Introduction
Although there has been an overall worldwide decline
in incidence, gastric cancer (GC) remains one of the most common
types of cancer and is the second leading cause of cancer-related
mortality (1). In China, a large
number of GC patients are diagnosed following tumor metastasis and,
despite a large number of clinical trials with conventional and
targeted therapies, current treatments only offer limited benefits.
Thus, strategies are required to overcome this life-threatening
disease.
Although a number of molecular markers have been
associated with the metastasis of human carcinoma, one of the most
important factors contributing to malignancy is the loss of
epithelial differentiation. This phenomenon is manifested as an
epithelial-mesenchymal transition (EMT), which promotes cancer
invasion and metastasis (2). During
EMT, the epithelial-specific junction protein, epithelial-cadherin
(E-cadherin), is downregulated and mesenchymal proteins, such as
neural cadherin (N-cadherin), are upregulated (3). Therefore, epithelial cells become
individual, non-polarized, motile and invasive mesenchymal cells
(4).
EMT is a dynamic process and is triggered by the
interplay of extracellular signals, as well as a number of secreted
soluble factors, including transforming growth factor-β, nuclear
factor κB, platelet-derived growth factor, Wnt (including a variety
of isoforms), microRNAs and others (5–9).
Furthermore, the Notch signaling pathway has been reported to be
involved in the acquisition of EMT (10).
Notch signaling is known to regulate a number of
cellular processes, including cell proliferation, apoptosis,
migration, invasion and angiogenesis (11). In addition, Notch expression has
been reported to be upregulated in a number of human malignancies
(12). However, the function of
Notch in the EMT processes of GC remain largely unknown. Therefore,
the focus of the present study was to determine the role of the
Notch1 signaling pathway in EMT.
Materials and methods
Human tissue specimens and cell
lines
The human tissue specimens, including 45 samples of
human GC (22 samples with metastasis and 23 samples without
metastasis) and 25 samples of adjacent normal mucosal tissues, were
collected from 70 patients who underwent surgery at the First and
Second Affiliated Hospital of Chongqing Medical University
(Chongqing, China) between 2011 and 2013. The study complied with
the regulations of the Ministry of Health, World Health
Organization Research Ethics Review Committee international
guidelines for research involving humans and the Declaration of
Helsinki on the Ethical Principles for Medical Research Involving
Human Subjects. In addition, written informed consent was obtained
from the patients prior to the procedures and Institutional Review
Board approval was granted from the First and Second Affiliated
Hospitals of Chongqing Medical University.
The human GC AGS cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA) and the MKN-45
and GES1 cell lines were purchased from the Type Culture Collection
of the Chinese Academy of Sciences (Shanghai, China). The cell
lines were cultured in RPMI-1640 (Hyclone, Logan, Utah, USA)
supplemented with 10% fetal bovine serum (FBS; Hyclone), and
maintained at 37°C in a humidified atmosphere of 5%
CO2/95% air. For the in vitro experiment, the
cells were treated with γ-secretase inhibitor DAPT (Sigma-Aldrich,
St. Louis, MO, USA) at a concentration of 10 μM (13) or with dimethyl sulfoxide (DMSO; as a
control) and analyzed after 72 h.
Immunoblotting
The total protein for the immunoblots was extracted
from the cell lines and tissue specimens using the
radioimmunoprecipitation assay lysis buffer (Beyotime, Shanghai,
China), according to the manufacturer’s instructions. Following the
quantification of the protein extracts in a bicinchoninic acid
protein assay, equivalent amounts of lysates were resolved using
10% SDS-polyacrylamide gel (Beyotime) electrophoresis and
transferred onto a polyvinylidene fluoride membrane (Beyotime). The
membrane was then blocked in 5% non-fat milk in Tris-buffered
saline (Beyotime) and Tween 20 (Beyotime) for 1 h at 4°C. The blots
were then incubated with primary antibodies and subsequently
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibodies. The signals were then detected by an enhanced
chemiluminescence reagent (Millipore, Billerica, MA, USA).
