Introduction
Surgery is the preferred treatment for non-small
cell lung cancer (NSCLC). However, without satisfactory surgical
results, the overall five-year survival rate is ~30–40%. The
majority of patients succumb following surgery due to tumor
metastasis and recurrence. The brain metastases-associated
clinicopathological features and molecular biological markers are
clinically significant as they may determine a more accurate
prognosis for the patient, allowing postoperative adjuvant
therapies to be targeted. However, there is currently no effective
method for identifying high-risk NSCLC patients with brain-specific
metastasis.
Brain-specific metastasis is one of the primary
causes of recurrence following complete resection of NSCLC, and the
underlying mechanism remains unclear. The present study was
designed to investigate the correlation between C-X-C chemokine
receptor type 4 (CXCR4) expression and brain-specific metastasis of
NSCLC.
The homing theory states that tumor cell metastasize
to specific organs, due to an organ-specific capacity to arrest or
attract specific types of cancer cells via chemotaxis (1). Therefore, the spreading of certain
tumors is considered to be selective, rather than random This
hypothesis is further affirmed by the observations of
organ-specific metastasis in certain cancer types, such as prostate
cancer, where the tumor cells are more likely to metastasize to
bone (2). Previous studies have
identified that CXCR4 and its ligand, CXCL12, are associated with
organ-specific metastasis (2–4). Our
previous preliminary study demonstrated that CXCR4 overexpression
in tumor tissue is correlated with NSCLC at the homeochronous and
heterochronic phases of solitary brain metastasis (5).
Based on previous studies, the present study
retrospectively investigated NSCLC patients with brain metastasis
following complete resection. Immunohistochemical methods were used
to detect the expression of CXCR4 within tumor tissues. Through
matched-pair analysis the correlation between CXCR4 overexpression
and brain metastasis of postoperative NSCLC patients was
investigated. The function of CXCR4 in brain-specific metastasis of
NSCLC, according to the controlled analysis of patients with
brain-specific metastasis and patients with other organ metastases,
was also examined.
Patients and methods
Patients
Between January 1998 and June 2008, 5,117 patients
underwent surgical resection of NSCLC at the Qingdao Municipal
Hospital (Qingdao, China). A total of 105 (2.1%) patients who
underwent complete tumor resection were retrospectively reviewed.
The patients in the present study did not receive any neoadjuvant
therapy prior to surgery. This study was approved by the ethics
committee of Qingdao Municipal Hospital (Qingdao, China). Patients
provided written informed consent.
The sample group included 34 NSCLC patients with
brain-specific metastasis during the follow-up period, which
consisted of 22 males and 12 females, with a median age of 56 years
(range, 37–75 years). Histological analysis revealed that there
were 14 squamous cell carcinomas, 17 adenocarcinomas and three
large cell lung cancers. According to the 2009 International
Association for the Study of Lung Cancer tumor, node, metastasis
(TNM) classification, seven patients were classified as Stage I, 11
patients were classified as Stage II and 16 patients were
classified as Stage III. A total of 30 patients received 4–6 cycles
of cisplatin-based chemotherapy (Table
I).
 | Table IClinicopathological features of
patients with brain-specific metastasis and patients in control
group 1. |
Table I
Clinicopathological features of
patients with brain-specific metastasis and patients in control
group 1.
| Clinicopathological
feature | Brain metastasis (no.
of cases) | Control group 1 (no.
