suggesting it is
a disease associated with lifestyle (1). With changing diet in China, the
incidence and mortality of colorectal cancer are increasing
markedly. Although surgical resection, radiotherapy, chemotherapy
and targeted therapies are available for the treatment of
colorectal cancer, recurrence and metastasis (20–40% of patients
present with liver metastases at diagnosis) remain a cause of poor
prognosis.
It has been proposed that a small group of cells
exists in tumors with properties of stem cells, including
self-renewal, multilineage differentiation, multidrug resistance
and tumorigenesis. These cells are considered cancer stem cells and
the source of the recurrence and metastasis of colorectal cancer
(2,3). Previous studies have revealed that
epithelial cell adhesion molecule-high
(EpCAMhigh)/cluster of differentiation
(CD)44+ colorectal cancer cells have such stem cell-like
properties, leading to tumorigenesis, invasion and metastasis of
colorectal cancer (4–6). This suggests that
EpCAMhigh/CD44+ may be used as a marker of
colorectal cancer stem cells (4).
In order to further understand the clinical
significance of EpCAMhigh/CD44+ colorectal
cancer cells, the present study used double immunohistochemical
staining to detect the presence of
EpCAMhigh/CD44+ cells in 80 cases of
colorectal cancer and 10 cases of corresponding liver metastases to
explore the correlation between these cells and the biological
behavior of colorectal cancer.
Materials and methods
Patients and tissue samples
Clinical data were collected from medical records at
the First and Second Affiliated Hospitals of Dalian Medical
University (Dalian, China). Formalin-fixed paraffin-embedded tumor
samples from 80 cases of colorectal cancer and 10 cases of
corresponding liver metastases obtained between 2003 and 2010 were
used in this study. In total, 30 cases of normal intestinal mucosa
were used as the controls. Paraneoplastic intestinal mucosa samples
were used as controls. There were 38 male cases and 42 female
cases. Patient age ranged between 19 and 83 years (mean age, 60
years), and 44 cases were >60 years old and 36 were ≤60 years
old. Tumor diameter in 30 cases was >5 cm and <5 cm in 50
cases. Histologically, there were 10 highly differentiated, 51
moderately differentiated and 19 poorly differentiated cases.
According to tumor, lymph node and metastasis (TNM) staging, 18
cases were stage T1 or T2, 28 cases were T3 and 34 cases were T4.
Dukes’ staging described 16 cases of Dukes’ A, 16 cases of Dukes’
B, 22 cases of Dukes’ C and 26 cases of Dukes’ D. Metastases were
present in 48 cases and absent in 32.
All histological diagnoses were independently
confirmed by two experienced pathologists. No patient had received
chemotherapy or radiotherapy prior to surgery. All tumors were
classified using the 2010 criteria of the World Health Organization
(WHO) (7,8). The study was approved by the Regional
Ethics Committee of Dalian Medical University and performed in
accordance with the Declaration of Helsinki. Patients provided
written informed consent.
Double immunohistochemical staining
Embedded specimens were sectioned at a thickness of
4 μm. Double immunohistochemical staining was performed
according to DouSP™ double staining kit (MaiXin Bio, Fuzhou, China)
procedures. Following deparaffinization and dehydration, endogenous
peroxidases were blocked briefly with 3%
H2O2. Non-specific antibody binding was
blocked with goat serum for 10 min at room temperature. Sections
were sequentially incubated with primary antibody [mouse anti-human
EpCAM monoclonal antibody (MaiXin Bio)] overnight at 4°C, followed
by the secondary antibody [goat anti-mouse/rabbit polyclonal IgG
(MaiXin Bio)] for 15 min at room temperature. Sections were
incubated with alkaline phosphatase for 15 min at room temperature.
