Introduction
Gastrointestinal stromal tumor (GIST) is the most
common type of mesenchymal tumor in the gastrointestinal (GI)
tract, with a disease incidence of 10–20 per million individuals
worldwide (1–3). GIST can occur in any region of the
digestive tract and the incidence of GIST in the stomach, small
intestine, large intestine and esophagus is reported to be 60–70,
20–30, 18.1 and 1.4%, respectively. According to the tumor
location, GISTs are classified as endoluminal, exoluminal,
intramural and mixed types (4).
Immunohistochemical findings and the ultramicrostructure of GIST
cells are similar to those of Cajal cells, which are autonomous
nerve-related GI pacemaker cells that regulate intestinal motility
(5,6). On diagnosis, immunohistochemical
analysis revealed the presence of cluster of differentiation
(CD)117-positive and CD34-positive/negative tumor cells. The most
typical characteristic of malignancy is infiltration of neighboring
organs or lymph node metastasis. Infiltration of the lamina propria
mucosae or the muscular layer is an important indicator for the
diagnosis of malignant GIST. In addition, manifestation of the
malignancy includes a large tumor size (diameter of >5 cm for
gastric tumors and >4 cm for small intestine tumors), obvious
mitosis count [>5/50 high-power fields (HPFs)] (7), high density of cells, infiltration of
the lamina propria mucosae, presence of coagulative tumor necrosis
(8), high Ki-labeling index
(>5%) (9,10), recurrence and metastasis. Patient
provided written informed consent.
Case report
A 78-year-old man presenting with abdominal
distension and a poor appetite was diagnosed with a pancreatic mass
and referred to the Renji Hospital (Shanghai, China) for treatment
on November 10, 2008. Hematological testing showed that tumor
antigen and routine blood test results were normal. Abdominal
ultrasonography revealed a hypoechoic mass with an uneven irregular
surface and a clear boundary in the middle upper abdomen; the tumor
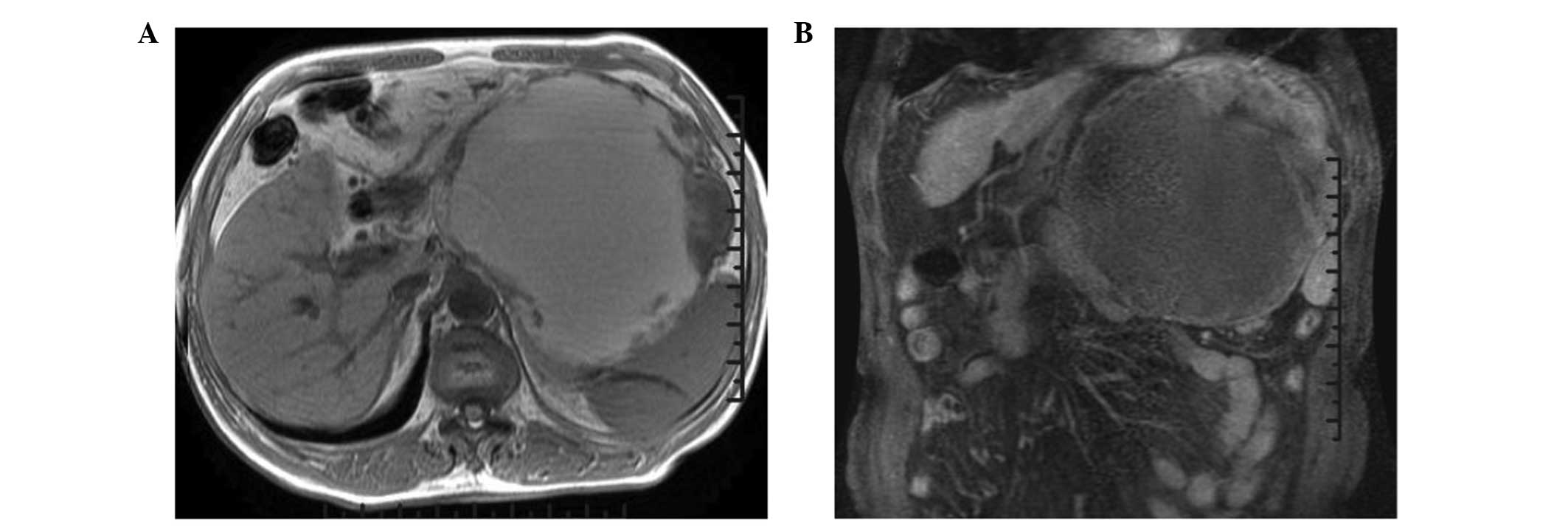
showed mixed echogenicity. Magnetic resonance imaging (MRI)
revealed a large cystic-solid mass that had grown into the lesser
omental bursa (Fig. 1A) and the
stomach had changed shape due to compression (Fig. 1B). The observed mass was likened to
pancreatic cystadenocarcinoma or canceration of cystadenoma.