of cases) |
|---|
| Age (years) | | |
| <40 | 4 | 3 |
| 40–50 | 6 | 5 |
| 50–60 | 15 | 15 |
| 60–70 | 7 | 8 |
| >70 | 2 | 3 |
| Gender | | |
| Male/Female | 22/12 | 21/13 |
| Surgical pattern | | |
| R. upper lobe | 8 | 9 |
| R. lower lobe | 7 | 8 |
| R. middle lobe | 2 | 1 |
| R. middle and lower
lobes | 3 | 2 |
| R. whole lung | 1 | 1 |
| L. upper lobe | 6 | 6 |
| L. lower lobe | 5 | 6 |
| L. whole lung | 2 | 1 |
| Pathological
type | | |
| Adenocarcinoma | 17 | 18 |
| Squamous cell
carcinoma | 14 | 14 |
| Large cell
carcinoma | 3 | 2 |
| Degree of
differentiation | | |
| Well
differentiated | 13 | 13 |
| Poorly
differentiated | 18 | 19 |
|
Undifferentiated | 3 | 2 |
| T status | | |
| T1/T2/T3/T4 | 5/16/8/5 | 6/17/8/3 |
| N status | | |
| N0/N1/N2 | 7/15/12 | 8/17/9 |
| Post-operative
adjuvant chemotherapy | | |
| Yes/No | 30/4 | 31/3 |
| Diagnosis of brain
metastases | | |
|
Pathology/Imaging | 16/18 | 0 |
| Transfer time from
surgery | | |
| <6 months | 2 | |
| 6–12 months | 6 | |
| 12–24 months | 12 | |
| 24–36 months | 9 | |
| >36 months | 5 | |
Follow-up and diagnosis of
metastases
A complete patient follow-up was performed on a
regular basis. Every 3–6 months, the patients received
comprehensive medical examinations as follows: Brain and thorax
X-ray computed tomography (CT) scans; upper abdomen enhanced CT
scans; and/or liver, gallbladder, pancreas, spleen, kidney and
adrenal B-mode ultrasound; electroconvulsive therapy scans in the
patients presenting with bone pain; and whole body PET-CT in
certain patients. The newly identified lesions in the brain and
other organs were diagnosed as metastases after eliminating the
possibility of benign lesions. The pathological investigation
following surgical resection confirmed brain-specific metastasis in
16 patients and the remaining patients were clinically diagnosed
with brain-specific metastasis.
Research design and statistical
analysis
A total of 34 eligible NSCLC patients without
metastases during the follow-up period served as control group 1
(Table I) and 37 NSCLC patients
with other organ metastases, excluding brain-specific metastasis,
during the follow-up period served as control group 2 (Table II). All of these patients were
screened in the following order: TNM stage, histological tumor
type, degree of tumor differentiation, gender, surgical pattern,
adjuvant therapy post-surgery and age. When these
clinicopathological characteristics did not exactly match with the
experimental group, the maximum extent of consistency principle was
followed.
 | Table IIClinicopathological features of
patients with other organ metastases in control group 2. |
Table II
Clinicopathological features of
patients with other organ metastases in control group 2.
| Location of
metastasesa |
|---|
|
|
|---|
| Clinicopathological
features | Lung and pleura
(n=14) | Liver (n=10) | Bone (n=6) | Adrenal glands
(n=5) | Other (n=2) |
|---|
| Surgical pattern |
| Lobe/Whole lung | 12/2 | 9/1 | 6/0 | 5/0 | 1/1 |
| Pathological
type |
| Adenocarcinoma | 7 | 5 | 2 | 1 | 0 |
| Squamous cell
carcinoma | 5 | 5 | 4 | 3 | 2 |
| Large cell
carcinoma | 2 | 0 | 0 | 1 | 0 |
| Degree of
defferentiation |
| Well
differentiated | 5 | 6 | 3 | 2 | 2 |
| Poorly
differentiated | 7 | 3 | 3 | 3 | - |
|
Undifferentiated | 2 | 1 | 0 | 0 | 0 |
| TNM stage |
| I | 2 | 1 | 0 | 1 | 0 |
| II | 5 | 4 | 2 | 1 | 2 |
| III | 7 | 5 | 4 | 3 | 0 |
| Diagnosis of
metastases |
|
Pathology/Imaging | 6/8 | 1/9 | 1/5 | 3/2 | 2/0 |
Pearson’s χ2 test was used to evaluate
differences in CXCR4 expression between the patients with
brain-specific metastasis and the patients without hematogenous
metastases; this enabled examination of the correlation between
CXCR4 expression and postoperative brain-specific metastasis of
NSCLC. The patients who received complete tumor resection at the
same period as the other two groups and exhibited other organ
metastases during the follow-up period were selected for control
group 2. The χ2 test was used to evaluate differences in
CXCR4 expression between brain-specific metastasis patients and
other organ metastases patients in order to further examine the
role of CXCR4 in postoperative brain-specific metastasis of
NSCLC.