EpCAM was detected with 5-bromo-4-chloro-3-indolyl phosphate,
p-toluidine salt (BCIP)/nitro blue tetrazolium (NBT) at room
temperature. Positively stained cells exhibited a blue-black
precipitate in the cytoplasm. Sections were subsequently incubated
with double staining enhancement solution for 10 min at room
temperature, then blocked with goat serum for 10 min, also at room
temperature. Sections were then sequentially incubated with primary
antibody [rabbit anti-human CD44 polyclonal antibody (Proteintech
Group, Inc., Chicago, IL, USA)] overnight at 4°C and secondary
antibody [goat anti-mouse/rabbit IgG (MaiXin Bio)] for 15 min at
room temperature. CD44 was detected with aminoethylcarbazole (AEC)
at room temperature. Positively stained cells exhibited red
precipitate in the cytomembrane. In order to prove the reliability
of double staining of EpCAMhigh/CD44+,
immunohistochemical staining of
EpCAMhigh/CD44−,
EpCAMlow/CD44+ and
EpCAMlow/CD44−, respectively were used as
controls.
Assessment of double immunohistochemical
staining
EpCAMhigh/CD44− cells were
positive for BCIP/NBT staining but negative for AEC staining;
EpCAMlow/CD44+ cells were negative for
BCIP/NBT staining but positive for AEC staining;
EpCAMlow/CD44− cells were negative for the
two stains; and EpCAMhigh/CD44+ cells were
positive for the two stains (Fig.
1). The results were assessed according to Lin et al
(9). Briefly, CD44 staining was
detected mainly in the membrane and EpCAM staining was detected
mainly in the cytoplasm. The results of EpCAM/CD44 cells proportion
were classified into four groups,
EpCAMhigh/CD44+,
EpCAMhigh/CD44−,
EpCAMlow/CD44+, EpCAMlow/CD44-.
The proportion of EpCAMhigh/CD44+ tumor cells
were defined as the percentage of cells positive for both
blue-black and red staining, EpCAMhigh/CD44−
were positve for blue-black staining [5-bromo-4chloro-3-indolyl
phosphate, p-toluidine salt (BCIP)/nitro blue tetrazolium (NBT);
MaiXin Bio], EpCAMlow/CD44+ were positive for
the red staining [3-amino 9-ethylcarbazole (AEC); MaiXin Bio] and
EpCAMlow/CD44− were negative for the two
stains. The results of EpCAMhigh/CD44+ cells
were based on the median value of their proportion checked for 10
visions under the microscope (BX51, Olympus Corporation, Tokyo,
Japan). The labeling index (the percentage of positively stained
cells) of each specimen was recorded and the values were
averaged.
Statistical analysis
SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was used for
statistical analysis. Differences in the means of continuous
variables between the groups were analyzed using analysis of
variance or t-tests. P-values from two-tailed tests are reported
and in all analyses P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of EpCAM/CD44 in samples of
colorectal cancer and their corresponding liver metastases
Double immunohistochemical staining was used to
detect the expression of EpCAM/CD44. The expression of EpCAM was
predominantly cytoplasmic, whereas the expression of CD44 was
primarily in the cytomembrane.