The rabbit monoclonal antibodies against vimentin,
E-cadherin and N-cadherin were purchased from Abcam (Cambridge,
UK); the mouse monoclonal antibodies against Snail and GAPDH were
purchased from BD Biosciences (Franklin Lakes, NJ, USA); and the
monoclonal HRP-conjugated goat anti-mouse and anti-rabbit IgG were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA)
The following antibody dilutions were used: 1:10,000
for anti-Hes1; 1:1,200 for anti-Notch1; 1:1,200 for anti-vimentin;
1:1,200 for anti-E-cadherin; 1:1,200 for anti-N-cadherin; 1:500 for
anti-Snail and anti-GAPDH; and 1:7,000 for HRP-conjugated IgG.
Quantitative polymerase chain reaction
(qPCR)
The RNA was purified from cell lines and tissue
specimens using RNAiso (Takara Bio, Inc., Shiga, Japan), and cDNA
was synthesized using a PrimeScript™ RT reagent kit (Takara Bio,
Inc.). The qPCR was performed using the CFX96™ Real-Time PCR
Detection system (Bio-Rad, Hercules, CA, USA) with SYBR®
Premix Ex Taq™ II (Takara Bio, Inc.). The PCR conditions used were
as follows: 95°C for 30 sec followed by 40 cycles of 95°C for 5 sec
and 60°C for 30 sec. The results were normalized against β-actin
RNA and the sequences of PCR primers used for each of the gene
transcripts were as follows: Sense, 5′-TGCCGAACCAATACAACCCTC-3′ and
anti-sense, 5′-TGGTAGCTCATCATCTGGGACA-3′ for Notch1; and sense,
5′-CCACGAAACTACCTTCAACTCC-3′ and anti-sense,
5′-GTGATCTCCTTCTGCATCCTGT-3′ for β-actin.
Cell viability
The cells were seeded into 96-well plates at a
density of 5×103 cells/well and incubated overnight.
Following the treatment with DAPT at a concentration of 10 μM or
with DMSO (as a control), the cells were incubated for 72 h. Next,
20 μl MTT solution (5 mg/ml) was added to the cultures (200 μl)
prior to a 4-h incubation at 37°C. Following the removal of the
culture medium, the remaining crystals were dissolved in DMSO and
the absorbance of the plates were read at 570 nm.
Clonality assays
For the colony formation assays, the cells treated
with DMSO and DAPT were seeded at a low density (1,000 cells/plate)
and cultured until visible colonies appeared. The colonies were
then stained with Giemsa and counted.
Migration and invasion assays
AGS and MKN45 (10×104 cells per 500 μl of
serum-free media) cells treated with DAPT and DMSO (as a control)
were added to the upper chambers, and the lower chambers were
filled with 750 μl of media containing 10% FBS. The cells were then
incubated for 24 h at 37°C in a humidified atmosphere of 5%
CO2 in a tissue culture incubator. After 24 h, the
non-migrated/invading cells were removed from the upper sides with
cotton-tipped swabs. The migrated/invaded cells on the lower sides
of the inserts were then stained and the absorbances were read at
560 nm, according to the manufacturer’s instructions.