Computed tomography (CT) revealed a tumor in the body of the
pancreas, which was closely attached to the gastric wall. Surgical
treatment was performed to excise the tumor on September 15, 2008.
The mass was ~17×15×16 cm in size with a thick wall that was
completely attached to gastric wall tissue. The tumor was
multicystic and partly solid, contained watery brown fluid,
compressed the pancreas and had a smooth outer surface. In
addition, an ulcer measuring ~0.5 cm in diameter was observed where
the mass adhered to the gastric wall. The mass, part of the
stomach, the spleen and the greater omentum were surgically
removed.
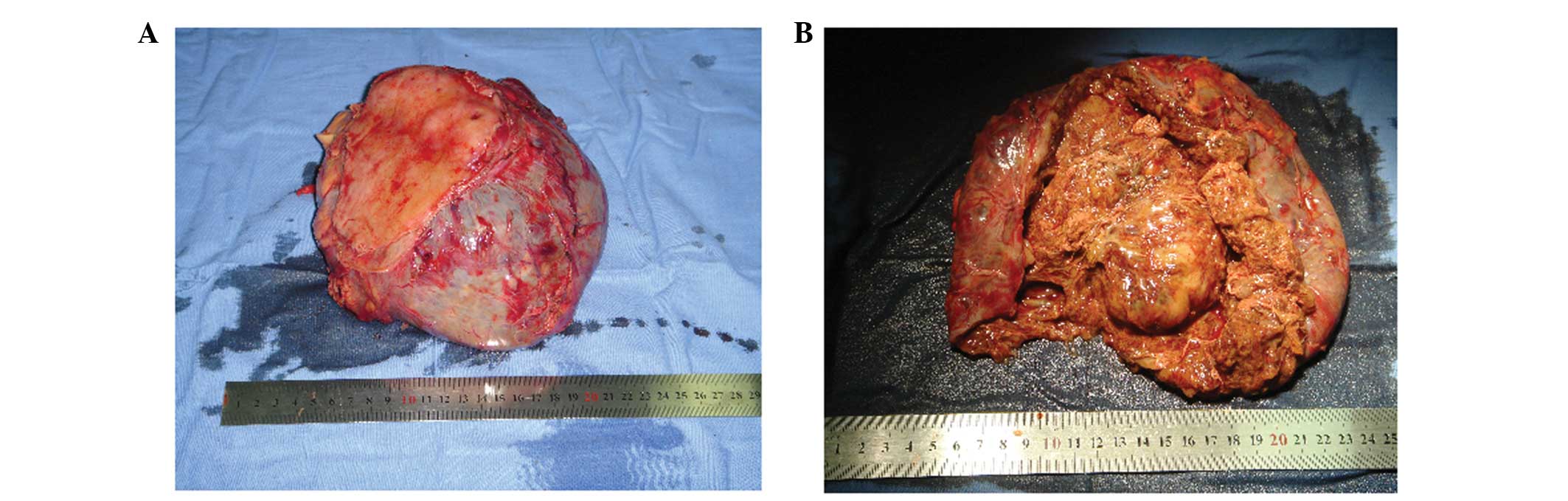
The resected tumor was a well-circumscribed mass,
measuring 15×17×13 cm in size. An ulcer was found on the resected
gastric wall where it was attached to the tumor (Fig. 2A). The solid portion was pink-gray
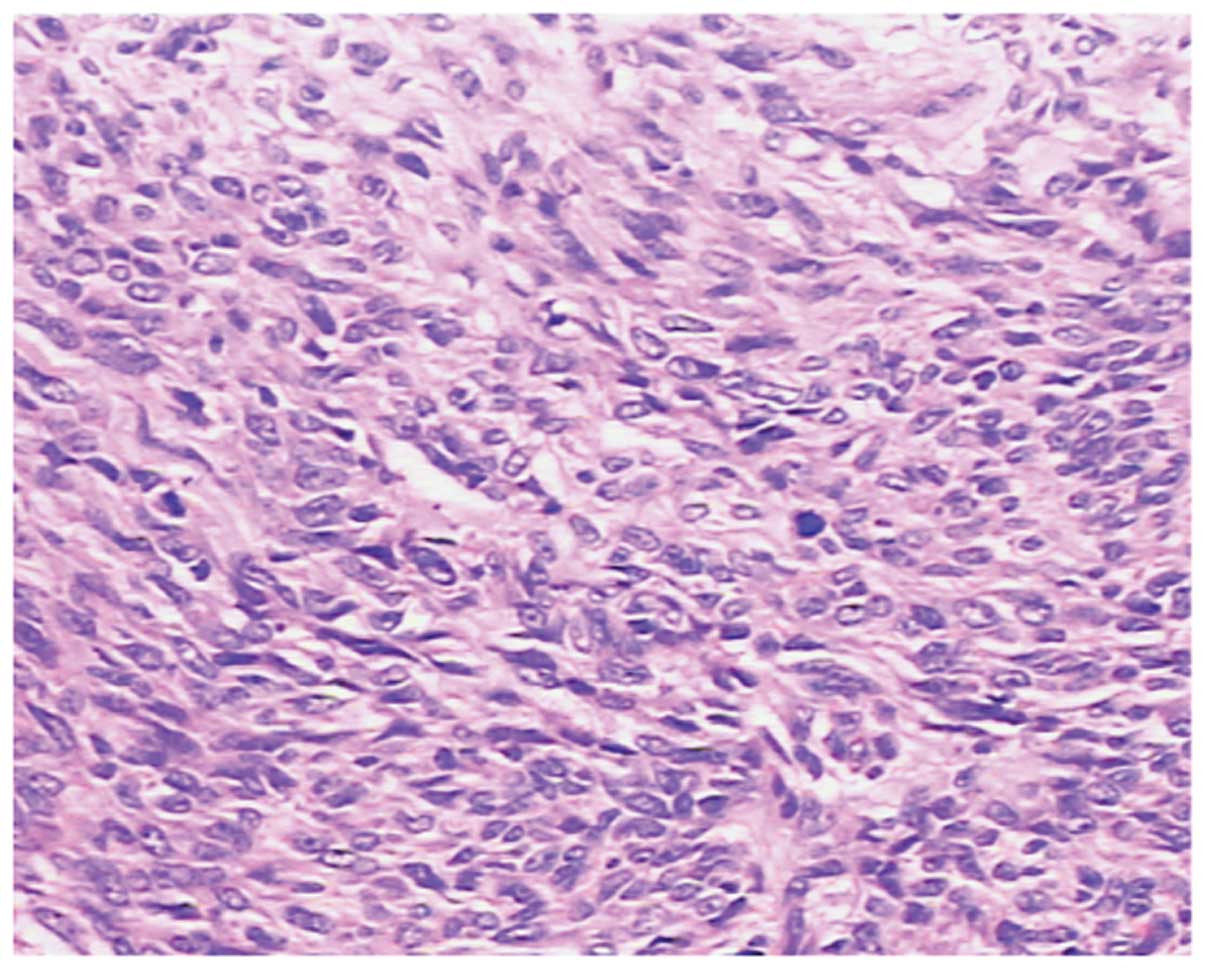
in color, soft and had a scaly appearance (Fig. 2B). Microscopically, the tumor cells
