Introduction
The term carcinoid is synonymous with the term well
differentiated neuroendocrine tumor. Recently, carcinoids of the
colon and the rectum have been grouped together. However, rectal
carcinoids tend to behave less aggressively than colon carcinoids.
Rectal carcinoids are often only diagnosed incidentally during
routine lower endoscopy procedures due to the fact that such tumors
are small, submucosal in location and rarely metastatize (1–3). In
addition, rectal carcinoids are uncommon tumors. The
age-standardized incidence of such carcinoids was ~0.3–1.2 cases
per 100,000 individuals annually between 1992 and 1999, which has
increased in recent decades due to improvements in diagnostic
technology (such as endoscopy) and as a result of increased medical
awareness (4). Rectal carcinoids
tend to grow more slowly than same-site adenocarcinomas and harbor
a favorable five-year survival rate. Gastrointestinal carcinoids
are associated with a high incidence of second primary malignancy,
found to occur in ~13.1% of all rectal carcinoids (4). The identification of an incidental
gastrointestinal carcinoid during surgical treatment of another
malignancy will usually only require resection without additional
treatment, having little effect on prognosis (5). However, the treatment of unexpected
carcinoids identified in the surgical margin of rectectomy
specimens has not been previously described in any reported
literature. In the present study three such cases have been
presented and the current literature has been reviewed.
Materials and methods
In total, three pathology reports, which revealed
incidental carcinoids in the surgical margin of radical rectectomy
specimens were retrieved from the pathology database of The First
Affiliated Hospital (Hangzhou, China), from January, 2007 to April,
2013. Clinical details were acquired from medical records
retrospectively, such details included patient gender, age, medical
history, symptoms at presentation, modality of diagnosis and
treatment. The pathological features were identified from the
pathology reports and the carcinoid tumor size was reported as the
largest diameter that was recorded microscopically. The follow-up
was conducted via telephone interview and through examination of
laboratory, endoscopy and imaging results that were obtained during
clinical visits. The examination results were documented in the
laboratory and imaging databases, which were also reviewed. This
study was approved by the institutional ethics committee of The
First Affiliated Hospital, College of Medicine, Zhejiang University
(Hangzhou, China). Informed consent was obtained from the patients
families.
Results
Patient and primary adenocarcinoma