Statistical analysis
All experiments were repeated three times and the
results were analyzed using SPSS 16.0 software (SPSS, Inc.,
Chicago, IL, USA). Data are presented as the mean ± standard
deviation and group comparisons were performed using Student’s
t-test and one-way analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
Notch1 expression is upregulated in GC
cell lines
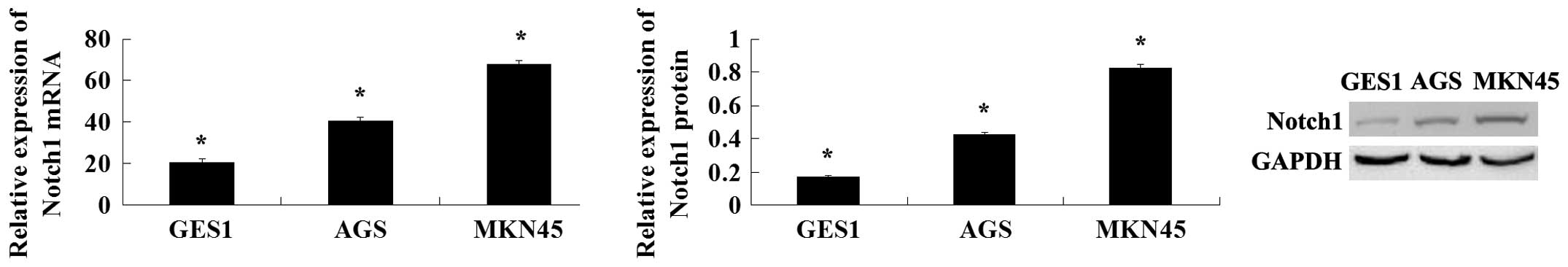
To explore the expression of Notch1 in human GC cell
lines, its expression was analyzed in two cancer cell lines (AGS
and MKN45) and in a normal gastric mucosa cell line (GES1). The
expression of Notch1 was found to increase in the GC cells compared
with the normal gastric mucosa cells. The AGS cells were derived
from non-metastatic tissue and the MKN45 cells were derived from
metastatic tissue, and Notch1 expression was increased in MKN45
cells compared with the AGS cells (Fig.
1). Thus, Notch1 may be important in GC.
Notch1 expression is upregulated in GC
tissues and increased Notch1 expression is associated with
metastatic GC
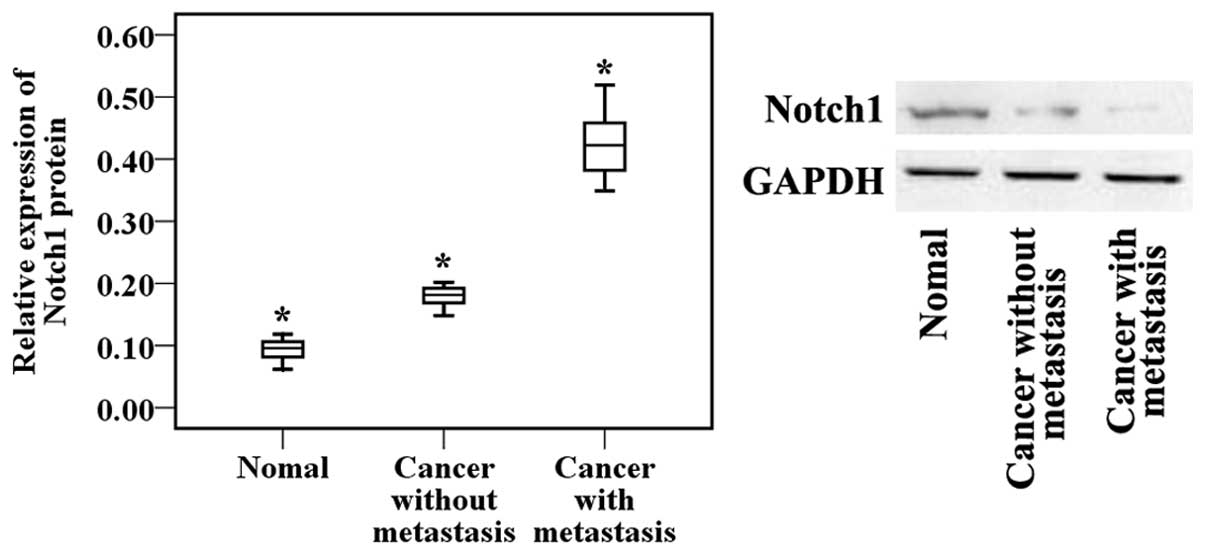
Therefore, to explore the role of Notch1 in human GC
development, its expression levels were detected in 45 human GC
tissue samples and 25 adjacent normal mucosa tissue samples.