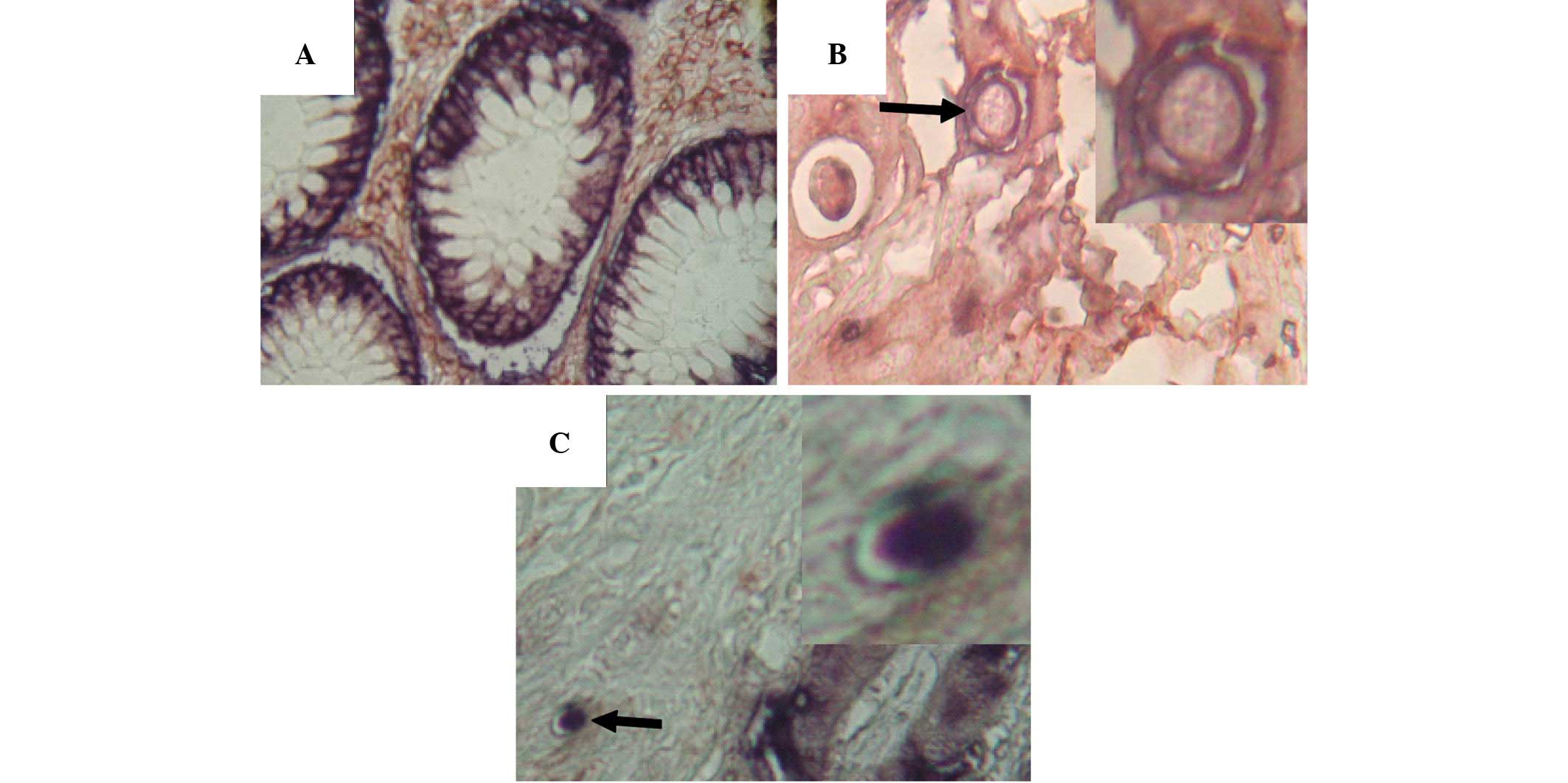
EpCAMhigh/CD44+ double-positive cells were
not present in the normal intestinal mucosa adjacent to colorectal
tumors (Fig. 2A). However, in
samples of colorectal cancer and their corresponding liver
metastases, EpCAMhigh/CD44+ cells were
visible (Fig. 2B and 2C).
Double-positive cells accounted for 0.8–3.1% of cells in colorectal
tumors. The expression of EpCAM/CD44 was significantly correlated
with degree of differentiation, tumor stage, depth of invasion
(Dukes’ stage) and metastatic status (P<0.05), while there was
no correlation with gender, age or the magnitude of the tumor
(P>0.05) (Table I).
 | Table IExpression of EpCAM/CD44 in
colorectal cancer. |
Table I
Expression of EpCAM/CD44 in
colorectal cancer.
