Introduction
In 1962, Cohen identified epidermal growth factor
(EGF) while studying nerve growth factor in submaxillary glands of
male Swiss-Webster mice (1). EGF is
known to be produced in human salivary glands. It modulates the
proliferation and differentiation of various cancer cells, as well
as normal epithelial cells. EGF has been shown to enhance bladder
cancer cell growth (2–4). Furthermore, it is known that EGF
stimulates matrix metalloproteinases (MMPs), which have been
previously reported to be associated with metastases in various
tumors (5–7). Advances in molecular biology have
revealed that MMPs are particularly important in cancer progression
(8), and the expression levels of
MMPs correlate with cancer invasion and metastasis (9). We previously reported that EGF
increases the promoter activities of the MMP9 gene in HSC-3 cells
(10). These results suggested that
EGF increases the invasive activity of oral cancer cells partly by
increasing MMP9 (10).
Cytokeratin (CK) 19 is a particularly intriguing
member of the CK intermediate filament superfamily (11). Unlike other type I acidic CKs, CK19
does not generally form heterodimers with any of the type II basic
proteins (12). CK19 is expressed
in the non-keratinizing stratified squamous epithelium exemplified
by the oral cavity (13) and tends
to reflect the state of differentiation of the tumor (14). For example, the expression of CK19
is more intense in poorly differentiated lesions than in other
lesions. In a previous study, keratin 19 downregulation in oral
squamous cell carcinoma cell lines increased their invasive
potential (15).
The present study analyzed the effects of EGF on the
invasive activity of a cultured oral cancer cell line and assessed
the transcription of MMP1. In addition, the expression of CK19 with
and without EGF was examined.
Materials and methods
Cell culture
The human oral squamous cell carcinoma (SCC) cell
lines, HSC-3, SAS and Ca9-22, were obtained from the RIKEN
BioResource Center (Ibaraki, Japan). HSC-3, SAS and Ca9-22 cells
were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Nissui,
Tokyo, Japan) containing 10% fetal bovine serum (FBS) and 5 μg/ml
ampicillin at 37°C in humidified 5% CO2 in air. Human
recombinant EGF (Upstate Biotechnology, Lake Placid, New York, USA)
was added to the medium at concentrations of 0, 10 and 100 ng/ml,
and the medium was changed every day. Following EGF addition, cell
lines were cultured for six days.
Observation of cell morphology
HSC-3 or SAS cells (15×104) were seeded
in 1 ml DMEM containing 1% FBS and EGF (0, 10 or 100 ng/ml) in a
35-mm dish for six days, during which the medium was changed every
day.
Matrigel invasion assay
The assays were performed according to the
manufacturer’s instructions. In conclusion, HSC-3 and SAS cells
(2×104/well) were seeded in a six-well Bio Coat
Matrigel® Invasion Chamber (Beckton-Dickinson, Bedford,
MA, USA) in Eagle’s minimal essential medium containing 10% (v/v)
heat-inactivated fetal calf serum, with 0, 10 and 100 ng/ml EGF.
Following 48 h of incubation, the non-invading cells were removed
from the upper surface of the membrane by scrubbing and the
membrane was stained using a Diff-Quick stain kit (Sysmex, Kobe,
Japan). Subsequently, invading cells on the lower surface were
counted using a microscope (BX50, Olympus Corporation, Tokyo,
Japan). Data are presented as the average of experiments performed
in triplicate.
RNA isolation and northern blot
analysis
HSC-3 and SAS cells (30×104) were seeded
on a 100-mm gelatin-coated dish. Following 48 h of incubation, EGF
was added to the medium at a concentration of 10 ng/ml and the
medium was changed every other day. Total cellular RNA was prepared
following six days of culture using the Isogen® RNA
isolation kit (Nippon Gene, Tokyo, Japan). For northern blot
analysis, 15 μg of total RNA was electrophoresed on
formaldehyde-agarose gel in 3-(N-morpholino)propanesulfonic acid
buffer and transferred to nitrocellulose filters (Nitroplus-2000,
Micron Separations Inc., Westborough, MA, USA) in 20X SSC (1.5 M
NaCl and 0.15 M sodium citrate). The filters were prehybridized in
50% formamide, 4X SSC, 5X Denhardt’s solution [0.1% Ficoll (GE
Healthcare Japan Corporation, Tokyo, Japan), 0.1%
polyvinylpyrrolidone (Sigma-Aldrich Japan K.K., Tokyo, Japan) and
0.1% bovine serum albumin (Takara Bio Inc., Shiga, Japan)], 0.2%
sodium dodecyl sulfate (SDS; Wako Pure Chemical Industries, Ltd.,
Osaka, Japan) and denatured sonicated salmon sperm DNA (120 μg/ml;
BioDynamics Laboratory Inc., Tokyo, Japan), at 42°C for 2 h and
hybridized with radiolabeled DNA probes (CK19, EGFR and GAPDH)
under the same conditions for 24 h. Following hybridization, the
filters were washed at 55°C with 0.1X SSC containing 0.1% SDS.