According to the results of the western blot analysis, the
expression of Notch1 was significantly upregulated in the tumor
tissues compared with the adjacent normal mucosa tissues.
Furthermore, to explore whether Notch1 expression is associated
with the metastasis of GC, the Notch1 expression levels were
examined in 45 gastric tumor samples. These tumors were divided
into the following two groups: i) Tumors resected from 22 patients
with lymph node or distant organ metastases; and ii) tumors
resected from 23 patients without metastases. The western blot
analysis also demonstrated that the expression of Notch1 was
significantly increased in the patients with metastasis compared
with the patients without metastasis (Fig. 2). These results showed that the
Notch1 signaling pathway is involved in the development and
metastasis of GC.
γ-secretase inhibitor DAPT prevents the
Notch-induced proliferation, migration and invasion of GC cell
lines
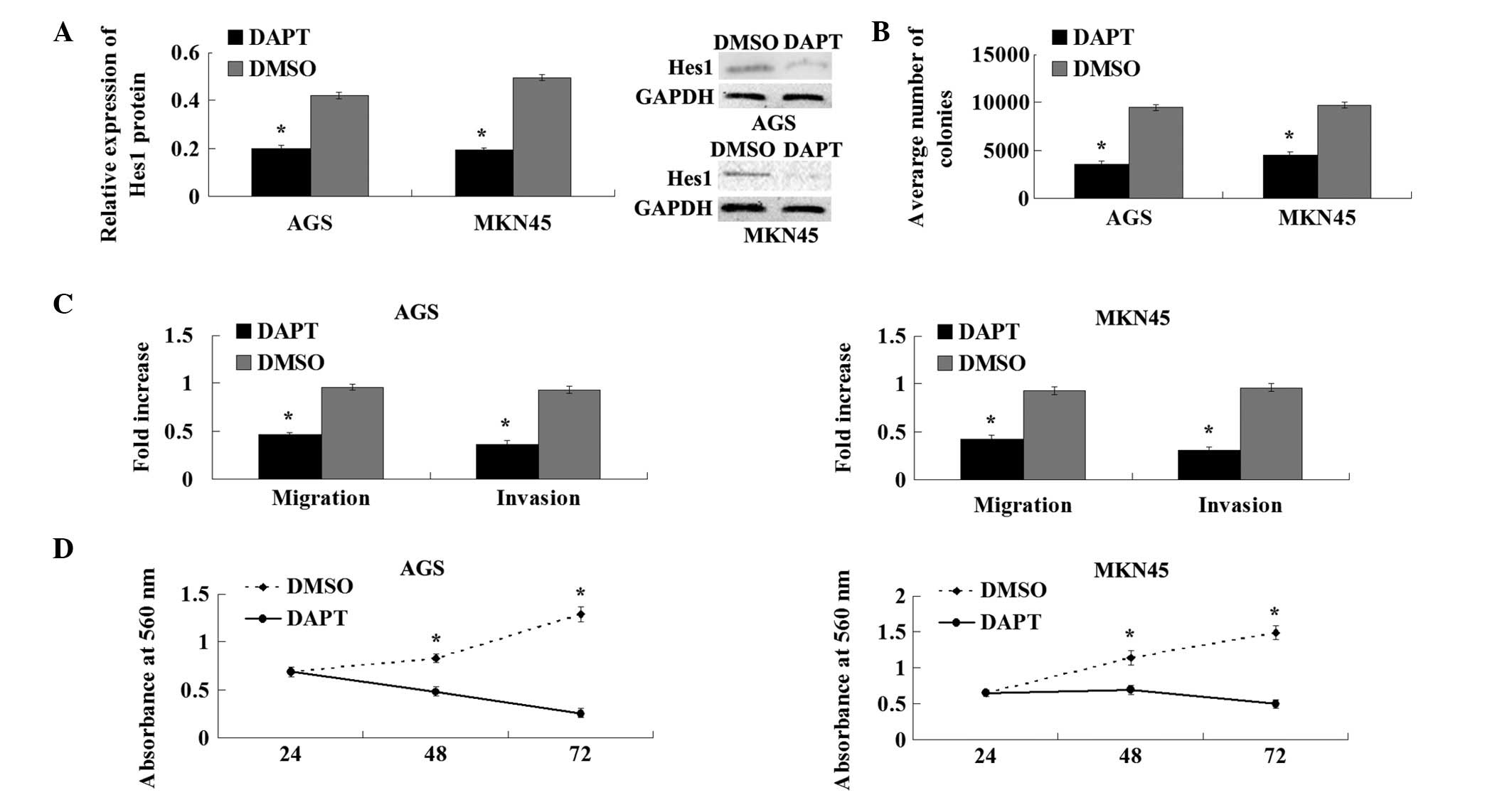
To explore the role of the Notch1 signaling pathway
in the development and progression of GC, AGS and MKN45 cells were
treated with DAPT. The western blot analysis showed that DAPT
treatment markedly suppresses the expression of the Notch1
