Introduction
Gastrointestinal stromal tumors (GISTs) are the most
common tumors of the gastrointestinal (GI) tract. GISTs originate
from the interstitial cell of Cajal, an intestinal pacemaker cell
in the gut. These cells are known to express the KIT gene (detected
as the cluster of differentiation [CD]117 antigen), which is
important for distinguishing GIST from other mesenchymal neoplasms
(1). In total, approximately
two-thirds of GISTs occur in the stomach and approximately
one-fifth in the small intestine, occasionally they occur in the
rectum, colon and esophagus (2).
GISTs that arise primarily outside the GI tract are termed
extragastrointestinal stromal tumors (EGISTs). EGISTs are known to
arise from various anatomic sites, such as the omentum, mesentery,
retroperitoneum and gall bladder. Notably, large, typical,
completely differentiated GISTs are rare in the extra GI tract
(2). EGISTs that arise in the
prostate are extremely rare and only a single case has previously
been reported indicating that the prostate is the primary site for
GIST (3–7). The current study reports an additional
case of a prostatic EGIST, including its presentation, diagnosis,
mutation analysis and the type of surgery that was performed, as
well as a review of the issues associated with GIST of the
prostate.
Case report
A 55-year-old male presented to the Department of
Urology at the Xiangya Hospital of the Central South University
(Changsha, China) with dysuria and urinary frequency that had
persisted for approximately six months. The patient’s review of
symptoms and medical history were otherwise unremarkable. A digital
rectal examination revealed that the prostate was markedly enlarged
with a smooth, bulging surface and unusual consistency on
palpation. The patient’s prostate-specific antigen (PSA) level was
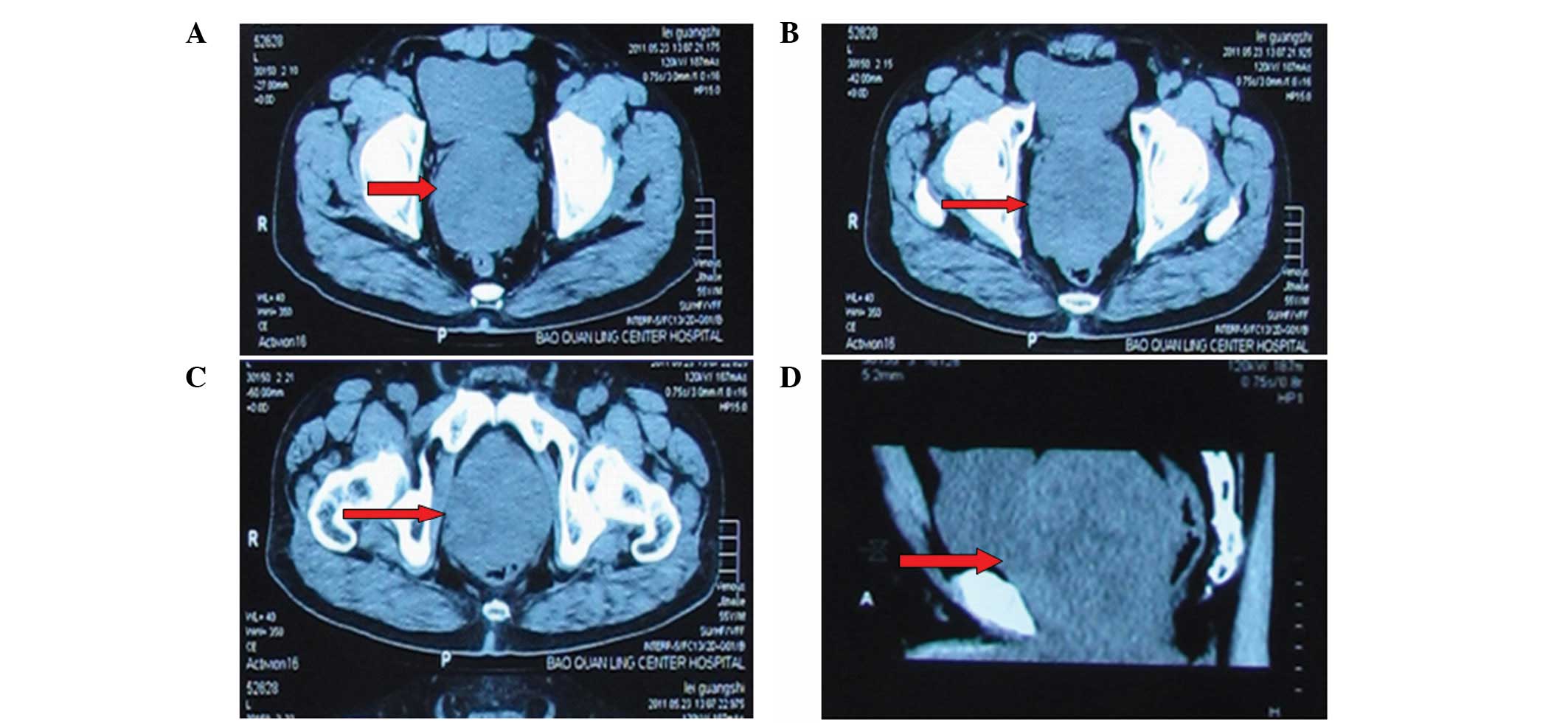
2.01 ng/ml; all other laboratory values were normal. Computed
tomography (CT) showed an enlarged prostate measuring 10×10.5×9.5
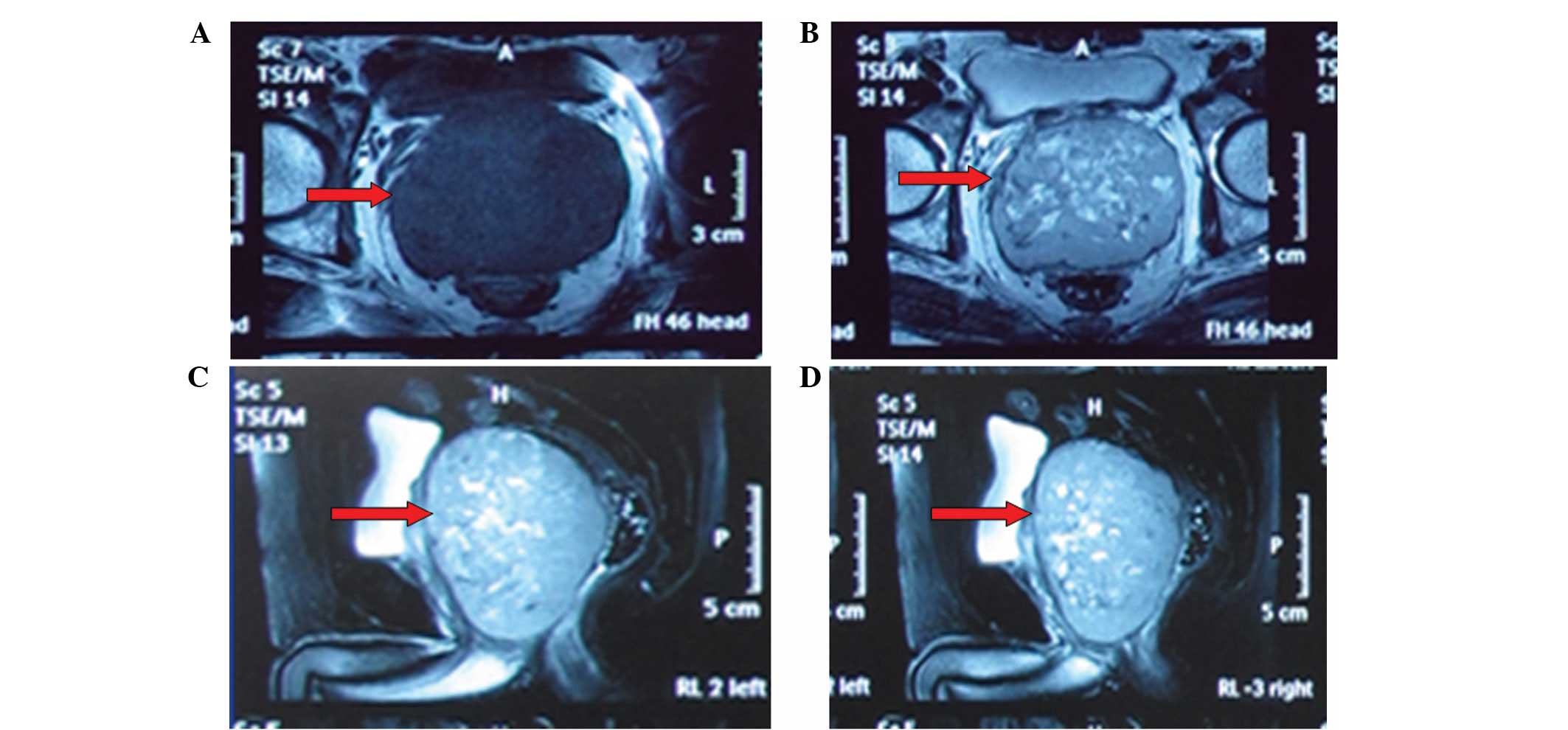
cm, but the prostatic capsule was intact (Fig. 1). Magnetic resonance imaging (MRI)
showed that the tumor was slightly hypointensive on the T1-weighted
images and hyperintensive on the T2-weighted images, with internal
irregular fluid-intense areas (Fig. 2A
and B), without evidence of infiltration of the rectum and
seminal vesicles. However, due to the location and volume of the
tumor, the rectum was severely compressed (Fig. 2C and D). No metastatic focus was
observed on the chest X-ray or emission CT examination of the
skeleton. The patient underwent a preoperative transperineal biopsy
for pathological dialysis. The result was highly indicative of
EGIST, and high cellularity, high mitotic count, marked nuclear
atypism and necrosis supported the high-risk nature of this tumor.
To detect the expression of c-kit (CD117), S-100, desmin, CD34,
cytokeratin, smooth muscle actin (SMA), DOG1 and vimentin (Vim)
proteins, formalin-fixed and paraffin-embedded tissue sections were
immunostained with anti-CD117, -CD34, -DOG1 and -Vim antibodies
(1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or
anti-cytokeratin, -SMA, -S-100 and -desmin antibodies (1:100;
Abcam, Cambridge, UK). All images were captured using a Nikon E1000
microscopic imaging system (Nikon Corporation, Tokyo, Japan). The
results of immunohistochemical staining were positive for CD34,
DOG1, Vim and CD117, and negative for cytokeratin, SMA, S-100
protein and desmin.
During surgery, the tumor was circumscribed and no
associations with the neighboring organs and structures were
identified. The tumor was easily separated from the adjacent
structures and no enlarged pelvic lymph nodes were detected.