DNA probes
A human CK19 cDNA clone in pUC18 (EcoRI
cloning site; 1.5-kb insert) and a human EGF receptor (EGFR)
cDNA clone in pBR322 (ClaI cloning site; 2.4-kb insert; pE7)
were used as probes and a human glyceraldehyde 3-phosphate
dehydrogenase cDNA clone in pUC18 (EcoRI cloning site;
1.2-kb insert) was used as a control probe.
Western blot analysis
Lysates obtained from 2.0 μg/ml CRN197-treated HSC-3
cells incubated for 24 h were resuspended in 0.2 ml
radioimmunoprecipitation assay buffer (Wako Pure Chemical
Industries, Ltd.) (0.1% Nonidet-P40, 1 mM CaCl2, 1 mM
MgCl2, 0.1% sodium azide, 1 mM phenylmethylsulfonyl
fluoride, 0.03 mg/ml aprotinin and 1 mM NaVO4). Proteins
were separated on 7 and 14% SDS polyacrylamide gels (Nacalai
Tesque, Inc., Kyoto, Japan), transferred overnight at 20 V and
incubated for 2 h at room temperature with mouse monoclonal
antibody, followed by incubation with secondary horseradish
peroxidase-conjugated antibodies (Anti-Mouse IgG, whole Ab from
Sheep; GE Healthcare UK Ltd., Amersham, UK) at a dilution of
1:1,000. Primary mouse monoclonal antibodies included anti-MMP1
(Calciobiochem, Darmstadt, Germany). Immunoreactivity was detected
using enhanced chemiluminescence. Densitometric analysis was
performed with ChemiDoc using Quantity One software (Bio-Rad,
Tokyo, Japan).
Chloramphenicol acetyl transferase (CAT)
assay
In summary, MMP1-CAT was constructed by ligating a
563-bp fragment (−518 to +45 of MMP1 genomic DNA) to the bacterial
CAT assay performed in HSC-3 cells using a transient transfection
system (Lipofectamine™; Invitrogen Life Technologies, Carlsbad, CA,
USA). Cells (1.5×106) were seeded in a 100-mm dish and
10 μg of DNA was co-transfected with 5 μg of pCH110 plasmid as an
internal control using the calcium phosphate co-precipitation
method. The culture medium was changed 16 h following transfection
to 10% DMEM with or without 10 ng/ml EGF. A cell lysate was
prepared 48 h following transfection and the reaction was
performed. Data are presented as the average of experiments
performed in triplicate.
Statistical analysis
The Mann-Whitney U test was used to assess the
statistical significance of differences between samples. P<0.05
was considered to indicate a statistically significant
difference.