features
All three patients were male and were aged 62, 66
and 54 years at presentation. They all experienced hematochezia,
leading to endoscopic evaluation and biopsy. This resulted in the
identification of adenocarcinoma and subsequently a radical
rectectomy (Dixon’s operation) was performed. Features of the
primary adenocarcinomas are displayed in Table I.
 | Table IFeatures of primary
adenocarcinoma. |
Table I
Features of primary
adenocarcinoma.
| Case | Histological
type | Differentiation | Tumor size (cm) | Depth of
invasion | TNM stage |
|---|
| 1 | Adenocarcinoma | Intermediate | 3.5×3.0 | Within submucosa | T1N0M0 |
| 2 | Adenocarcinoma | Intermediate | 4.0×3.5 | Within serosaa | T3N0M0 |
| 3 | Adenocarcinoma | Intermediate | 4.5×3.5 | Within
adventitiab | T3N0M0 |
Incidental carcinoid features
The distal margins of the resection specimens were
2–3 cm from the respective adenocarcinoma and were removed during
surgical procedures. Sections of each distal margin revealed an
unexpected microcarcinoid tumor within it. Their sizes were all
<0.5 mm in diameter (0.2, 0.2 and 0.3 mm, respectively). All
tumors invaded the submucosa only, without lymphovascular invasion,
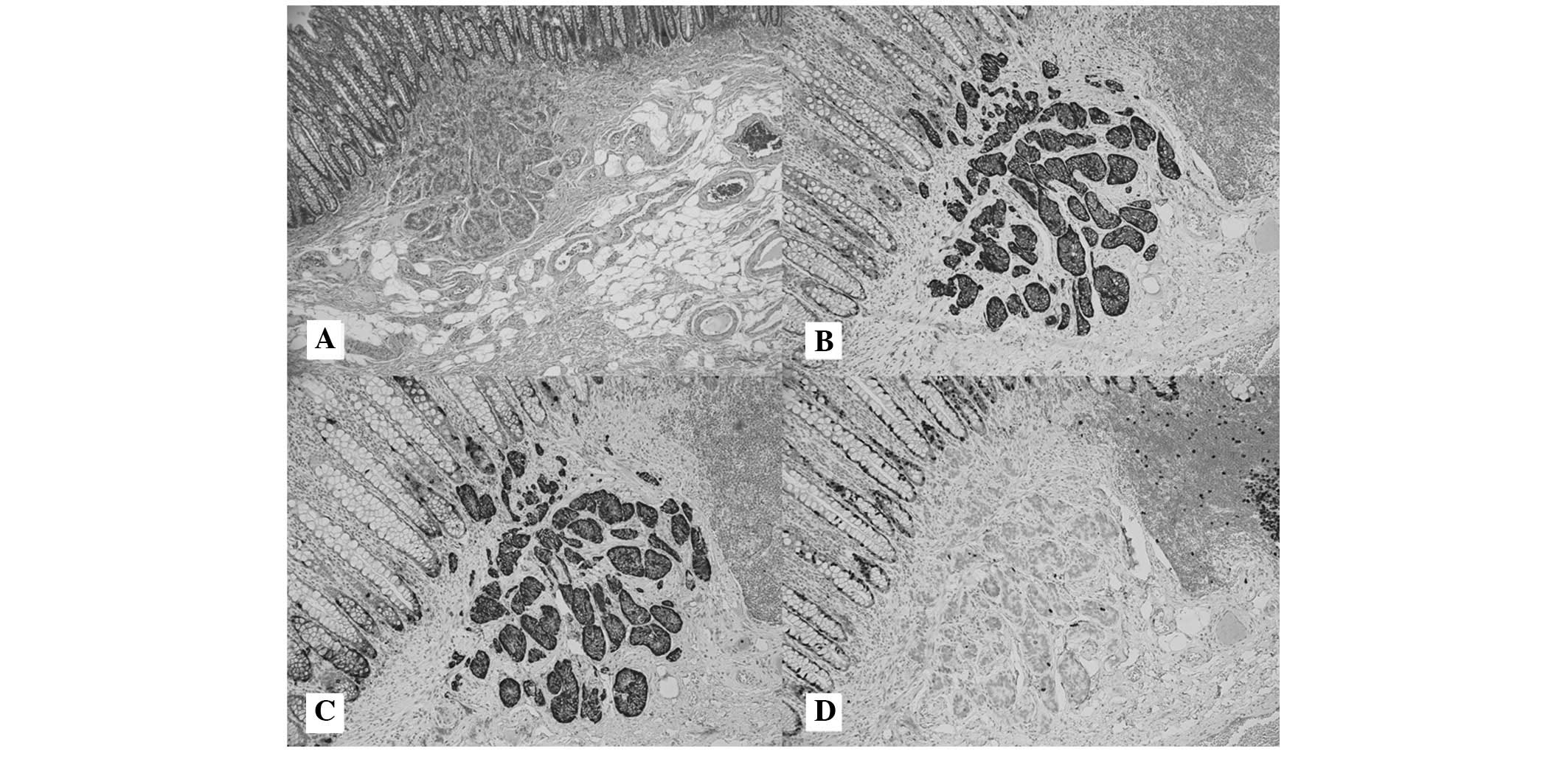
lymph node involvement or distant metastasis. Immunohistochemical
staining was performed on all carcinoid tumors. The tumor cells
were identified to be positive for synaptophysin, chromogranin A
and cluster of differentiation 56, but were negative for Ki-67
(Fig. 1).
Treatment and follow-up
Following diagnosis, patients were treated without
further surgical or endoscopic resection. This decision was made by
the patient and was based on the consideration that benign-like
carcinoids have little effect on patient prognosis compared with
adenocarcinoma. None of the patients underwent chemotherapy or
radiation following the radical rectectomy. Patients were followed
up for 38, 26 and 14 months, respectively. Case three suffered
hepatic metastasis of the adenocarcinoma in the 13th month
following the rectectomy and underwent partial resection of the
liver. However, none of the three patients demonstrated carcinoid
recurrence or metastasis.
Discussion
In 2008, Landry et al (6) proposed a novel staging system for
adenocarcinoma based on the assessment of 4,701 rectal carcinoid
tumors, considering variables, such as tumor size, depth of
invasion, lymph node involvement and distant metastasis. In the
study, the five-year survival rates for patients with stage I, II,
III or IV disease were 97, 84, 27 and 20%, respectively. Overall,
patients with rectal carcinoids exhibit a favorable five-year
survival rate (88.3%), as reflected by a large percentage of
localized tumors (82%) (4).