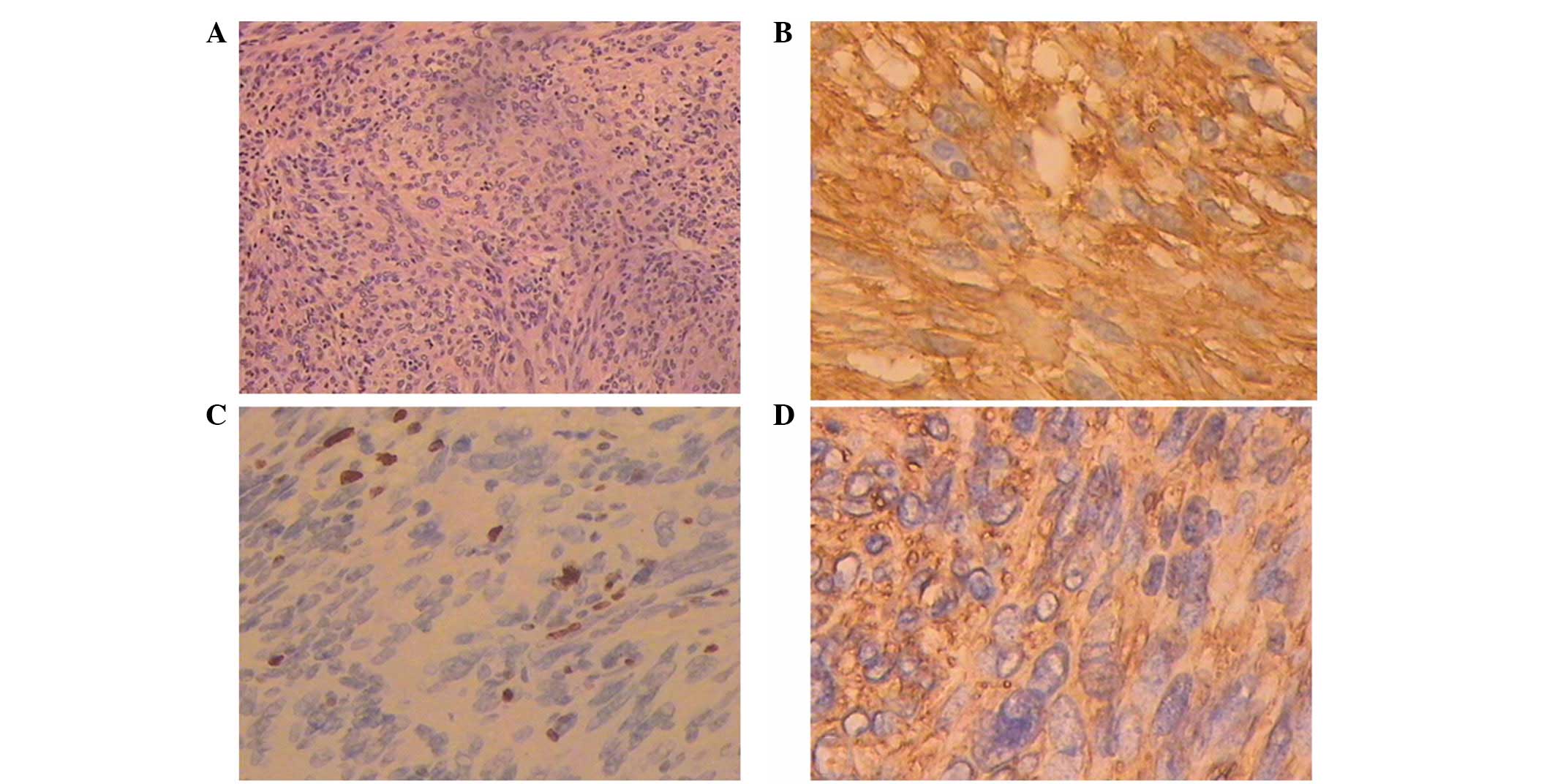
Microscopically, the tumor consisted of
spindle-shaped cells with 8/50 high-power fields (HPFs) of mitotic
activity (Fig. 3A) and regions of
myxoid degeneration. Immunohistochemistry showed immunoreactivity
for CD117, CD34, DOG1 and Vim, however, no immunoreactivity was
identified for S-100, desmin or SMA (Fig. 3B–D). In addition, the Ki-67 labeling
index was low (<1%).