Results
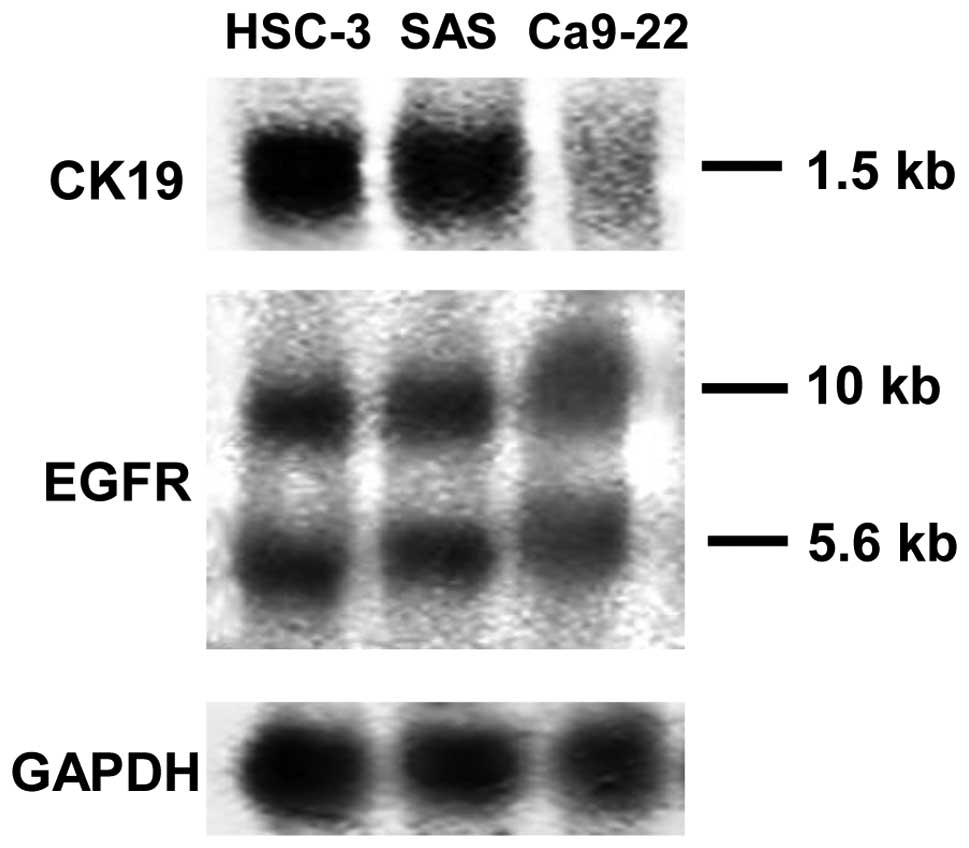
Expression of CK19 and EGFR mRNA in HSC-3
and SAS
EGF stimulates the proliferation of target cells
through interaction with its surface receptor. Previous studies
have shown that EGFR mRNA is expressed in a large number of oral
SCC cell lines. In total, 10- and 5.6-kb EGFR mRNA was detected in
the present study from HSC-3, SAS and Ca9-22 cells. This result
confirmed the observations of previous studies for HSC-3 cells, but
this is the first report of EGFR mRNA detection in SAS cell lines.
Similar levels of expression of EGFR mRNA were detected in HSC-3
and SAS cells (Fig. 1).
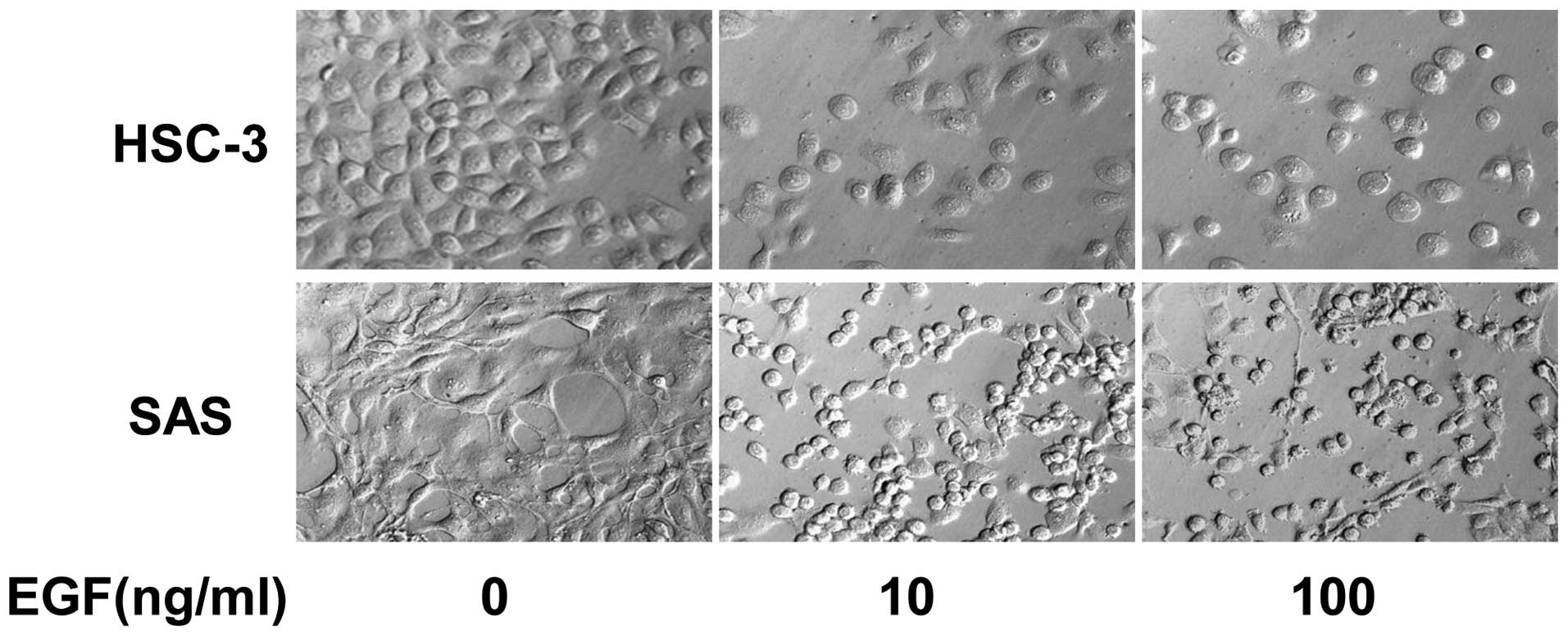
Morphological change in HSC-3 and SAS in
medium containing EGF
EGF produces various changes in cells. Therefore,
morphological change in HSC-3 and SAS was observed under EGF
stimulation. HSC-3 and SAS exhibited typical proliferation in the
absence of EGF. By contrast, HSC-3 and SAS exhibited morphological
change in the presence of EGF. It was shown that their form changed
