Introduction
Endothelial progenitor cells (EPCs) are bone
marrow-derived cells that can be found in peripheral and umbilical
cord blood. The cells were first isolated in the study by Asahara
et al (1997), where it was demonstrated that cluster of
differentiation 34-positive (CD34+) hematopoietic
progenitor cells from adults can differentiate ex vivo into
the endothelial phenotype (1).
These cells express endothelial markers and are incorporated into
the neoformation vessels in ischemic areas. Data in the literature
have supported the presence of circulating hemangioblasts in
adults, and EPCs are defined as CD34- and VEGFR2-expressing
elements (2,3). CD133, also known as prominin or AC133,
is a conserved antigen with unknown biological activity, which is
expressed by hematopoietic stem cells, but is absent in mature
endothelial cells and in the monocyte line (4). Under these conditions,
CD133+/VEGFR2+ cells are likely to reflect
immature progenitors and the cells interspersed in the vascular
endothelium.
In the group of circulating blood mononuclear cells
there may be several sources of EPCs, including hematopoietic stem
cells, myeloid cells that can differentiate on endothelial cells by
growing, other progenitor circulating cells and mature endothelial
circulating cells. The first evidence of the existence of several
circulating EPCs was reported by Lin et al (5).
Although the existence of EPCs has been
demonstrated, with regard to malignant tumors the data is
controversial on the pre-existing endothelium insertion rate and
the extent to which these cells contribute to tumor angiogenesis.
From these points of view, the results obtained so far vary between
the extremely wide limits of 0 and 72 % for various human tumors.
So far, no such studies have reported the contribution of EPCs in
ovarian tumors. For this reason, the present study evaluated the
expression of two markers, AC133 and tyrosine kinase with
immunoglobulin-like and EGF-like domains 2 (Tie2), which signal the
presence of EPCs in the pre-existing endothelium.
Materials and methods
Patient selection
In total, 62 female patients who were diagnosed with
ovarian tumors were retrospectively selected over a four-year
period. The patients had complete clinicopathological and
post-surgical evaluation data, and were well characterized with
regard to the invasion (local and distant) and surgical protocols.
Signed consent was obtained from each patient. All procedures were
carried out according to the principles embodied in the Declaration
of Helsinki and were approved by the Institutional Review Board of
‘Victor Babeş’ University of Medicine and Pharmacy, Timişoara,
Romania.
Specimens and histopathological primary
processing
Tumor specimens were surgically removed and the most
representative sections were carefully selected to include tumor
and adjacent normal ovarian tissues. Tumor sections with necrosis
and extensive hemorrhages were avoided. Small tumor tissues
(10×10×3-mm biopsies) were washed in saline solution, fixed in 10%
buffered formalin for 24 h and then paraffin embedded. For each
paraffin-embedded specimen, 5-μm serial sections were mounted on
silanized slides. One slide from each case was stained with
hematoxylin and eosin using a routine method for histopathological
evaluation and also for case selection for the immunohistochemical
procedures.
Immunohistochemistry
Heat-induced epitope retrieval was performed with a
citrate-based solution (pH 6.0; Novocastra Laboratories, Ltd.,
Newcastle upon Tyne, UK) for 30 min. Endogenous peroxidase blocking
was carried out with 3% hydrogen peroxide for 5 min, followed by
incubation for 30 min with Tie2 (dilution 1:300, mouse monoclonal
clone 9; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
AC133 (dilution 1:300, rabbit polyclonal clone H-284; Santa Cruz
Biotechnology, Inc.) as primary antibodies. The Bond Polymer Refine
Detection System (Leica Biosystems, Newcastle upon Tyne, UK) was
used for visualization. 3,3 Diaminobenzidine dihydrochloride was
applied as a chromogen and hemotoxylin was used as a counterstain.
The entire immunohistochemical procedure was performed with the
Leica Bond-Max autostainer (Leica Biosystems).