The clinical and pathological data were compiled
into an SPSS 15.0 database (SPSS, Inc., Chicago, IL, USA). An
estimation of survival rate was calculated using the Kaplan-Meier
method and statistical differences were analyzed with the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Techniques
Immunohistochemistry (streptavidin-peroxidase [SP]
method) was performed to detect the CXCR4 expression levels in
every tissue specimen. The primary antibody employed was the mouse
anti-human CXCR4 monoclonal antibody at 1:100 dilution (Abcam
Ltd.). Secondary processing of the tissue samples was performed
with an SP kit and a universal secondary antibody kit according to
the manufacturer’s instructions (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China). Briefly, following
incubation overnight with the primary antibody, the secondary
biotinylated antibody and subsequent avidin-biotin complex reagent
were incubated for 30 min, respectively. Staining was visualized
using diaminobenzidine.
Paraffin-embedded specimens of the surgically
removed tumor tissue were collected and all of the stained sections
were examined by two independent experienced pathologists who had
been double blinded to the clinical data. The immunohistochemical
score was calculated by combining the proportion score (percentage
of positive stained cells) with the staining intensity score. The
proportion score ranged from 0 to 4, as follows: 0 (no staining), 1
(1–24%), 2 (25–49%), 3 (50–74%), 4 (>75%). The staining
intensity was scored as follows: 0 (negative), 1 (weak), 2
(moderate) and 3 (strong). The proportion score and staining
intensities score were subsequently multiplied to generate the
total IHS score for each case. The total score ranging 4–12 was
considered to be positive expression. The tissue specimens were
scored according to a combination of the intensity and the
proportion of positive-stained tumor cells, as follows: i) The
number of positive tumor cells was determined by assesment of l0
high-power fields of dense tumor cell areas, which were randomly
selected for counting. A minimum of 600 tumor cells were counted
and observed and the proportion of the positive-stained tumor cells
was evaluated according to the following scale: 0=0–5%; 1=6–10%;
2=11–50%; and 3≥50%. ii) The staining intensity was evaluated
according to the following scale: 0=No reactivity; 1=low;
2=moderate; and 3=strong. A total score of 3–6 was considered to
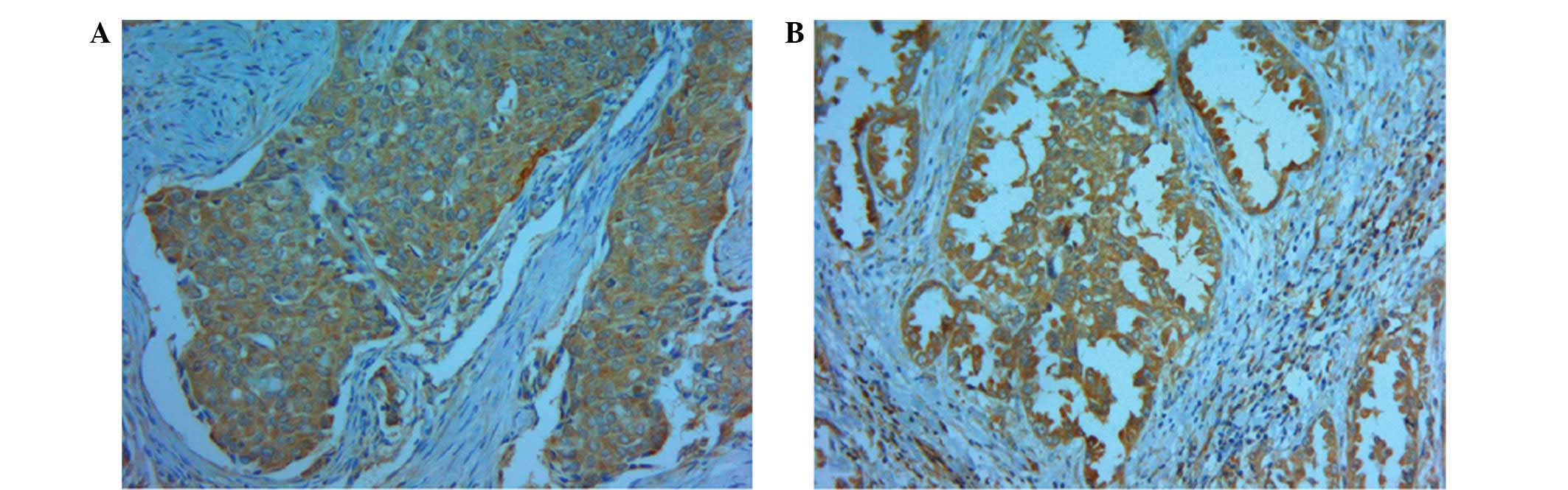
indicate positive staining of CXCR4 (Fig. 1) (6).