to circular; in addition, cells showed detachment, with the loss of
cell adhesion. Fig. 2 shows the
morphology of HSC-3 and SAS on day six. As bladder cell carcinoma
cell lines piled up into discrete colonies due to EGF, differences
were identified among the oral SCC cell lines. This suggested the
possibility of variable cell morphological changes among the cell
lines under EGF stimulation. These observations suggested that EGF
affects the degree of cell-cell adhesion of tumor cells.
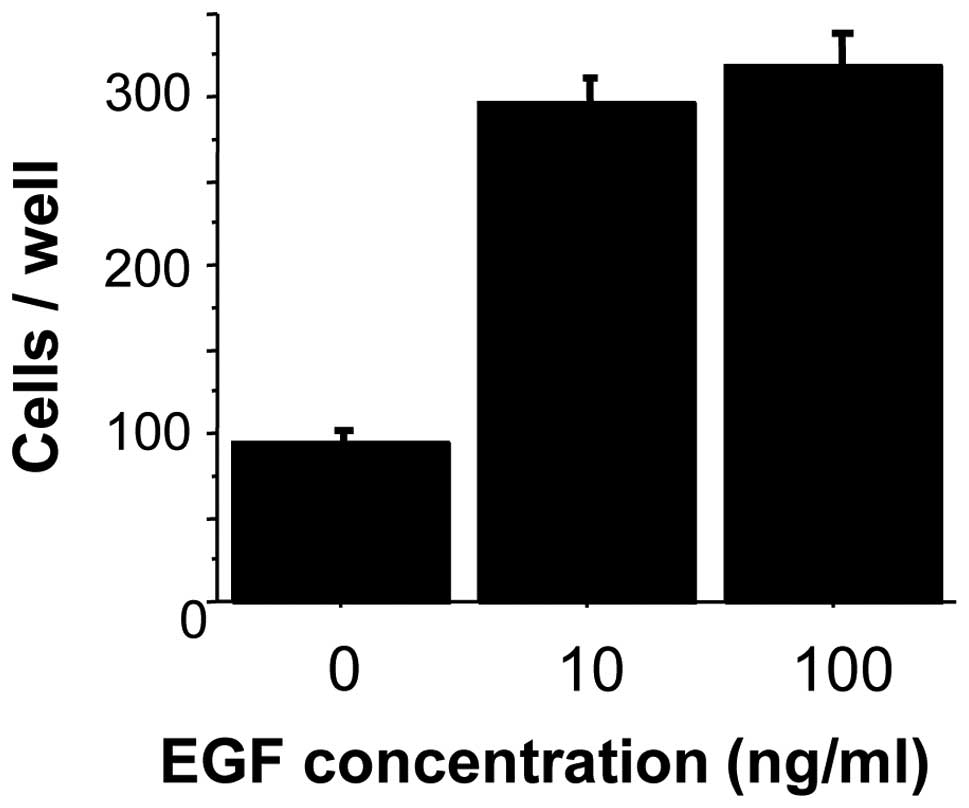
Invasiveness and tumorigenicity
To determine whether EGF increases the invasive
activity of oral cancer, the invasiveness of EGF-stimulated HSC-3
cells was investigated using Matrigel invasion chambers that
included extracellular matrix components. The number of HSC-3 cells
penetrating the Matrigel membrane was approximately three-fold
higher in EGF-stimulated (10 mg/ml) cells than in unstimulated
cells. No significant difference was identified in the penetration
of HSC-3 cells with the various concentrations of EGF used (10 and
100 mg/ml) (Fig. 3).