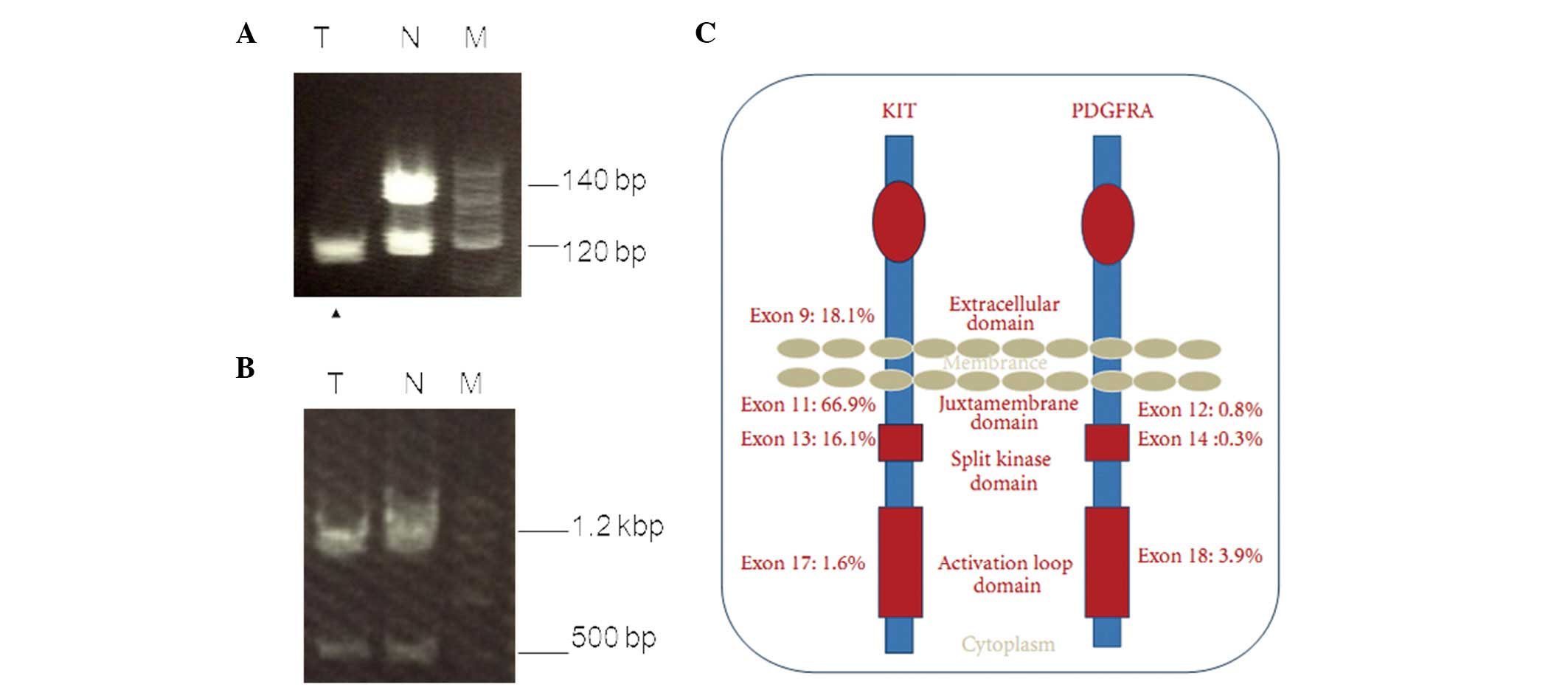
A mutation analysis of exons 9 and 11 of the c-kit
gene and those of exons 12 and 18 of the platelet-derived growth
factor receptor-α (PDGFRA) gene were examined. Microsatellite
markers proximal to the c-kit gene were performed via polymerase
chain reaction of the tumor tissue and normal specimens (8). Microsatellite analysis revealed a loss
of heterozygosity (LOH) of the c-kit gene in the tumor (Fig. 4A), however, no changes were
identified in the PDGFRA gene (Fig.
4B).
The patient had an uneventful postoperative course.
As an adjuvant to the postoperative molecular targeted
chemotherapy, the patient was treated with an oral administration
of 400 mg/day of imatinib (IM). No local recurrence or distant
metastasis was observed at the 12-month follow-up.
In accordance with the regulations of the Human
Investigation Committee of the Central South University (Changsha,
China), written informed consent was obtained from the patient for
publication of the current report and any accompanying images.