| Variable | Samples, n |
EpCAMhigh/CD44+
cells, % | P-value |
|---|
| Gender | | | 1.000 |
| Male | 38 | 0.99±1.11 | |
| Female | 42 | 0.99±0.90 | |
| Patient age,
years | | | 0.212 |
| >60 | 44 | 0.84±1.04 | |
| ≤60 | 36 | 1.12±0.96 | |
| Tumor magnitude,
cm | | | 0.051 |
| ≤5×5 | 50 | 0.80±0.73 | |
| >5×5 | 30 | 1.31±1.29 | |
|
Differentiation | | | <0.001a |
| High | 10 | 0.53±0.93 | |
| Moderate | 51 | 0.74±0.68 | |
| Low | 19 | 1.90±1.23 | |
| Tumor stage | | | 0.002a |
| T1+T2 | 18 | 0.49±0.43 | |
| T3 | 28 | 0.80±0.43 | |
| T4 | 34 | 1.41±1.00 | |
| Dukes’ stage | | | <0.001a |
| A | 16 | 0.42±0.39 | |
| B | 16 | 0.62±0.74 | |
| C | 22 | 0.89±0.66 | |
| D | 26 | 1.66±1.26 | |
| Metastasis | | | <0.001a |
| Negative | 32 | 0.52±0.59 | |
| Positive | 48 | 1.31±1.09 | |
Discussion
Cancer stem cells have the capacity for infinite
proliferation and self-renewal, and account for 0.3–2.2% of
colorectal cancer cells. They are considered the cause of
recurrence, metastasis and drug resistance in tumors (8). Therefore the identification of cancer
stem cells and treatments targeting these cells facilitates
elimination of the tumor.
EpCAM, a 40 kDa glycoprotein, functions as an
epithelial cell adhesion molecule (11); its expression has been reported as
localized to the epithelium along the basolateral surface of the
majority of gastrointestinal tract mucosa. EpCAM can inhibit
differentiation and promote proliferation (12). It is expressed in 85% of colorectal
carcinomas and is one of the earliest tumor markers to appear that
is involved in signal transduction, regeneration of tissue and
other biological functions. EpCAM can upregulate the expression of
the oncogene c-myc, induce acceleration of the cell cycle and
promote proliferation (12). The
overexpression of EpCAM enhances the proliferative and invasive
capacities of tumors, while downregulation by RNA interference
inhibits these functions (13).
CD44 is a multifunctional class I transmembrane
glycoprotein located on the cytomembrane (14,15).
As a cell adhesion molecule, CD44 is mainly involved in cell-cell
and cell-matrix interactions (16).
CD44 plays a vital role in the regulation of cell adhesion, growth,
differentiation, migration and angiogenesis, and contributes to
tumor progression by promoting invasion and metastasis (14,15).
Knockdown of CD44 in primary colon cancer cell lines reduces
clonogenicity in vitro and tumorigenicity in vivo
(17). Schulenburg et al
isolated CD44+ and CD44− cells from colon
cancer samples. The authors found that CD44+ cells had
characteristics of stem cells and showed much higher capacity for
proliferation and invasion than CD44− cells (18). Thus, CD44 is considered a marker of
cancer stem cells. In combination with other surface markers, CD44
can also be used to discriminate between a variety of cancer
subsets (19).
Previous studies have revealed that the composite
structure of EpCAM and CD44 can promote invasion and metastasis of
tumors more strongly than any single adhesion molecule (20–22).
In 2008, Marhaba et al proposed that
EpCAMhigh/CD44+ cells are a marker of
colorectal cancer stem cells (23),
and Dalerba et al found that the
EpCAMhigh/CD44+ phenotype of colorectal
cancer cells has stem cell-like properties (4). The authors reported that 200–500
EpCAMhigh/CD44+ cells promoted tumorigenesis
in non-obsese diabetic/severe combined immunodeficient mice, while
no tumor was produced by injection of 104
EpCAMlow/CD44− cells. Since the
EpCAMhigh/CD44+ phenotype of colorectal
cancer cells, which has stem cell-like properties, was confirmed
(24), it has been regarded as an
effective marker of colorectal cancer stem cells (25). The identification of cancer stem
cells improves the understanding of tumorigenesis.
In the present study, 80 cases of colorectal cancer
and their corresponding liver metastases were examined.