Results
The difference in CXCR4 protein
expression between patients with brain-specific metastasis and the
control groups
In the group of patients with brain-specific
metastasis, CXCR4 overexpression was observed in 31 samples of
NSCLC tissue (91.2%), whereas of the 34 patients without
metastases, CXCR4 overexpression was observed in five samples of
NSCLC tissue (14.7%), which was significantly lower compared with
that of patients with brain-specific metastasis according to the
χ2 tests (P<0.001; Table III).
 | Table IIIComparison of CXCR4 expression in
non-small cell lung cancer patients. |
Table III
Comparison of CXCR4 expression in
non-small cell lung cancer patients.
| Expression of
CXCR4 |
|---|
|
|
|---|
| Location of
metastases | Positive | Negative | Total |
|---|
| Brain | 31 | 3 | 34 |
| Other organ | 27 | 10 | 37 |
| No metastases | 5 | 29 | 34 |
In the group of patients with other organ
metastases, CXCR4 overexpression was observed in 27 samples of
NSCLC tissue (73.0%), which was significantly higher compared with
that of patients without metastases (14.7%; P<0.001; Table III).
The CXCR4 expression levels of the brain-specific
metastasis group and other organ metastases group (control group 2)
were compared. According to the χ2 test (Pearson method)
the brain-specific metastasis patients exhibited a higher
expression of CXCR4 (Table III),
which was identified to be a statistically significant difference
(P=0.048).
Analysis of survival rate among NSCLC
patients with and without metastases
All patients with lung cancer were closely
followed-up, with a mean follow-up time of 59.6 months (range,
7–116 months). The three- and five-year cumulative survival rates
of 34 patients with brain-specific metastasis were 61.8 and 38.2%,
respectively and the three- and five-year cumulative survival rates
of patients with other organ metastases were 59.5 and 32.4%,
respectively. The log-rank test demonstrated that there were no
statistically significant differences identified between the
survival rates of the two groups (P>0.05; Fig. 2). The three- and five-year
cumulative survival rates of the 34 patients without metastases
were 97.1 and 94.1%, respectively.
Discussion
Clinicopathological feature analyses have revealed
that, for NSCLC patients presenting with brain-specific metastasis
alone, metastatic lesion resection or other adjuvant therapies may
improve quality of life and prolong survival time (7–9).
Therefore, identifying patients with a high risk of brain-specific
metastasis following lung cancer resection is clinically
significant for predicting the prognosis and selecting the most
appropriate adjuvant therapy.
Currently, the mechanism(s) that promote
brain-specific metastasis have not been clearly elucidated. When
NSCLC attacks the pulmonary vein, tumor cells are able to directly
enter the blood circulation without pulmonary capillary bed
involvement. Hematogenous spread may lead to multiple organ
metastases, with the brain being the most common metastatic site
clinically. Further studies are required to determine why NSCLC
cells remain in the brain by hematogenous metastases and the
underlying mechanisms regarding the specific affinity of NSCLC
cells to the brain.
In recent years, the homing theory has been proposed
as a result of investigations into tumor metastases to specific
organs. The theory states that different organs chemotactically
capture or attract particular types of tumor cells, which is termed
homing and results in metastasis to specific organs (1). In 2001, Müller et al (3) proposed that tumor cells metastasize by
a specific combination of chemotactic factors (chemokines) and
receptors (chemokine receptor) to specific organs. It was
identified that breast cancer cells express CXCR4 highly and that
the ligand of CXCR4, CXCL12, is primarily expressed in the lungs,
liver and bone marrow. It is also these same organs that the
majority of breast cancer cells often metastasize to, providing
strong correlational evidence in support of the homing theory.
Clinically, NSCLC most commonly metastasizes to the brain. Whether
this is also due to an interaction between chemotactic factors and
their receptors requires further investigation.