Discussion
GIST is a non-epithelial, mesenchymal tumor of the
GI tract, occurring predominantly in the stomach and small and
large intestines. A mutation in c-kit exons 9, 11, 13 and 17, and
PDGFRA exons 12, 14 and 18 is responsible for activation of the
gene signaling system, which results in uncontrolled
phosphorylation and tissue growth (Fig.
4C) (9). EGISTs are
histologically and immunohistochemically comparable with their GI
counterparts, however, exhibit an aggressive course, which
resembles that of small intestinal stromal tumors. In addition,
EGISTs have little or no connection to the abdominal wall or
serosal surface of the GI tract (10).
Urologists and urologic pathologists must be
particularly aware that certain apparent stromal tumors of the
prostate may be rectal GISTs, which involve the prostate in a
secondary manner. Previously, Hansel et al (11) showed that EGISTs may involve the
prostate via a direct extension from the abdominal wall. In
addition, Ghobadi et al (12) concluded that anorectal GISTs mimic
the presentation of prostate cancer. In the current case, the
patient did not present any abdominal pain or change in bowel
habits. However, clinical and radiological observations did not
identify any other primary site of disease or an apparently direct
extension from the rectum. The tumor cells diffusely and strongly
expressed CD117, CD34 and DOG1 (Fig.
3B–D); therefore, the prostate was considered to be the origin
of the tumor.
The clinicopathological features and treatment
outcomes of previously described prostatic GISTs, including the
present case, are presented in Table
I. Dysuria, urinary frequency, hematuria, and pelvic or
perineal pain are the common clinical presenting symptoms for
prostatic GIST, which has a predilection for adults of >49 years
old. GIST is often clinically silent in the initial stages and
grows slowly until it reaches a large size. Digital rectal
examination and imaging studies often reveal a significantly
enlarged prostate. However, the PSA level is almost always within
the normal range and previously, one case was identified with
multiple liver metastases at diagnosis (4). Immunohistochemistry demonstrated that
the mitotic rate was high (5/10 HPFs) in the tumors of the patient
in the study by Yinghao et al (5) and the present case (8/50 HPFs).
However, all cases were considered at high risk of recurrence or
metastasis according to the criteria by Yamamoto et al
(13), and based on tumor size and
the histopathological mitotic count (14). However, all four cases demonstrated
intense immunoreactivity for CD117 and CD34, and negative staining
for S-100. Immunoreactivity for Vim, desmin and SMA was variable in
the tumor cells.
 | Table IComparison of clinicopathological
observations, treatment and follow-up data of reported cases of
prostatic extragastrointestinal stromal tumors. |
Table I
Comparison of clinicopathological
observations, treatment and follow-up data of reported cases of
prostatic extragastrointestinal stromal tumors.
| First author
(ref) | Age, years | Prostate volume,
cm | Presentation | PSA, ng/ml | Metastasis | Pathology | Treatment | Follow-up,
months |
|---|
| Vander et al
(4) | 49 | 14.2×9.6×14.0 | Urinary
retention | 1.36 | Liver | Mitotic count:
abundant; CD117 (+), CD34 (+), SMA (+), S-100 (−) and desmin
(−) | IM mesylate | 25 |
| Lee et al
(3) | 75 | 6.7×5.6×5.5 | Urinary
retention | 1.36 | (−) | Mitotic count: 15/50
HPFs; CD117 (+), CD34 (+), and disuria Vim (+), SMA (−) and S-100
(−) | Radical
prostatectomy | 6 |
| Yinghao et al
(5) | 49 | 8.5×7.0×6.0 | Perineum pain | 1.1 | (−) | Mitotic count:
>5/10 HPFs; CD117 (+), CD34 (+), Vim (+), SMA (−), S-100 (−) and
desmin (−) | Radical
prostatectomy | 14 |
| Present case | 55 | 10.0×10.5×9.5 | Disuria | 2.01 | (−) | Mitotic count: 8/50
HPFs; CD117 (+), CD34 (+), Vim (+), SMA (−), S-100 (−), desmin (−)
and DOG1 (+) | Radical prostatectomy
and IM mesylate | 12 |
In the current case, DOG1, a novel marker originally
identified in GIST via gene profiling analysis, exhibited positive
immunoreactivity. DOG1 is strongly expressed on the cell surface of
GIST and is rarely expressed in other soft tissue tumors; it is
also expressed ubiquitously in GIST irrespective of the c-kit or
PDGFRA mutation status (15). The
reactivity for DOG1 may aid in the diagnosis of EGIST. However,
previous studies have established that activating mutations, in KIT
and PDGFRA genes, are present in GIST (9,15). In
addition, the current study first characterized the gene expression
patterns of c-kit and PDGFRA in prostatic EGIST and proposed that
the LOH of the c-kit gene may be involved in prostatic EGIST.