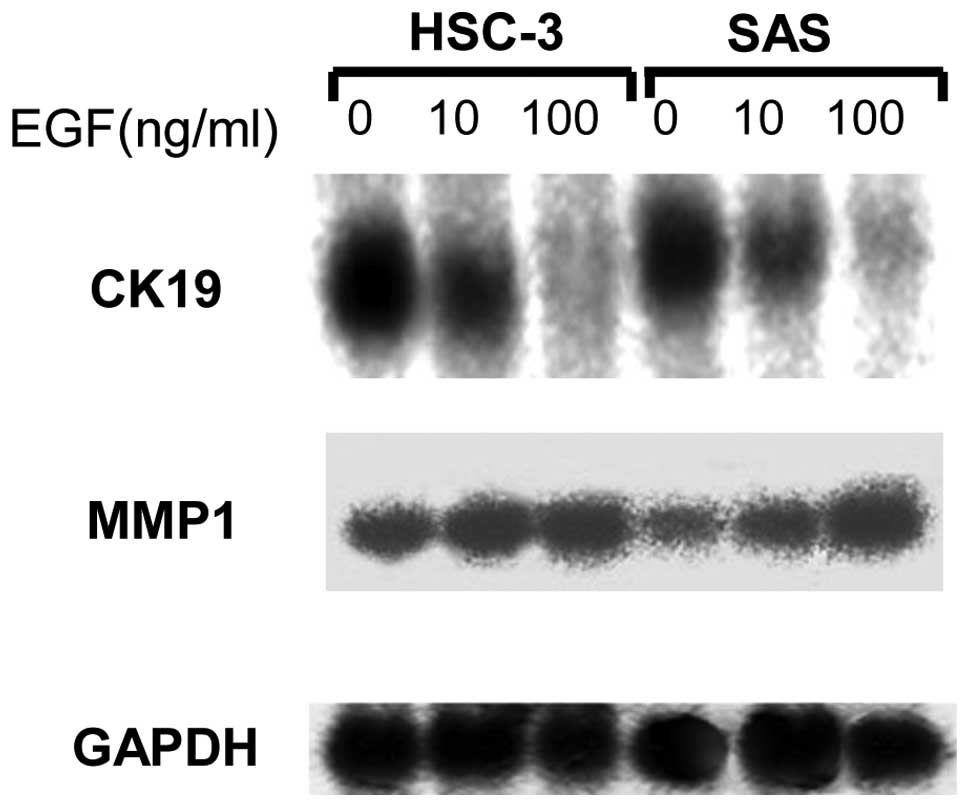
Northern blot analysis
Northern blot analysis was used to analyze the
influence of EGF on the expression of MMP1. EGF increased the
expression of MMP1. Furthermore, the expression of CK19 was
analyzed to investigate the correlation between cell conformation
and CK expression. The expression of CK19 mRNA was evidently
reduced by EGF, even at the lowest concentration of 10 ng/ml
(Fig. 4).