EpCAMhigh/CD44+ cells were not present in the
adjacent normal intestinal mucosa. However, in colorectal cancer
tissue and their corresponding liver metastases,
EpCAMhigh/CD44+ cells were visible. The
percentage of double-positive cells was 0.8–3.1% in colorectal
cancer, which is consistent with a previous study (9). Further analysis found that the
percentage of EpCAMhigh/CD44+ cells in poorly
differentiated tumors was higher than that in highly or moderately
differentiated tumors. In addition, the percentage of
EpCAMhigh/CD44+ cells in the T-stage 4 and
Dukes’ D groups or in cases of metastasis was higher than that at
other stages or in the group without metastases. Statistical
analysis revealed that EpCAMhigh/CD44+
correlated with degree of differentiation, clinical stage, depth of
invasion and metastasis. These results demonstrate that
EpCAMhigh/CD44+ expression is significantly
correlated with invasion and metastasis, and confirm
EpCAMhigh/CD44+ cells as effective markers
for colorectal cancer stem cells. These findings support the
proposal that cancer stem cells may be the cause of recurrence and
metastasis.
Based on the theory that cancer stem cells are the
source of tumorigenesis, recurrence and metastasis in tumors,
targeting cancer stem cells should effect the elimination of
tumors. Specific drugs (catumaxomab and edrecolomab) targeting
EpCAM have been developed, which can significantly reduce the size
of tumor used alone or in combination with standard treatment,
demonstrating the potential of such targeting strategies (26,27).
Further study and the development of targeted drugs is required to
elucidate the mechanisms of colorectal cancer stem cells and
improve the clinical prognosis of colorectal cancer.
EpCAMhigh/CD44+, which is
regarded as a marker of colorectal cancer stem cells, is
significantly correlated with the invasion and metastasis of
colorectal cancer, suggesting that this molecular marker may
promote the progression of tumors.
Acknowledgements
The skillful technical assistance of the Department
of Pathology, The Second Affiliated Hospital of Dalian Medical
University (Dalian, China) in preparing sections for
immunohistochemistry is gratefully acknowledged. The authors thank
laboratory members for help with photographic work.
References
|
1
|
Yamaki M, Shinozaki K, Sakaguchi T, Meseck
M, Ebert O, Ohdan H and Woo SL: The potential of recombinant
vesicular stomatitis virus-mediated virotherapy against metastatic
colon cancer. Int J Mol Med. 31:299–306. 2013.
|
|
2
|
Christ B and Stock P: Mesenchymal stem
cell-derived hepatocytes for functional liver replacement. Front
Immunol. 3:1682012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lakowski J, Han YT, Pearson RA, et al:
Effective transplantation of photoreceptor precursor cells selected
via cell surface antigen expression. Stem Cells. 29:1391–1404.
2011.
|
|
4
|
Dalerba P, Dylla SJ, Park IK, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lugli A, Iezzi G, Hostettler I, et al:
Prognostic impact of the expression of putative cancer stem cell
markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer.
Br J Cancer. 103:382–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piscuoglio S, Lehmann FS, Zlobec I, et al:
Effect of EpCAM, CD44, CD133 and CD166 expression on patient
survival in tumours of the ampulla of Vater. J Clin Pathol.
65:140–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fléjou JF: WHO Classification of digestive
tumors: the fourth edition. Ann Pathol. 31:S27–S31. 2011.(In
French).
|
|
8
|
Aust DE: WHO classification 2010 for the
lower gastrointestinal tract: what is new? Pathologe. 32:326–331.
2011.(In German).
|
|
9
|
Lin Y, Zhong Y, Guan H, Zhang X and Sun Q:
CD44+/CD24− phenotype contributes to
malignant relapse following surgical resection and chemotherapy in
patients with invasive ductal carcinoma. J Exp Clin Cancer Res.
31:592012.
|
|
10
|
Haraguchi N, Inoue H, Tanaka F, et al:
Cancer stem cells in human gastrointestinal cancers. Hum Cell.