However, cancer-specific survival rates are comparable between
rectal carcinoid and adenocarcinoma if tumors exhibit lymph node or
distant metastasis (7).
Furthermore, the metastatic rate of early-stage rectal carcinoids
may be higher than those of rectal carcinomas if tumors are >10
mm in diameter (7,8).
Certain studies have identified various risk factors
hypothesized to be involved in rectal carcinoid metastasis, such as
a tumor size >10 mm, muscularis propria infiltration, the
presence of lymphovascular invasion, the mitotic rate increased and
the Ki-67 index increased (7,
9–12). Mani et al (3) evaluated >200 studies of rectal
carcinoids and noted that tumor size and muscularis propria
invasion were the two most important predictors of neoplastic
malignancy. The majority of rectal carcinoids are <10 mm at the
time of diagnosis with a median size of 6 mm (6). These lesions metastasized in <2% of
patients (3). However, when a tumor
reaches a size >10 mm, the metastatic rate increases notably.
For example, in tumors measuring between 10 and 20 mm, the
metastatic rate increases to 10–15%, whereas tumors measuring
>20 mm have a metastatic rate of 60–80% (3). Another study showed that metastases
were present in only 2% of tumors, which were <20 mm and did not
exhibit muscularis propria invasion, compared with 48% in tumors
that had invaded the muscularis layer (13). In addition, a large study in Asia
revealed that a tumor size of >10 mm and the presence of
lymphatic invasion were independently predictive of lymph node
metastasis, whereas a tumor size >20 mm and the presence of
venous invasion were independently predictive of distant metastasis
(7).
Synchronous or metachronous secondary primary
malignancies (SPM) are a common observation in carcinoid tumor
patients (4,5,14–17).
In addition, delayed metachronous SPM is common and incidence is
eight times higher in carcinoid tumor patients compared with in the
normal population (16). Of the
gastrointestinal tract carcinoids, it was noted that a high
percentage of associated tumors occurred with small intestinal
carcinoids (29.0%), whereas a lower percentage was identified with
rectal carcinoids (13.1%) (4). The
majority of associated secondary malignancies were found to be
located in the gastrointestinal tract (4,5,14–17),
which occurred in 32–62% of tumors, followed by the genitourinary
tract and the lung/bronchial system.
The etiology of this associated high risk of SPM
remains unclear. It may be hypothesized that neuroendocrine factors
secreted by carcinoids enhance the development or growth of other
neoplastic tissue (18). In support
of this hypothesis, higher levels of neuroendocrine factors are
observed in small intestinal carcinoids (midgut) when compared with
colorectal carcinoids (hindgut), in which SPMs are less common.
However, it is difficult to explain the high incidence of delayed
SPM following excision of carcinoid tumors. It is also speculated
that carcinoid patients may exhibit an increased susceptibility to
all forms of malignancy (16).
It is generally accepted that rectal carcinoids
>20 mm in size require radical resection (19–22)
due to the high rate of metastases (in ~3/4 cases) as described in
the literature (13,14). However, controversy remains with
regards to the management of tumors measuring <20 mm. For small
rectal tumors (<10 mm), local removal is considered to be
sufficient (20,22). However, certain tumors, including
those measuring <5 mm, metastasize regionally and distally
(7,8,23,24).
In general, small rectal carcinoids are known as tumors with little
metastatic risk, rendering local treatment desirable and reserving
radical surgery for patients that display risk factors that are
associated with metastasis. However, the aforementioned risk
factors are difficult to identify during preoperative evaluation.
In addition, the clinical role of preoperative imaging remains
uncertain. Currently, treatment of small rectal carcinoids remains
controversial as tumors measuring between 10 and 20 mm demonstrate
unpredictable behavior and metastatic risk (20). However, it is reasonable to perform
radical rectal resection for tumors, which are 10–20 mm in size and
exhibit high-risk features, such as muscular and lymphovascular
invasion (7,24).
The necessity for achieving a microscopically
negative margin has been questioned in the past (25). Currently it is recommended that a
negative margin is to be achieved if possible (24–26).