Results
Upon microscopic evaluation of the hematoxylin and
eosin-stained tumor specimens, four main histopathological types of
ovarian tumors were identified: Serous carcinomas (62%), mucinous
carcinomas (18%), clear cell carcinomas (6%) and ovarian germ cells
tumors (8%) and undifferentiated carcinomas (6%). The majority of
the aforementioned ovarian tumors exhibited a G2 tumor grade (58%),
followed by grades G3 (39%) and G1 (3%).
In evaluating AC133 and Tie2 expression, the
location of the positive cells was examined and only elements with
a positive cytoplasmic reaction that defined the lumens of the
blood vessels were subjectively assessed. AC133 was positive in 18
out of 62 specimens (29.03%), and Tie2 was positive in 21 of the
specimens (33.87%). Co-expression of the markers was noted in 17
cases (27.42%), in which it was considered that the positive
reaction reflected the insertion of the endothelial progenitor
cells into the pre-existing endothelium. The presence of
endothelial progenitor cells did not exhibit a statistically
significant correlation with vascular microdensity, vessel type or
histopathological form.
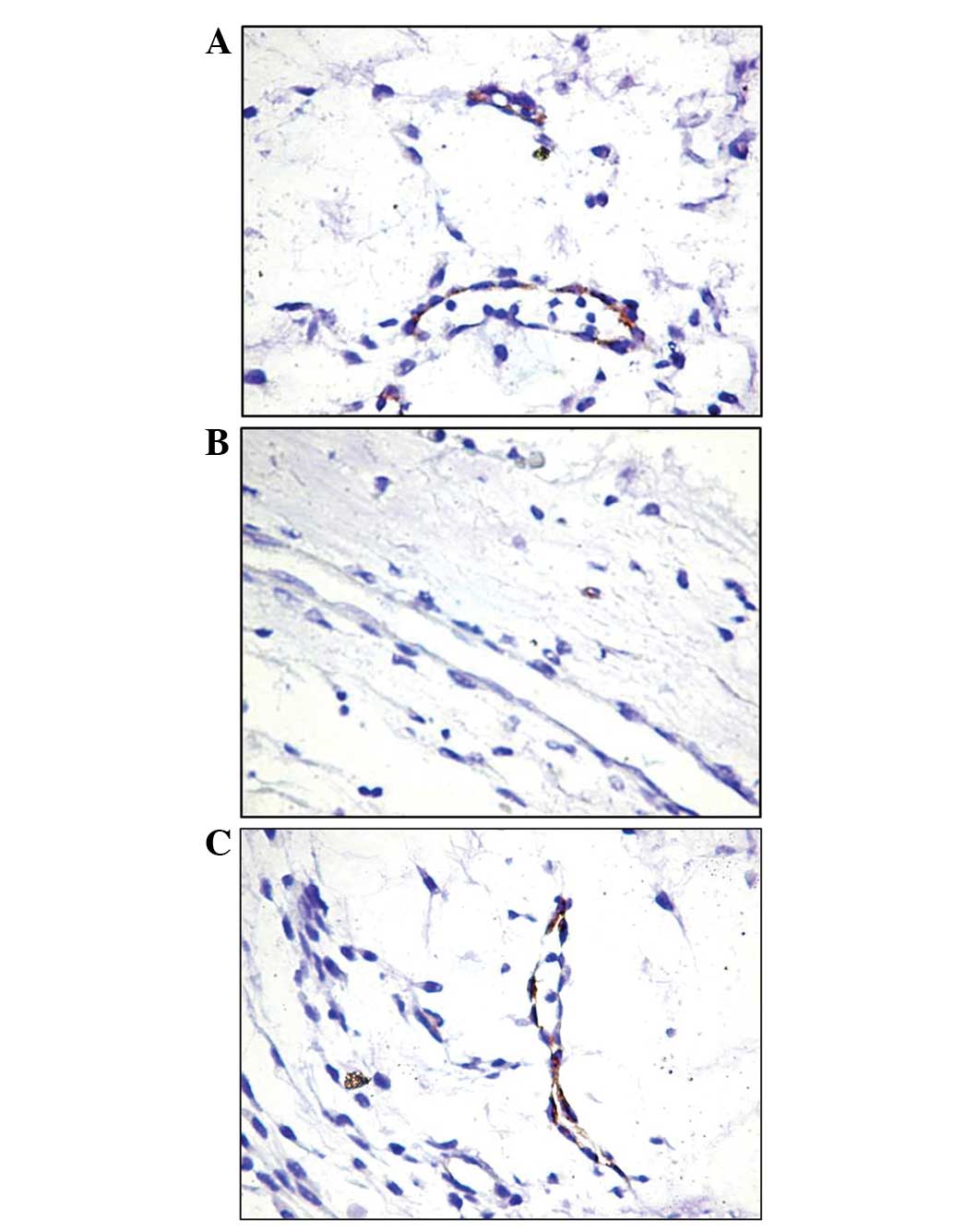
For the expression of AC133, the positive reaction
was constantly evident in the vessels of the tumor area. These
vessels were small, and relatively frequent positive endothelial
cells lined the majority of the lumens (Fig. 1A). Notably, the endothelial cells
were the only AC133-positive cells in the majority of the tumor
stroma cases. In the peritumoral area, the blood vessels were
predominantly AC133-negative, particularly when their morphology
was indicative of a mature character. Occasionally, in extremely
small vessels, a positive reaction was observed (Fig. 1B). The most frequently observed
aspect in the intratumoral area was the heterogeneous model with
alternating AC133-positive and -negative cells (Fig. 1C).
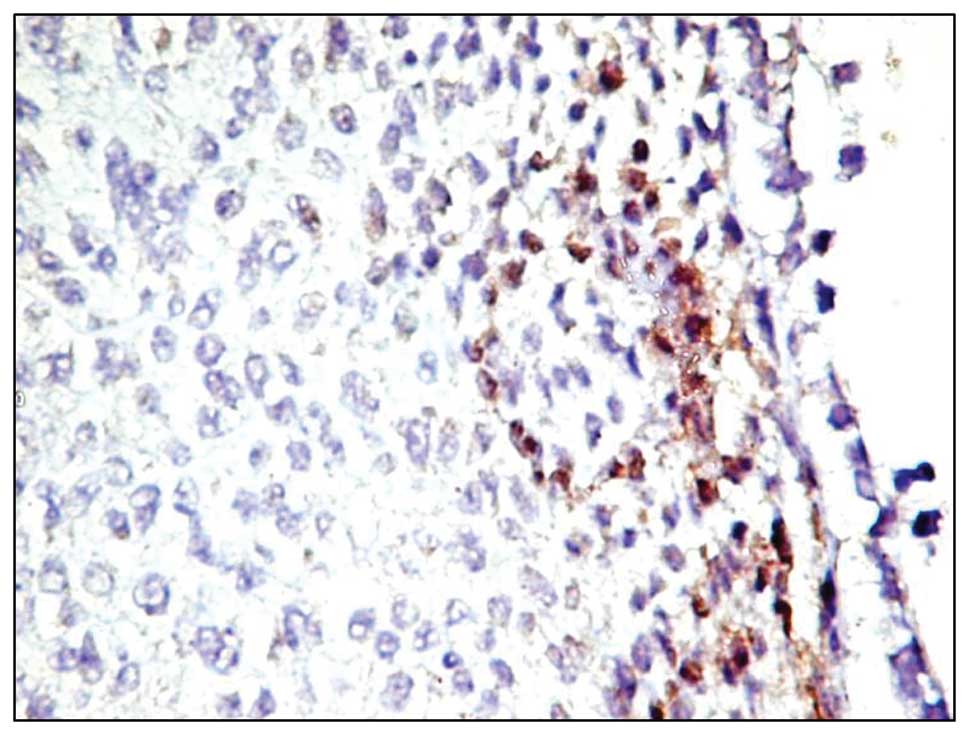
In only two out of the 62 cases, AC133-positive
neoplastic cells were focally observed in the intratumoral area.