were epithelioid or spindle-shaped and arranged in an ill-defined
fascicular pattern. Pathologically, the mitosis count was >10/50
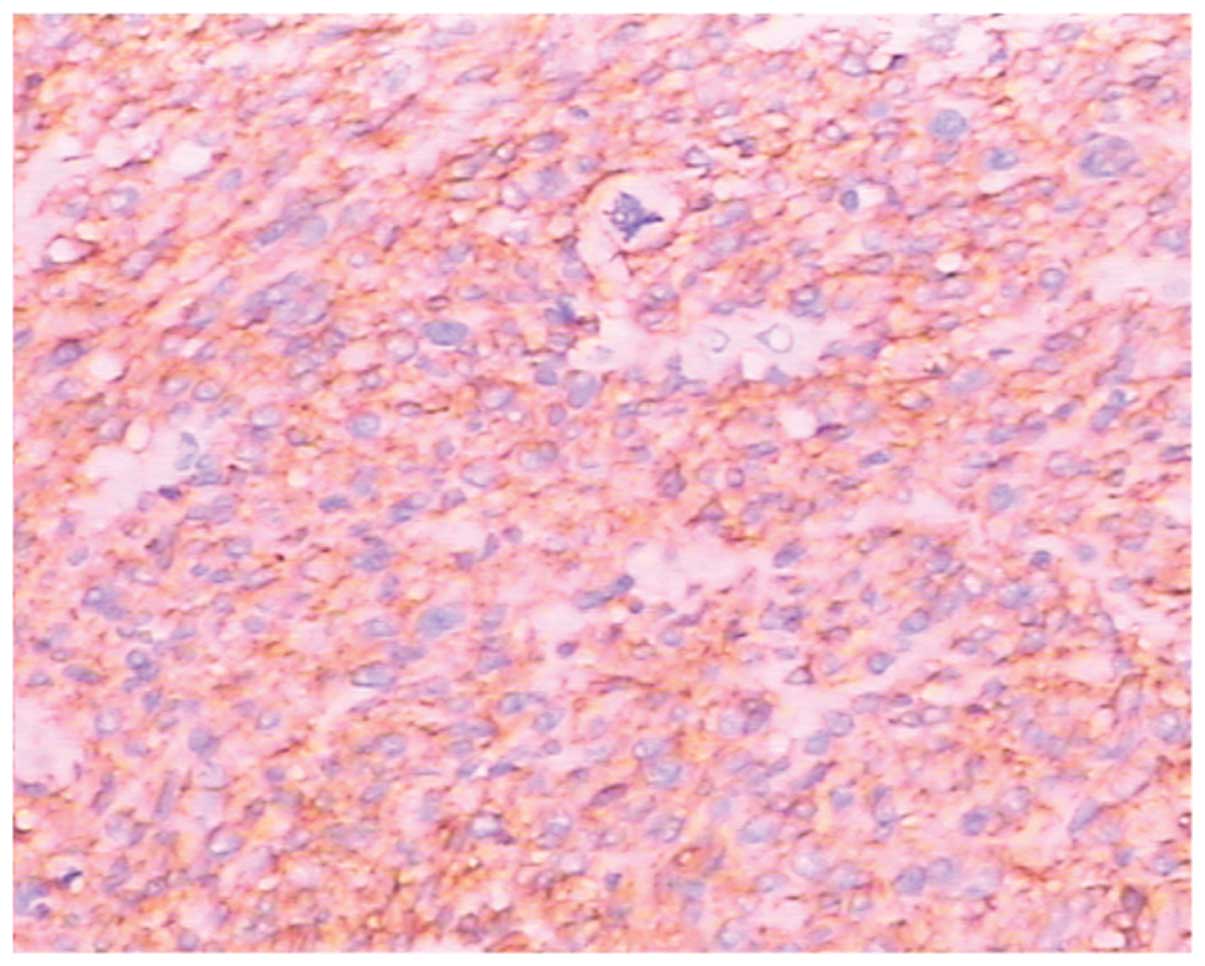
HPFs (Fig. 3). Immunohistochemical
staining revealed that the tumor cells were CD34-positive,
CD117-positive (Fig. 4),
Ki67-positive, S-100-positive/negative, smooth muscle
actin-negative and p53-negative. Thus, the final diagnosis was GIST
that was highly malignant.