Reasons for this are as follows: i) Malignancy criteria for rectal
carcinoid are not well established and numerous benign-like tumors
may evolve over a prolonged period of time resulting in late
recurrences (24,27); ii) once a tumor has spread,
chemotherapy and radiotherapy are not effective (19); whereas complete resection of the
local disease offers the only chance of a cure; and iii) residual
tumors may continue to release factors that enhance the development
or growth of other neoplastic tissue.
The identification of an incidental gastrointestinal
carcinoid during the operative treatment of another malignancy does
not worsen the prognosis of the individual; complete resection is
sufficient for therapy (5).
However, occasionally it is difficult to identify minute rectal
carcinoids during operative treatment of another malignancy,
rendering a positive resection margin possible for the
microcarcinoid. In the present study, no recurrence of carcinoids
were observed in any of the three cases; however, during the
follow-up period one patient suffered hepatic metastasis of
adenocarcinoma in the 13th month following rectectomy. This
indicated that it is reasonable to conduct a follow-up without
further excision as such incidental microcarcinoids are usually
absent of risk factors and are unlikely to recur prior to another
malignancy.
Examination following resection of small rectal
carcinoids is controversial. The latest consensus with regards to
treatment guidelines for small rectal carcinoids is that tumors
without lymph node involvement require no long-term follow up
(21,22); however, exceptions do exist. Kwaan
et al (24) reported that
two patients with small rectal carcinoid tumors presented distant
metastasis in the 5th and 13th year after resection. Conversely,
small rectal carcinoids are usually treated by local excision at
first, which renders it difficult to determine whether there is any
lymph node involvement. In addition, synchronous or delayed SPM is
a common finding in patients with carcinoid tumors. Thus, certain
individuals may argue that long-term follow-up is recommended for
delayed metastasis and secondary malignancies with a central point
of focus on the gastrointestinal tract (17).
In conclusion, patients with rectal carcinoids, in
particular those that are low-risk, have a favorable five-year
survival rate. Such carcinoids behave less aggressively than
same-site adenocarcinomas. Furthermore, the identification of an
incidental rectal carcinoid during operative treatment of another
malignancy is possible. However, it is possible to forgo further
excision as incidental carcinoids are usually absent of risk
factors and are unlikely to recur prior to another malignancy.
References
|
1
|
Shields CJ, Tiret E and Winter DC;
International Rectal Carcinoid Study Group. Carcinoid tumors of the
rectum: a multi-institutional international collaboration. Ann
Surg. 252:750–755. 2010. View Article : Google Scholar
|
|
2
|
Yoon SN, Yu CS, Shin US, et al:
Clinicopathological characteristics of rectal carcinoids. Int J
Colorectal Dis. 25:1087–1092. 2010. View Article : Google Scholar
|
|
3
|
Mani S, Modlin IM, Ballantyne G, Ahlman H
and West B: Carcinoids of the rectum. J Am Coll Surg. 179:231–248.
1994.
|
|
4
|
Modlin IM, Lye KD and Kidd M: A 5-decade
analysis of 13,715 carcinoid tumors. Cancer. 97:934–959. 2003.
View Article : Google Scholar
|
|
5
|
Gerstle JT, Kauffman GL Jr and Koltun WA:
The incidence, management, and outcome of patients with
gastrointestinal carcinoids and second primary malignancies. J Am
Coll Surg. 180:427–432. 1995.
|
|
6
|
Landry CS, Brock G, Scoggins CR, et al: A
proposed staging system for rectal carcinoid tumors based on an
analysis of 4701 patients. Surgery. 144:460–466. 2008. View Article : Google Scholar
|
|
7
|
Konishi T, Watanabe T, Kishimoto J, Kotake
K, Muto T and Nagawa H; Japanese Society for Cancer of the Colon
and Rectum. Prognosis and risk factors of metastasis in colorectal
carcinoids: results of a nationwide registry over 15 years. Gut.
56:863–868. 2007.
|
|
8
|
Soga J: Early-stage carcinoids of the
gastrointestinal tract: an analysis of 1914 reported cases. Cancer.