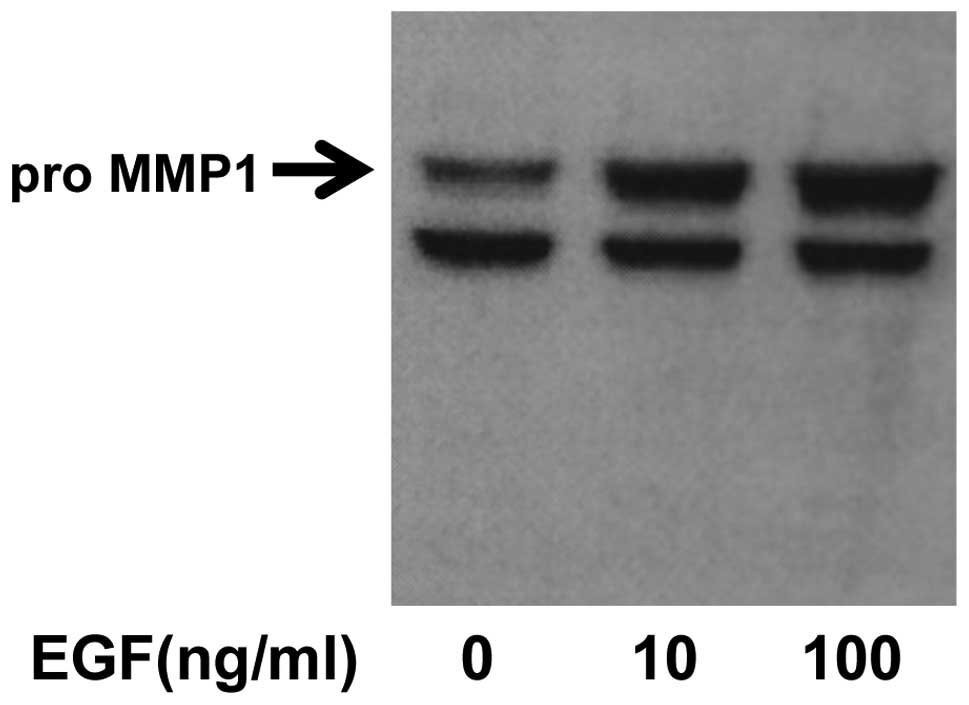
MMP1 expression in western blot
analysis
To confirm an increase in MMP1 protein levels,
western blot analysis was performed using an anti-MMP1 monoclonal
antibody. EGF increased the expression of MMP1 in an EGF
concentration-dependent manner (Fig.
5).
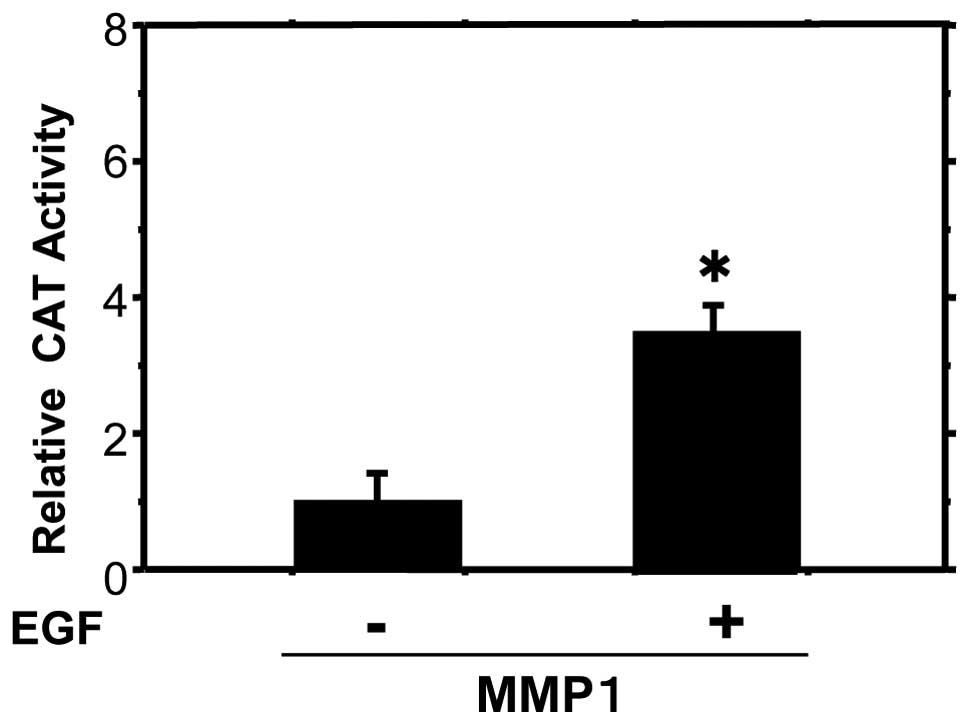
EGF stimulates MMP1 expression
As EGF increased the invasiveness of the HSC-3 oral
cancer cell line, it was assessed whether it is also likely to
modulate the expression of MMP1. CAT reporter genes driven by MMP1
promoters were transiently transfected into HSC-3 cells. MMP1-CAT
activities were increased 3.7-fold following EGF stimulation
compared with the controls (Fig.