The distribution pattern of the positive reaction was diffuse,
cytoplasmic and not predominantly in the membrane (Fig. 2). These cells formed a distinct
population of tumor cells, preferentially located at the tumor
proliferation front, which could represent tumor stem cells. In the
present study tumor stem cells were positive for this marker, but
the method of detection is not specific enough and further studies
are required to demonstrate their character.
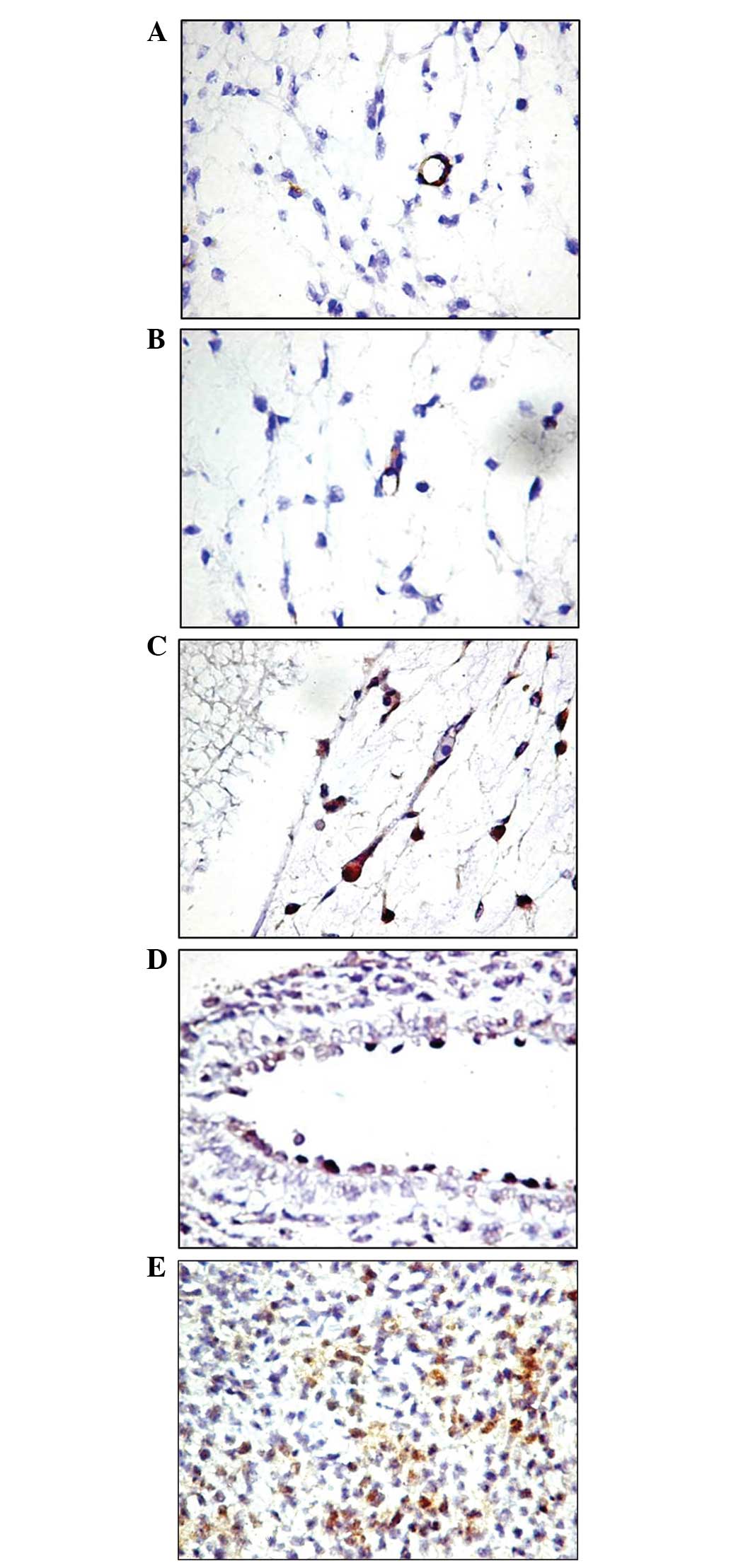
The immunoreaction for Tie2 was also selective for
cells that defined the blood vessel lumens. Even under these
conditions, a small number of vessels with Tie2-positive
endothelial cells were identified in the tumor area, and the
distribution model was found to be homogeneous in the small vessels
(Fig. 3) and heterogeneous in the
larger vessels with relatively large lumens (Fig. 3C). Unlike the reaction for AC133,
Tie2 expression was positive in the endothelium of pre-existing
mature blood vessels, which were larger in size (Fig. 3D). The immunoreaction was found to
be restricted to the endothelium and did not stain perivascular
cells. Since it was not possible to quantify the Tie2-positive
cells compared with the Tie2-negative cells at the endothelial
level, based on subjective observations it appears that Tie2 is
less selective in identifying EPCs, and this most likely indicates
the presence of pre-existing activated endothelial cells. The two
cases in which AC133-positive tumor cells were identified were also
Tie2-positive, but the number of positive cells was significantly
higher.
Discussion
Tumor neovascularization represents a key point in
tumor progression, and has been extensively demonstrated to result
from the process of angiogenesis (6). The role ascribed to the cancer cells
during the process of tumor angiogenesis is the initiation of the
angiogenic switch, which is a critical step in tumor progression
(7).
Treatment for ovarian cancer is now shifting from
conventional chemotherapy to molecular-targeted therapies (8). An example of one such therapy is the
inhibition of the specific cytokines essential for tumor
vascularization (9).
Antiangiogenesis therapy has thus become a novel strategy for