downstream target, Hes1 (Fig. 3).
The colony-forming and proliferation abilities in the cells treated
with DAPT were also reduced significantly compared with the cells
treated with the DMSO control (Fig. 4A
and B). The Transwell migration and Matrigel invasion assays
demonstrated that DAPT reduces the migration and invasion
capacities of AGS and MKN45 cells (Fig.
4C). These results showed that γ-secretase inhibitor DAPT
suppresses the Notch1 signaling pathway and inhibits the
proliferation, migration and invasion of GC cell lines.
γ-secretase inhibitor DAPT prevents EMT
in GC cell lines
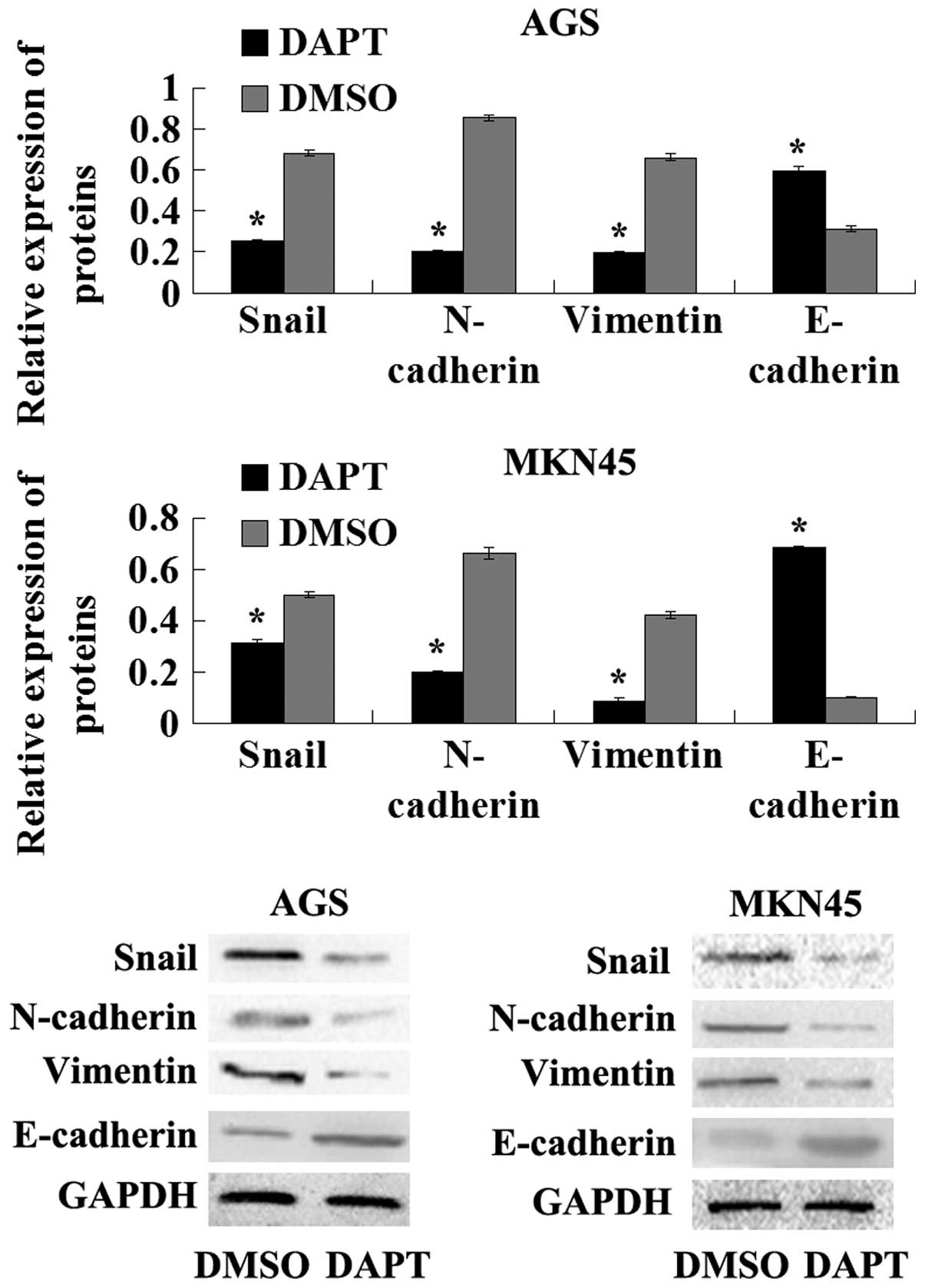
To further explore the molecular mechanism of the
inhibition of DAPT on EMT in human GC cell lines, the expression of
the epithelial marker, E-cadherin and mesenchymal markers, such as
vimentin, N-cadherin and Snail, were examined in AGS and MKN45
cells in the presence of DAPT or DMSO. The results of the western
blot analysis showed that the protein levels of N-cadherin,
vimentin and Snail were decreased in the cells treated with DAPT.
Furthermore, E-cadherin expression was upregulated in the cells
treated with DAPT compared with the cells treated with the DMSO
control. Overall, these results indicated that the γ-secretase
inhibitor DAPT impairs EMT in GC cells.
Discussion
Emerging evidence suggests that Notch receptors and
their ligands are upregulated in cervical, lung, colon, head and
neck and renal carcinomas, acute myeloid, Hodgkin’s and large-cell
lymphomas, and pancreatic cancer (14–16).
Furthermore, the high expression levels of Notch1 and its ligand,
Jagged-1, have been associated with a poor prognosis in breast
cancer, bladder cancer, leukemia, intrahepatic cholangiocarcinoma
and prostate cancer (17–22). In pancreatic cancer cell lines, the
activation of Notch1 signaling has been found to contribute to
invasion and metastasis by EMT (23,24).
In the present study, the expression of Notch1 was found to
increase in the GC AGS and MKN45 cell lines compared with the
normal gastric mucosa GES1 cell line. In addition, Notch1
expression was significantly higher in the tumor tissues than that
in the adjacent normal mucosa tissues, as well as in the metastatic
patients compared with the non-metastatic patients. The results
showed that Notch1 signaling correlates with the invasion and
metastasis of GC and are consistent with the results of several
studies that have been previously conducted (25).