6).
Discussion
MMPs are considered to be important in cancer
invasion and metastasis (8,16,17).
MMPs degrade various types of collagen, namely, stromal type 1
collagen and type 4 collagen of the basement membrane, and
proteoglycans. The expression of matrix-degrading proteinases is
elevated in a variety of invasive carcinomas (18,19).
However, the activating mechanisms of these invasion-associated
genes remain poorly understood. Certain previous studies have shown
that EGF stimulates the expression of MMP1 and MMP9 in several
cancers (16–18). In the present study, EGF stimulated
HSC-3 and SAS cells to invade Matrigel membranes. Additionally, EGF
stimulated HSC-3 and SAS cells to produce MMP1 mRNA. Notably, it
has been previously reported that SCC exhibits overexpression of
EGFRs (18,19). These previous studies suggest that
EGF and EGFRs are likely regulatory factors for the production of
MMP1. In addition, the present study found by CAT assay that EGF
stimulates the MMP1 promoter. These observations suggested that the
stimulation of MMP1 expression by EGF is likely to be one of the
mechanisms that increases the invasive activity of HSC-3 and SAS
cells.
CK8, CK18 and CK19 are CKs that are part of the cell
skeleton and arrange the cell conformation similar to actins. CKs
are also involved in maintaining desmosomal junctions through
connections with certain cadherins, including desmogleins and
desmocollins. Thus, CKs are essential for cell-cell adhesion
similar to E-cadherin. In addition, CK8 and CK18 have been
previously reported to be expressed at high levels in various
cancers (20). Certain previous
studies have reported the reduced expression of CK19 in poorly
differentiated skin and breast cancer (21). The observations of the current study
showed that EGF reduced the expression of CK19 mRNA in oral cancer
cell lines. Reduction of CK19 may be an additional pathway through
which EGF increases the invasive activity of oral cancer cell
lines.
The standard care for oral cancer is a combination
of surgery, radiation and chemotherapy. Commonly, carboplatin,
cisplatin and paclitaxel are used for chemotherapy. However, in
specific patients, these drugs have no effect due to the
development of drug resistance. In oral cancer, overexpression of
EGFR has been associated with chemoresistance and poor prognosis.
Targeting EGF, as an abundant EGFR ligand, may be favorable for the
treatment of chemoresistant oral cancer. It has also been noted
that EGF inactivates CK19. Therefore, EGF may be a better target
for the therapy of oral cancer than EGFR.
In conclusion, the current study identified two
pathways of EGF effects on oral carcinoma cell lines. Firstly, that
the expression of MMP1 results in degradation of the extracellular
matrix. Secondly, that changes in cytoskeletal proteins, such as
CK19, may allow changes in morphology that promote movement into
vessels and lymphatics. The results of the present study suggested
that EGF may promote the invasive activity of oral cancer cells by
activating these two pathways.
References
|
1
|
Cohen S: Isolation of a mouse submaxillary
gland protein accelerating incisor eruption and eyelid opening in
the new-born animal. J Biol Chem. 237:1555–1562. 1962.
|
|
2
|
Kawamata H, Azuma M, Kameyama S, Nan L and
Oyasu R: Effect of epidermal growth factor/transforming growth
factor alpha and transforming growth factor beta 1 on growth in
vitro of rat urinary bladder carcinoma cells. Cell Growth Differ.
3:819–825. 1992.
|
|
3
|
Kuranami M, Tamaguchi K, Fuchigami M, et
al: Effect of urine on clonal growth of human bladder cancer cell
lines. Cancer Res. 51:4631–4635. 1991.PubMed/NCBI
|
|
4
|
Ishikawa J, Maeda S, Sugiyama T, et al:
EGF stimulates anchorage-independent growth of a human bladder
carcinoma cell line (KU1) with an amplified and over-expressed EGF
receptor gene. Int J Cancer. 44:1000–1004. 1989. View Article : Google Scholar
|
|
5
|
Chen LL, Narayanan R, Hibbs MS, et al:
Altered epidermal growth factor signal transduction in activated
Ha-ras transformed human keratinocytes. Biochem Biophys Res Commun.