The patient was discharged from hospital 14 days
following surgery and was not treated with imatinib
(Gleevec®; Novartis AG, Basel, Switzerland) due to
financial reasons. Follow-up revealed that the patient is alive
three years after surgery with no evidence of tumor recurrence.
Discussion
GIST typically appears as a regular, soft, solid
mass and rarely presents with cystic changes as the main clinical
manifestation. Exophytic stromal tumors with cystic changes have
been previously reported; however, large cystic mesenchymal tumors
are rarely observed. As the number of available studies on
exophytic stromal tumors with cystic degeneration are currently
limited, almost all authors suggest that, during preoperative
diagnosis, these masses may be mistaken to have derived from the
liver or pancreatic tissue. In our case, the patient presented with
abdominal distension and anorexia with no other gastrointestinal
symptoms, such as vomiting or melena. Upper gastrointestinal barium
meal imaging revealed no obvious abnormalities and preoperative MRI
and CT suggested the presence of a tumor derived from the
pancreatic body and tail. Therefore, the tumor was misdiagnosed as
a pancreatic body and tail tumor. The use of ultrasound-guided
endoscopy may have provided further diagnostic evidence.
In the present case, the size of the tumor was >5
cm, which is the standard size used to discriminate benignity from
malignancy for GIST. In accordance with the current criterion for
benignity and malignancy in GIST (11), the present case was classified into
the malignancy group, a high-risk group with a poor prognosis.
However, in the absence of imatinib treatment, the results of the
follow-up examinations were unexpected, as no evidence of tumor
recurrence or metastasis was reported three years after surgery.
Thus, the current criterion for the benignity and malignancy of
GIST may be debatable. A review of the currently available
literature suggests that in cases of cystic stromal tumors, tumor
size is not a true indicator of benignity or malignancy. However,
the solid part of the tumor may be included in the criteria for
indicating benignity or malignancy. Wang et al (12) suggested that the size of the tumor
is difficult to determine objectively. In cases of GIST with cystic
degeneration, the larger the area of the cystic component, the
lower the objectivity in determining tumor size (13). In addition, Kim et al
(14) found that among cases of
GIST with a diameter of <5 cm, almost half of the tumors showed
internal bleeding, necrosis or cystic degeneration and the
prognosis was not necessarily associated with tumor size. GIST with
cystic changes may be observed in the following situations: i)
primary cystic GIST, in which the main structure comprises cystic
tissue with a pseudocapsule, rarely invading the surrounding
organs; ii) malignant GIST with cystic degeneration, caused by
rapid growth of the tumor, which due to insufficiency of the
internal blood supply results in necrosis and liquefaction; iii)
when the tumor metastasizes to the liver and pancreas, the
metastatic lesion is always cystic in nature, often confused with
liver cysts and pancreatic cysts; and iv) on treatment with
imatinib, malignant GISTs show cystic degeneration (15).