The Notch genes encode proteins that are activated
by interacting with a family of ligands. Upon activation, Notch is
cleaved, releasing the intracellular domain of Notch (ICN) through
a cascade of proteolytic cleavages by the metalloprotease tumor
necrosis factor-α converting enzyme and the γ-secretase complex
(26,27). Therefore, inhibiting the γ-secretase
function is likely to prevent the cleavage of the Notch receptor
and block the Notch signaling pathway. Furthermore, in pancreatic
cancer cell lines, the inhibition of Notch1 signaling prevents
migration and invasion (13). In
the present study, following treatment of GC cell lines with the
γ-secretase inhibitor DAPT, the expression of the Notch1 target
gene, Hes1, was significantly decreased, E-cadherin was upregulated
and mesenchymal proteins, such as N-cadherin and vimentin, were
downregulated. In addition, the inhibition of Notch1 signaling with
DAPT significantly decreased the colony formation, migration and
invasion of GC cell lines compared with the cells treated with the
DMSO control.
A crucial step in impairing GC cell migration and
invasion through the inhibition of Notch1 signaling may be the
downregulation of E-cadherin expression during the acquisition of
the EMT phenotype, which reduces cell-cell adhesion and
destabilizes the epithelial architecture. Furthermore, E-cadherin
gene repression has been attributed to the function of Snail, which
is activated during the acquisition of EMT. Snail may bind to the
two E-boxes of the E-cadherin promoter and function as a repressor
of E-cadherin expression (28).
Therefore, any biological processes that trigger Snail
overexpression are likely to downregulate E-cadherin expression,
leading to the acquisition of EMT. The effects of Notch1 on
E-cadherin expression are mediated through ICN via the regulation
of Snail expression. In addition, it has been reported that the
overexpression of Notch-1 induces Snail expression, which yields
attenuated E-cadherin expression and the acquisition of EMT.
Therefore, the γ-secretase inhibitor DAPT can inhibit this process
(29). In the present study, the
expression of Hes1 was also found to significantly decrease
following treatment with the γ-secretase inhibitor DAPT. In
addition, Snail expression was downregulated and EMT was impaired
in cells treated with DAPT.
In conclusion, the present study identified that the
Notch1 signaling pathway is closely associated with the growth,
invasion and metastasis of GC. Furthermore, the results
demonstrated that the suppression of Notch1 with the γ-secretase
inhibitor DAPT restrains the growth, invasion and metastasis of GC
by inhibiting EMT.
Acknowledgements
The authors would like to thank the surgeons and
nurses at the Department of General Surgery of the First and Second
Affiliated Hospital of Chongqing Medical University for providing
the gastric specimens. The authors would also like to thank
Xiao-Qiu Xiao at the Institutes for Biological Science, Chongqing
Medical University for useful suggestions, incisive comments and
constructive criticism, which have greatly contributed to the
completion of the study.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Cano CE, Motoo Y and Iovanna JL:
Epithelial-to-mesenchymal transition in pancreatic adenocarcinoma.
ScientificWorldJournal. 10:1947–1957. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang H, Liu L, Wang Y, Zhao G, Xie R, Liu
C, Xiao X, Wu K, Nie Y, Zhang H and Fan D: KLF8 involves in
TGF-beta-induced EMT and promotes invasion and migration in gastric
cancer cells. J Cancer Res Clin Oncol. 139:1033–1042. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Le Bras GF, Taubenslag KJ and Andl CD: The
regulation of cell-cell adhesion during epithelial-mesenchymal
transition, motility and tumor progression. Cell Adh Migr.
6:365–373. 2012.PubMed/NCBI
|
|
5
|
Lim S, Becker A, Zimmer A, Lu J, Buettner
R and Kirfel J: SNAI1-mediated epithelial-mesenchymal transition
confers chemoresistance and cellular plasticity by regulating genes
involved in cell death and stem cell maintenance. PLoS One.