193:167–174. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shima I, Sasaguri Y, Kusukawa J, et al:
Production of matrix metalloproteinase 9 (92 kDa gelatinase) by
human oesophageal squamous cell carcinoma in response to epidermal
growth factor. Br J Cancer. 67:721–727. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueda M, Fujii H, Yoshizawa K, Abe F and
Ueki M: Effects of sex steroids and growth factors on migration and
invasion of endometrial adenocarcinoma SNG-M cells in vitro. Jpn J
Cancer Res. 87:524–533. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liotta LA, Stetler-Stevenson WG and Steeg
PS: Cancer invasion and metastasis: positive and negative
regulatory elements. Cancer Invest. 9:543–551. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grigioni WF, D’Errico A, Fortunato C, et
al: Prognosis of gastric carcinoma revealed by interactions between
tumor cells and basement membrane. Mod Pathol. 7:220–225.
1994.PubMed/NCBI
|
|
10
|
Ohnishi Y, Lieger O, Attygalla M, Iizuka T
and Kakudo K: Effects of epidermal growth factor on the invasion
activity of the oral cancer cell lines HSC3 and SAS. Oral Oncol.
44:1155–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu L, Crowe DL, Rheinwald JG, Chambon P
and Gudas LJ: Abnormal expression of retinoic acid receptors and
keratin 19 by human oral and epidermoid squamous cell carcinoma
lines. Cancer Res. 51:3972–3981. 1991.PubMed/NCBI
|
|
12
|
Hu L and Gudas LJ: Activation of keratin
19 gene expression by a 3′ enhancer containing an AP1 site. J Biol
Chem. 269:183–191. 1994.
|
|
13
|
Lindberg K and Rheinwald JG: Suprabasal
40kd keratin (K19) expression as an immunohistochemical marker of
premalignancy in oral epithelium. Am J Pathol. 134:89–98. 1989.
|
|
14
|
Shinohara M, Hiraki A, Ikebe T, et al:
Immunohistochemical study of desmosomes in oral squamous cell
carcinoma : correlation with cytokeratin and E-cadherin staining,
and with tumour behavior. J Pathol. 184:369–391. 1998. View Article : Google Scholar
|
|
15
|
Crowe DL, Milo GE and Shuler CF: Keratin
19 Downregulation by oral squamous cell carcinoma lines increases
invasive potential. J Dent Res. 78:1256–1263. 1999. View Article : Google Scholar
|
|
16
|
Nakajima M, Welch DR, Belloni PN and
Nicolson GL: Degradation of basement membrane type IV collagen and
lung subendothelial matrix by rat mammary adenocarcinoma cell
clones of differing metastatic potentials. Cancer Res.
47:4869–4876. 1987.
|
|
17
|
Juarez J, Clayman G, Nakajima M, et al:
Role and regulation of expression of 92-kDa type-IV collagenase
(MMP-9) in 2 invasive squamous-cell-carcinoma cell lines of the
oral cavity. Int J Cancer. 55:10–18. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamata N, Chida K, Rikimaru K, et al:
Growth-inhibitory effects of epidermal growth factor and
overexpression of its receptor on human squamous cell carcinoma in
culture. Cancer Res. 46:1648–1653. 1986.PubMed/NCBI
|
|
19
|
Ozawa S, Ueda M, Ando N, Abe O and Shimizu
N: High incidence of EGF receptor hyperproduction in esophageal
squamous cell carcinoma. Int J Cancer. 39:333–337. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hendrix MJ, Seftor EA, Chu YW, et al:
Coexpression of vimentin and keratins by human melanoma tumor
cells: correlation with invasive and metastatic potential. J Natl
Cancer Inst. 84:165–174. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogden GR, Chisholm DM, Adi M and Lane EB:
Cytokeratin expression in oral cancer and its relationship to tumor
differentiation. J Ora Pathol Med. 22:82–86. 1993. View Article : Google Scholar : PubMed/NCBI
|