103:1587–1595. 2005. View Article : Google Scholar
|
|
9
|
Fujimoto Y, Oya M, Kuroyanagi H, Ueno M,
Akiyoshi T, Yamaguchi T and Muto T: Lymph-node metastases in rectal
carcinoids. Langenbecks Arch Surg. 395:139–142. 2010. View Article : Google Scholar
|
|
10
|
Fahy BN, Tang LH, Klimstra D, Wong WD,
Guillem JG, Paty PB, Temple LK, Shia J and Weiser MR: Carcinoid of
the rectum risk stratification (CaRRs): a strategy for preoperative
outcome assessment. Ann Surg Oncol. 14:1735–1743. 2007. View Article : Google Scholar
|
|
11
|
Hassan MM, Phan A, Li D, Dagohoy CG, Leary
C and Yao JC: Risk factors associated with neuroendocrine tumors: A
U.S.-based case-control study. Int J Cancer. 123:867–873. 2008.
View Article : Google Scholar
|
|
12
|
Hemminki K and Li X: Incidence trends and
risk factors of carcinoid tumors: a nationwide epidemiologic study
from Sweden. Cancer. 92:2204–2210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naunheim KS, Zeitels J, Kaplan EL, et al:
Rectal carcinoid tumors - treatment and prognosis. Surgery.
94:670–676. 1983.PubMed/NCBI
|
|
14
|
Soga J: Carcinoids of the rectum: an
evaluation of 1271 reported cases. Surg Today. 27:112–119. 1997.
View Article : Google Scholar
|
|
15
|
Tichansky DS, Cagir B, Borrazzo E, et al:
Risk of second cancers in patients with colorectal carcinoids. Dis
Colon Rectum. 45:91–97. 2002. View Article : Google Scholar
|
|
16
|
Schneider C, Wittekind C and Köckerling F:
An unusual incidence of carcinoid tumors and secondary
malignancies. Chirurg. 66:607–611. 1995.(In German).
|
|
17
|
Li AF, Hsu CY, Li A, Tai LC, Liang WY, Li
WY, Tsay SH and Chen JY: A 35-year retrospective study of carcinoid
tumors in Taiwan: differences in distribution with a high
probability of associated second primary malignancies. Cancer.
112:274–283. 2008.
|
|
18
|
Oberg K: Expression of growth factors and
their receptors in neuroendocrine gut and pancreatic tumors, and
prognostic factors for survival. Ann NY Acad Sci. 733:46–55. 1994.
View Article : Google Scholar
|
|
19
|
Modlin IM, Kidd M, Latich I, et al:
Current status of gastrointestinal carcinoids. Gastroenterology.
128:1717–1751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramage JK, Ahmed A, Ardill J, et al; UK
and Ireland Neuroendocrine Tumour Society. Guidelines for the
management of gastroenteropancreatic neuroendocrine (including
carcinoid) tumours (NETs). Gut. 61:6–32. 2012. View Article : Google Scholar
|
|
21
|
Anthony LB, Strosberg JR, Klimstra DS, et
al; North American Neuroendocrine Tumor Society (NANETS). The
NANETS consensus guidelines for the diagnosis and management of
gastrointestinal neuroendocrine tumors (nets): well-differentiated
NETS of the distal colon and rectum. Pancreas. 39:767–774. 2010.
View Article : Google Scholar
|
|
22
|
Ramage JK, Goretzki PE, Manfredi R, et al;
Frascati Consensus Conference participants. Consensus guidelines
for the management of patients with digestive neuroendocrine
tumours: well-differentiated colon and rectum tumour/carcinoma.
Neuroendocrinology. 87:31–39. 2008. View Article : Google Scholar
|
|
23
|
Seow-Cheoen F and Ho J: Tiny carcinoids
may be malignant. Dis Colon Rectum. 36:309–310. 1993. View Article : Google Scholar
|
|
24
|
Kwaan MR, Goldberg JE and Bleday R: Rectal
carcinoid tumors: review of results after endoscopic and surgical
therapy. Arch Surg. 143:471–475. 2008. View Article : Google Scholar
|
|
25
|
Moore JR, Greenwell B, Nuckolls K, et al:
Neuroendocrine tumors of the rectum: a 10-year review of
management. Am Surg. 77:198–200. 2011.PubMed/NCBI
|
|
26
|
Koura AN, Giacco GG, Curley SA, et al:
Carcinoid tumors of the rectum: effect of size, histopathology, and
surgical treatment on metastasis free survival. Cancer.
79:1294–1298. 1997. View Article : Google Scholar
|
|
27
|
Sippel RS and Chen H: Carcinoid tumors.
Surg Oncol Clin N Am. 15:463–478. 2006. View Article : Google Scholar
|