ovarian cancer treatment.
Su et al (2010) demonstrated that the levels
of EPCs are significantly increased in the blood of patients with
ovarian cancer and are correlated with cancer stage and residual
tumor size (8). It was also shown
that treatment reduces the levels of circulating EPCs in patients.
Previous clinical correlations have shown that a positive
correlation occurs between an increase in EPC circulation in
pancreatic, breast and ovarian cancer patients, and tumor stage and
size (10,11). The co-expression of AC133 and Tie2
occurred in 27.4% of cases in the present study. Bagley et
al (2011) revealed that tumor endothelial marker 7 (TEM-7) is a
vascular protein associated with angiogenic status and that it may
be a novel and attractive target for antiangiogenic therapy
(12).
The tumor microenvironment plays a significant role
in the activation of circulating EPCs and the mediation of
neovascularization. Stressors, including hypoxia, glucose
deprivation and reactive oxygen species, are activated in the tumor
microenvironment and result in the upregulation of the
transcription of angiogenic factors, including vascular endothelial
growth factor (VEGF), stromal cell-derived factor 1 monocyte
chemotactic protein-1 and erythropoietin, in EPCs (13–15).
In the present study it was noticed that in the majority of tumor
stroma cases, the endothelial cells were the only cells positive
for AC133.
EPCs are regarded as bone marrow-derived cells that
are able to migrate into the peripheral blood in response to
cytokines, such as VEGF (16). As
opposed to in ischemic conditions, the role of circulating EPCs in
tumor growth and angiogenesis is not clear. EPCs have been
identified as a potential marker for the response to antiangiogenic
therapies and neovascularization, and they also possess a high
proliferation potential (17).
Initially, Tie2 was found to be overexpressed in
tumoral vessels, and it is also expressed in several types of
cancer, including leukemia, and solid neoplasms, including gliomas
and gastric and breast tumors. Tie2 expression in various tumoral
compartments highlights this cellular receptor as an attractive
target for cancer therapy (18).