In our previous study, the correlation of CXCR4 and
solitary brain-specific metastasis at the homeochronous and
heterochronic phases was examined. The preliminary results
demonstrated that CXCR4 expression levels in the tumor tissue of
NSCLC patients with brain-specific metastasis is higher compared
with NSCLC patients without distant metastases (5). This indicated that CXCR4 may be
associated with brain-specific metastasis in NSCLC. In order to
further examine the correlation between CXCR4 overexpression and
brain-specific metastasis following NSCLC surgical resection and to
assess whether it is associated with brain-specific metastasis, the
present study examined more cases. In addition, a comparison
between patients with brain-specific metastasis and patients with
other organ metastases was performed.
In the group of patients with brain-specific
metastasis, CXCR4 overexpression was observed in 31 of the patients
with brain-specific metastasis and, of the 34 patients without
metastases, CXCR4 overexpression was observed in only five
patients, which was a statistically significant difference. In the
group of patients exhibiting other organ metastases, CXCR4
over-expression in tumor tissue was also higher compared with the
patients without metastases. The present study indicates that CXCR4
overexpression in NSCLC may be correlated with postoperative
hematogenous metastases. Further analysis by comparing patients
exhibiting brain-specific metastasis and other organ metastases
revealed that CXCR4 overexpression is higher in patients with
brain-specific metastasis compared with patients exhibiting other
organ metastases (P=0.048; Table
III). It was also demonstrated that a chemotaxis function may
mediate the homing of CXCR4-overexpressing NSCLC cells to the
brain, where the ligand CXCL12 is overexpressed.
Adopting statistical methods of matching comparison
reduced the experimental bias and enhanced the objectivity of the
present study. However, as a retrospective study, several
limitations remain, including: i) Following surgery, the adjuvant
chemotherapy scheme and medication-use time were not tightly
controlled between the patients; ii) the majority of organ
metastases patients were clinically diagnosed; iii) the number of
cases involved in this single-center study was limited; iv) due to
ethical considerations, it was not possible to obtain normal brain
tissue for the detection of normal CXCL12 expression levels.
However, the fact that CXCL12 is constitutively expressed in the
developing and mature central nervous system (10,11)
may support the results of the present study indirectly. Previous
studies have demonstrated that CXCL12 expression was normally
controlled at a relatively low level (12–17).
Under certain pathological situations, including HIV 1-associated
dementia, brain tumor, ischemia and neuroinflammation, CXCL12
expression may be briefly upregulated. Astrocytes and vascular
endothelial cells in the parenchyma have been proposed as two
primary cell sources for inducible CXCL12, and hypoxia-inducible
factor-1 (18) may regulate CXCL12
gene expression in endothelial cells, resulting in the selective
expression of CXCL12. Furthermore, interleukin-1β (16) induces CXCL12 in astrocytes by
extracellular signal-regulated kinase and
phosphatidylinositol-4,5-bisphosphate 3-kinase signaling pathways.
In the present study, although the majority of organ metastases
occurred within three years postoperatively and all of the patients
in the control groups had regular follow-up, no brain-specific
metastasis was identified; however, the possibility that
brain-specific metastasis may have occurred subsequently may not be
ruled out. These problems should be addressed in future
studies.
In conclusion, brain-specific metastasis is one of
the primary reasons for recurrence following complete resection of
NSCLC. At present, it is only possible to assess the occurrence of
brain-specific metastasis according to clinicopathological
features, which are considered to lack sensitivity. The present
study demonstrated that CXCR4 overexpression in patients with
brain-specific metastasis was higher when compared with the control
group patients, indicating that the CXCL12/CXCR4 signaling axis may
be involved in the brain-specific metastasis processes of NSCLC.
Therefore, further studies are required to examine whether CXCR4
may be a molecular marker in predicting brain-specific metastasis
associated with NSCLC.
References
|
1
|
Takeuchi H, Kitago M and Hoon DS: Effects
of chemokines on tumor metastasis. Cancer Treat Res. 135:177–184.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taichman RS, Cooper C, Keller ET, Pienta
KJ, Taichman NS and McCauley LK: Use of the stromal cell-derived
factor-1/CXCR4 pathway in prostate cancer metastasis to bone.
Cancer Res. 62:1832–1837. 2002.PubMed/NCBI
|
|
3
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, et al: Involvement of chemokine receptors in breast
cancer metastases. Nature. 410:50–56. 2001.PubMed/NCBI
|
|
4
|
Phillips RJ, Burdick MD, Lutz M, Belperio
JA, Keane MP and Strieter RM: The stromal derived
factor-1/CXCL12-CXC chemokine receptor 4 biological axis in
non-small cell lung cancer metastases. Am J Respir Crit Care Med.