Surgery remains the standard treatment for primary
resectable EGISTs (5,13). Whenever possible, complete en bloc
removal of the tumor and the surrounding organs that are involved
is required. The available methods include radical prostatectomy,
cystoprostatectomy and total pelvic exenteration. Conventional
chemotherapy and radiotherapy are not effective in the treatment of
EGISTs and GISTs, whereas IM, a tyrosine kinase inhibitor of c-kit,
and PDGFRA as methods of adjuvant therapy, have been proposed as
treatment for advanced, unresectable and metastatic GIST. In the
evaluation of patients with completely resected primary EGISTs,
assessing the risk of recurrence is important for optimal
postoperative management. The size, cellularity and mitotic
activity of EGISTs have been reported as the most accurate
predictors of an adverse outcome (14). Previously, Reith et al
(10) proposed in a multivariate
analysis, that mitotic activity and necrosis exhibit trends towards
independent predictive value.
In the present case, the large size (~15 cm), high
mitotic counts (8/50 HPFs) and extensive hemorrhagic necrosis of
the tumor were the malignant features. Therefore, this tumor
belongs to the high-risk group and the results indicated that IM
was required.
GISTs exhibit highly variable biological behavior.
Although only 10–30% of GISTs are clinically malignant, GISTs
harbor a slight malignant potential (16). Therefore, close follow-up is
required and must be based upon the risk of recurrence following
resection. Generally, high-risk GIST patients relapse early, in a
median time of two years following resection (14). Limited data are available to predict
the malignant potential of prostatic GIST. However, the available
information indicates that EGISTs possess a similar degree of risk
as intestinal GISTs (10). The
accurate radiological follow-up (abdominal and pelvic CT) is
considered to be the approach of choice in the control of EGIST of
the prostate.
In conclusion, the current study presents a rare
case of EGIST arising from the prostate and the following are
proposed: i) It is crucial to note possible adhesion to the rectal
wall during pathological examination; ii) immunohistochemistry,
using antibodies against CD117 (c-kit) and CD34, is valuable for
the diagnosis of EGIST; iii) DOG1 must be included in the routine
diagnostic immunohistochemical panel as it allows proper
classification of EGIST; iv) mutation analysis (of c-kit and
PDGFRA) is potentially significant in the diagnosis and treatment
of EGIST; v) IM therapy is recommended for high-risk patients
following complete surgical removal of EGIST; and vi) long-term
follow-up is necessary.