19:24–29. 2006. View Article : Google Scholar
|
|
11
|
Sankpal NV, Mayfield JD, Willman MW,
Fleming TP and Gillanders WE: Activator protein 1 (AP-1)
contributes to EpCAM-dependent breast cancer invasion. Breast
Cancer Res. 13:R1242011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin CW, Liao MY, Lin WW, Wang YP, Lu TY
and Wu HC: Epithelial cell adhesion molecule regulates tumor
initiation and tumorigenesis via activating reprogramming factors
and epithelial-mesenchymal transition gene expression in colon
cancer. J Biol Chem. 287:39449–39459. 2012. View Article : Google Scholar
|
|
13
|
Gaiser MR, Lämmermann T, Feng X, et al:
Cancer-associated epithelial cell adhesion molecule (EpCAM; CD326)
enables epidermal Langerhans cell motility and migration in
vivo. Proc Natl Acad Sci USA. 109:E889–E897. 2012. View Article : Google Scholar
|
|
14
|
Jaggupilli A and Elkord E: Significance of
CD44 and CD24 as cancer stem cell markers: an enduring ambiguity.
Clin Dev Immunol. 2012:7080362012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murai T, Maruyama Y, Mio K, Nishiyama H,
Suga M and Sato C: Low cholesterol triggers membrane
microdomain-dependent CD44 shedding and suppresses tumor cell
migration. J Biol Chem. 286:1999–2007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zarrabi K, Dufour A, Li J, et al:
Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer
cell migration. J Biol Chem. 286:33167–33177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kemper K, Grandela C and Medema JP:
Molecular identification and targeting of colorectal cancer stem
cells. Oncotarget. 1:387–395. 2010.PubMed/NCBI
|
|
18
|
Schulenburg A, Cech P, Herbacek I, Marian
B, Wrba F, Valent P and Ulrich-Pur H: CD44-positive colorectal
adenoma cells express the potential stem cell markers musashi
antigen (msi1) and ephrin B2 receptor (EphB2). J Pathol.
213:152–160. 2007. View Article : Google Scholar
|
|
19
|
Strauss R, Li ZY, Liu Y, et al: Analysis
of epithelial and mesenchymal markers in ovarian cancer reveals
phenotypic heterogeneity and plasticity. PLoS One. 6:e161862011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Belov L, Zhou J and Christopherson RI:
Cell surface markers in colorectal cancer prognosis. Int J Mol Sci.
12:78–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang SJ and Bourguignon LY: Role of
hyaluronan-mediated CD44 signaling in head and neck squamous cell
carcinoma progression and chemoresistance. Am J Pathol.
178:956–963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuhn S, Koch M, Nübel T, et al: A complex
of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins
promotes colorectal cancer progression. Mol Cancer Res. 5:553–567.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marhaba R, Klingbeil P, Nuebel T,
Nazarenko I, Buechler MW and Zoeller M: CD44 and EpCAM:
cancer-initiating cell markers. Curr Mol Med. 8:784–804. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dylla SJ, Beviglia L, Park IK, et al:
Colorectal cancer stem cells are enriched in xenogeneic tumors
following chemotherapy. PLoS One. 3:e24282008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biddle A, Liang X, Gammon L, Fazil B,
Harper LJ, Emich H, Costea DE and Mackenzie IC: Cancer stem cells
in squamous cell carcinoma switch between two distinct phenotypes
that are preferentially migratory or proliferative. Cancer Res.
71:5317–5326. 2011. View Article : Google Scholar
|
|
26
|
Münz M, Murr A, Kvesic M, et al:
Side-by-side analysis of five clinically tested anti-EpCAM
monoclonal antibodies. Cancer Cell Int. 10:442010.PubMed/NCBI
|
|
27
|
Bezan A, Hohla F, Meissnitzer T, et al:
Systemic effect of catumaxomab in a patient with metastasized
colorectal cancer: a case report. BMC Cancer. 13:6182013.
View Article : Google Scholar : PubMed/NCBI
|