167:1676–1686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen G, Wang Z, Liu XY and Liu FY:
High-level CXCR4 expression correlates with brain-specific
metastases of non-small cell lung cancer. World J Surg. 35:56–61.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spano JP, Andre F, Morat L, Sabatier L,
Besse B, Combadiere C, et al: Chemokine receptor CXCR4 and
early-stage non-small cell lung cancer: pattern of expression and
correlation with outcome. Ann Oncol. 15:613–617. 2004. View Article : Google Scholar
|
|
7
|
Koutras AK, Marangos M, Kourelis T,
Partheni M, Dougenis D, Iconomou G, et al: Surgical management of
cerebral metastases from non-small cell lung cancer. Tumori.
89:292–297. 2003.PubMed/NCBI
|
|
8
|
Girard N, Cottin V, Tronc F,
Etienne-Mastroianni B, et al: Chemotherapy is the cornerstone of
the combined surgical treatment of lung cancer with synchronous
brain metastases. Lung Cancer. 53:51–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paek SH, Audu PB, Sperling MR, Cho J and
Andrews DW: Reevaluation of surgery for the treatment of brain
metastases: review of 208 patients with single or multiple brain
metastases treated at one institution with modern neurosurgical
techniques. Neurosurgery. 56:1021–1034. 2005.
|
|
10
|
Li M and Ransohoff RM: Multiple roles of
chemokine CXCL12 in the central nervous system: a migration from
immunology to neurobiology. Prog Neurobiol. 84:116–131. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banisadr G, Skrzydelski D, Kitabgi P,
Rostène W and Parsadaniantz SM: Highly regionalized distribution of
stromal cell-derived factor-1/CXCL12 in adult rat brain:
constitutive expression in cholinergic, dopaminergic and
vasopressinergic neurons. Eur J Neurosci. 18:1593–1606. 2003.
View Article : Google Scholar
|
|
12
|
Rempel SA, Dudas S, Ge S and Gutiérrez JA:
Identification and localization of the cytokine SDF1 and its
receptor, CXC chemokine receptor 4, to regions of necrosis and
angiogenesis in human glioblastoma. Clin Cancer Res. 6:102–111.
2000.PubMed/NCBI
|
|
13
|
Rostasy K, Egles C, Chauhan A, Kneissl M,
Bahrani P, Yiannoutsos C, et al: SDF-1alpha is expressed in
astrocytes and neurons in the AIDS dementia complex: an in vivo and
in vitro study. J Neuropathol Exp Neurol. 62:617–626.
2003.PubMed/NCBI
|
|
14
|
Hill WD, Hess DC, Martin-Studdard A,
Carothers JJ, Zheng J, Hale D, et al: SDF-1 (CXCL12) is upregulated
in the ischemic penumbra following stroke: association with bone
marrow cell homing to injury. J Neuropathol Exp Neurol. 63:84–96.
2004.PubMed/NCBI
|
|
15
|
Miller JT, Bartley JH, Wimborne HJ, Walker
AL, Hess DC, Hill WD and Carroll JE: The neuroblast and angioblast
chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated
by reactive astrocytes in brain following neonatal hypoxic-ischemic
injury. BMC Neurosci. 6:632005. View Article : Google Scholar
|
|
16
|
Peng H, Erdmann N, Whitney N, Dou H,
Gorantla S, Gendelman HE, et al: HIV-1-infected and/or immune
activated macrophages regulate astrocyte SDF-1 production through
IL-1beta. Glia. 54:619–629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tabatabai G, Frank B, Möhle R, Weller M
and Wick W: Irradiation and hypoxia promote homing of
haematopoietic progenitor cells towards gliomas by
TGF-beta-dependent HIF-1alpha-mediated induction of CXCL12. Brain.
129:2426–2435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ceradini DJ, Kulkarni AR, Callaghan MJ,
Tepper OM, Bastidas N, Kleinman ME, et al: Progenitor cell
trafficking is regulated by hypoxic gradients through HIF-1
induction of SDF-1. Nat Med. 10:858–864. 2004. View Article : Google Scholar : PubMed/NCBI
|