Abbreviations:
|
EGIST
|
extragastrointestinal stromal
tumor
|
|
LOH
|
loss of heterozygosity
|
|
GIST
|
gastrointestinal stromal tumor
|
|
IM
|
imatinib
|
|
PDGFRA
|
platelet-derived growth factor
receptor-α
|
|
SMA
|
smooth muscle actin
|
|
HPF
|
high-power fields
|
|
GI
|
gastrointestinal
|
References
|
1
|
Mucciarini C, Rossi G, Bertolini F, Valli
R, Cirilli C, Rashid I, Marcheselli L, Luppi G and Federico M:
Incidence and clinicopathologic features of gastrointestinal
stromal tumors. A population-based study. BMC Cancer. 7:2302007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Appelman HD: Morphology of
gastrointestinal stromal tumors: historical perspectives. J Surg
Oncol. 104:874–881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee CH, Lin YH, Lin HY, Lee CM and Chu JS:
Gastrointestinal stromal tumor of the prostate: a case report and
literature review. Hum Pathol. 37:1361–1365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van der Aa F, Sciot R, Blyweert W, Ost D,
Van Poppel H, Van Oosterom A, Debiec-Rychter M and De Ridder D:
Gastrointestinal stromal tumor of the prostate. Urology.
65:3882005.PubMed/NCBI
|
|
5
|
Yinghao S, Bo Y and Xiaofeng G:
Extragastrointestinal stromal tumor possibly originating from the
prostate. Int J Urol. 14:869–871. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anagnostou E, Miliaras D and
Panagiotakopoulos V: Diagnosis of gastrointestinal stromal tumor
(GIST) on transurethral resection of the prostate: a case report
and review of the literature. Int J Surg Pathol. 19:632–636. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herawi M, Montgomery EA and Epstein JI:
Gastrointestinal stromal tumors (GISTs) on prostate needle biopsy:
A clinicopathologic study of 8 cases. Am J Surg Pathol.
30:1389–1395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kikuchi H, Yamamoto M, Hiramatsu Y, Baba
M, Ohta M, Kamiya K, Tanaka T, Suzuki S, Sugimura H, Kitagawa M, et
al: Effect of loss of heterozygosity of the c-kit gene on prognosis
after hepatectomy for metastatic liver gastrointestinal stromal
tumors. Cancer Sci. 98:1734–1739. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan CB, Zhi W, Shahzad G and Mustacchia P:
Gastrointestinal stromal tumors: a review of case reports,
diagnosis, treatment, and future directions. ISRN Gastroenterol.
2012:5959682012.PubMed/NCBI
|
|
10
|
Reith JD, Goldblum JR, Lyles RH and Weiss
SW: Extragastrointestinal (soft tissue) stromal tumors: an analysis
of 48 cases with emphasis on histologic predictors of outcome. Mod
Pathol. 13:577–585. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hansel DE, Herawi M, Montgomery E and
Epstein JI: Spindle cell lesions of the adult prostate. Mod Pathol.
20:148–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghobadi A, Kabbani W, Barker B and Dowell
JE: Rectal GI stromal tumor mimicking a prostate mass. J Clin
Oncol. 25:5827–5828. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto H, Oda Y, Kawaguchi K, Nakamura
N, Takahira T, Tamiya S, Saito T, Oshiro Y, Ohta M, Yao T and
Tsuneyoshi M: c-kit and PDGFRA mutations in extragastrointestinal
stromal tumor (gastrointestinal stromal tumor of the soft tissue).
Am J Surg Pathol. 28:479–488. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dematteo RP, Gold JS, Saran L, Gönen M,
Liau KH, Maki RG, Singer S, Besmer P, Brennan MF and Antonescu CR:
Tumor mitotic rate, size, and location independently predict
recurrence after resection of primary gastrointestinal stromal
tumor (GIST). Cancer. 112:608–615. 2008. View Article : Google Scholar
|
|
15
|
West RB, Corless CL, Chen X, Rubin BP,
Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R,
et al: The novel marker, DOG1, is expressed ubiquitously in
gastrointestinal stromal tumors irrespective of KIT or PDGFRA
mutation status. Am J Pathol. 165:107–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miettinen M, Sobin LH and Lasota J:
Gastrointestinal stromal tumors of the stomach: a
clinicopathologic, immunohistochemical, and molecular genetic study
of 1765 cases with long-term follow-up. Am J Surg Pathol. 29:52–68.
2005. View Article : Google Scholar : PubMed/NCBI
|