In summary, the results of the present study
revealed that 27.4% of ovarian tumor cases express AC133 and Tie2
in blood vessel endothelial cells. The expression of these two
markers did not correlate with any clinicopathological prognostic
parameters, including histological type, vascular microdensity and
vessel type. Co-expression of the markers most likely reflects the
insertion of endothelial progenitor cells into the pre-existing
endothelium. This phenomenon contributes to angiogenesis
progression in cases where the budding process is reduced or
absent, as shown by the inverse correlation with the rate of
endothelial cell proliferation.
References
|
1
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peichev M, Naiyer AJ, Pereira D, et al:
Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells
identifies a population of functional endothelial precursors.
Blood. 95:952–958. 2000.
|
|
3
|
Shi Q, Rafii S, Wu MH, et al: Evidence for
circulating bone marrow-derived endothelial cells. Blood.
92:362–367. 1998.PubMed/NCBI
|
|
4
|
Handgretinger R, Gordon PR, Leimig T, et
al: Biology and plasticity of CD133+ hematopoietic stem cells. Ann
NY Acad Sci. 996:141–151. 2003.
|
|
5
|
Lin Y, Weisdorf DJ, Solovey A and Hebbel
RP: Origins of circulating endothelial cells and endothelial
outgrowth from blood. J Clin Invest. 105:71–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sato Y: Molecular diagnosis of tumor
angiogenesis and anti-angiogenic cancer therapy. Int J Clin Oncol.
8:200–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folkman J and Hanahan D: Switch to the
angiogenic phenotype during tumorigenesis. Princess Takamatsu Symp.
22:339–347. 1991.PubMed/NCBI
|
|
8
|
Su Y, Zheng L, Wang Q, et al: Quantity and
clinical relevance of circulating endothelial progenitor cells in
human ovarian cancer. J Exp Clin Cancer Res. 29:272010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hefler LA, Mustea A, Könsgen D, et al:
Vascular endothelial growth factor gene polymorphisms are
associated with prognosis in ovarian cancer. Clin Cancer Res.
13:898–901. 2007. View Article : Google Scholar
|
|
10
|
Naik RP, Jin D, Chuang E, et al:
Circulating endothelial progenitor cells correlate to stage in
patients with invasive breast cancer. Breast Cancer Res Treat.
107:133–138. 2008. View Article : Google Scholar
|
|
11
|
Li A, Cheng XJ, Moro A, Singh RK, Hines OJ
and Eibl G: CXCR2-dependent endothelial progenitor cell
mobilization in pancreatic cancer growth. Transl Oncol. 4:20–28.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bagley RG, Rouleau C, Weber W, et al:
Tumor endothelial marker 7 (TEM-7): a novel target for
antiangiogenic therapy. Microvasc Res. 82:253–262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong F and Ha XQ: Effect of endothelial
progenitor cells in neovascularization and their application in
tumor therapy. Chin Med J (Engl). 123:2454–2460. 2010.PubMed/NCBI
|
|
14
|
Janic B and Arbab AS: The role and
therapeutic potential of endothelial progenitor cells in tumor
neovascularization. Scientific World Journal. 10:1088–1099. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ribatti D: The involvement of endothelial
progenitor cells in tumor angiogenesis. J Cell Mol Med. 8:294–300.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li B, Sharpe EE, Maupin AB, et al: VEGF
and PlGF promote adult vasculogenesis by enhancing EPC recruitment
and vessel formation at the site of tumor neovascularization. FASEB
J. 20:1495–1497. 2006. View Article : Google Scholar
|
|
17
|
Kawamoto A and Asahara T: Role of
progenitor endothelial cells in cardiovascular disease and upcoming
therapies. Catheter Cardiovasc Inter. 70:477–484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martin V, Liu D, Fueyo J, et al: Tie2: a
journey from normal angiogenesis to cancer and beyond. Histol
Histopathol. 23:773–780. 2008.PubMed/NCBI
|