On reviewing this case and the currently available
literature, we suggest that further pathological investigations of
cystic GIST are required to avoid potentially excessive or
inappropriate administration of imatinib.
References
|
1
|
Kim KM, Kang DW, Moon WS, Park JB, Park
CK, Sohn JH, Jeong JS, Cho MY, Jin SY, Choi JS and Kang DY;
Gastrointestinal Stromal Tumor Committee; Korean Gastrointestinal
Pathology Study Group. Gastrointestinal stromal tumors in Koreans:
it’s incidence and the clinical, pathologic and immunohistochemical
findings. J Korean Med Sci. 20:977–984. 2005.
|
|
2
|
Tryggvason G, Gíslason HG, Magnússon MK
and Jónasson JG: Gastrointestinal stromal tumors in Iceland,
1990–2003: the icelandic GIST study, a population-based incidence
and pathologic risk stratification study. Int J Cancer.
117:289–293. 2005.
|
|
3
|
Goettsch WG, Bos SD, Breekveldt-Postma N,
Casparie M, Herings RM and Hogendoorn PC: Incidence of
gastrointestinal stromal tumours is underestimated: results of a
nation-wide study. Eur J Cancer. 41:2868–2872. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miettinen M and Lasota J: Histopathology
of gastrointestinal stromal tumor. J Surg Oncol. 104:865–873. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirota S, Isozaki K, Moriyama Y, et al:
Gain-of-function mutations of c-kit in human gastrointestinal
stromal tumors. Science. 279:577–580. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heinrich MC, Corless CL, Duensing A, et
al: PDGFRA activating mutations in gastrointestinal stromal tumors.
Science. 299:708–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Emory TS, Sobin LH, Lukes L, Lee DH and
O’Leary TJ: Prognosis of gastrointestinal smooth-muscle (stromal)
tumors: dependence on anatomic site. Am J Surg Pathol. 23:82–87.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lerma E, Oliva E, Tugués D and Prat J:
Stromal tumours of the gastrointestinal tract: a
clinicopathological and ploidy analysis of 33 cases. Virchows Arch.
24:19–24. 1994.PubMed/NCBI
|
|
9
|
Rudolph P, Gloeckner K, Parwaresch R,
Harms D and Schmidt D: Immunophenotype, proliferation, DNA ploidy,
and biological behavior of gastrointestinal stromal tumors: a
multivariate clinicopathologic study. Hum Pathol. 29:791–800. 1998.
View Article : Google Scholar
|
|
10
|
Ando N, Goto H, Niwa Y, et al: The
diagnosis of GI stromal tumors with EUS-guided fine needle
aspiration with immunohistochemical analysis. Gastrointest Endosc.
55:37–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lewin KJ, Riddell RH and Weinstein WM:
Gastrointestinal Pathology and its Clinical Implications.
Igaku-Shoin; New York, NY: pp. 284–341. 1992
|
|
12
|
Wang CZ, Hou YY, Shen KT, et al:
Clinicopathological feature and prognosis of cystic
gastrointestinal stromal tumor. Chin J Gastro Surg. 14:599–602.
2011.PubMed/NCBI
|
|
13
|
Hou YY and Zhu ZX: Malignant degree of
gastrointestinal stromal tumor and the impact on biological
behavior. Chin J Pract Surg. 30:265–269. 2010.
|
|
14
|
Kim HC, Lee JM, Kim SH, et al: Small
gastrointestinal stromal tumours with focal areas of low
attenuation on CT: pathological correlation. Clin Radiol.
60:384–388. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bechtold RE, Chen MY, Stanton CA, Savage
PD and Levine EA: Cystic changes in hepatic and peritoneal
metastases from gastrointestinal stromal tumors treated with
Gleevec. Abdom Imaging. 28:808–814. 2003. View Article : Google Scholar
|