8:e665582013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Q, Hou X, Xia J, Qian X, Miele L,
Sarkar FH and Wang Z: Emerging roles of PDGF-D in EMT progression
during tumorigenesis. Cancer Treat Rev. 39:640–646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomson S, Petti F, Sujka-Kwok I, Mercado
P, Bean J, Monaghan M, Seymour SL, Argast GM, Epstein DM and Haley
JD: A systems view of epithelial-mesenchymal transition signaling
states. Clin Exp Metastasis. 28:137–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG and
Weiss SJ: Canonical Wnt signaling regulates Slug activity and links
epithelial-mesenchymal transition with epigenetic Breast Cancer 1,
Early Onset (BRCA1) repression. Proc Natl Acad Sci USA.
109:16654–16659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du R, Sun W, Xia L, Zhao A, Yu Y, Zhao L,
Wang H, Huang C and Sun S: Hypoxia-induced down-regulation of
microRNA-34a promotes EMT by targeting the Notch signaling pathway
in tubular epithelial cells. PLoS One. 7:e307712012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Li Y, Kong D and Sarkar FH: The
role of Notch signaling pathway in epithelial-mesenchymal
transition (EMT) during development and tumor aggressiveness. Curr
Drug Targets. 11:745–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim TH and Shivdasani RA: Notch signaling
in stomach epithelial stem cell homeostasis. J Exp Med.
208:677–688. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Penton AL, Leonard LD and Spinner NB:
Notch signaling in human development and disease. Semin Cell Dev
Biol. 23:450–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palagani V, El Khatib M, Kossatz U, Bozko
P, Müller MR, Manns MP, Krech T, Malek NP and Plentz RR: Epithelial
mesenchymal transition and pancreatic tumor initiating CD44+/EpCAM+
cells are inhibited by γ-secretase inhibitor IX. PLoS One.
7:e465142012.
|
|
14
|
Miele L, Miao H and Nickoloff BJ: NOTCH
signaling as a novel cancer therapeutic target. Curr Cancer Drug
Targets. 6:313–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Banerjee S, Li Y, Rahman KM, Zhang
Y and Sarkar FH: Down-regulation of Notch-1 inhibits invasion by
inactivation of nuclear factor-κB, vascular endothelial growth
factor, and matrix metalloproteinase-9 in pancreatic cancer cells.
Cancer Res. 66:2778–2784. 2006.
|
|
16
|
Wang Z, Zhang Y, Banerjee S, Li Y and
Sarkar FH: Inhibition of nuclear factor kappab activity by
genistein is mediated via Notch-1 signaling pathway in pancreatic
cancer cells. Int J Cancer. 118:1930–1936. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi TP, Xu H, Wei JF, Ai X, Ma X, Wang BJ,
Ju ZH, Zhang GX, Wang C, Wu ZQ and Zhang X: Association of low
expression of notch-1 and jagged-1 in human papillary bladder
cancer and shorter survival. J Urol. 180:361–366. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reedijk M, Pinnaduwage D, Dickson BC,
Mulligan AM, Zhang H, Bull SB, O’Malley FP, Egan SE and Andrulis
IL: JAG1 expression is associated with a basal phenotype and
recurrence in lymph node-negative breast cancer. Breast Cancer Res
Treat. 111:439–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dickson BC, Mulligan AM, Zhang H, Lockwood
G, O’Malley FP, Egan SE and Reedijk M: High-level JAG1 mRNA and
protein predict poor outcome in breast cancer. Mod Pathol.
20:685–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu YM, Zhao WL, Fu JF, Shi JY, Pan Q, Hu
J, Gao XD, Chen B, Li JM, Xiong SM, et al: NOTCH1 mutations in
T-cell acute lymphoblastic leukemia: prognostic significance and
implication in multifactorial leukemogenesis. Clin Cancer Res.
12:3043–3049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma D, Dong X, Zang S, Ma R, Zhao P, Guo D,
Dai J, Chen F, Ye J and Ji C: Aberrant expression and clinical
correlation of Notch signaling molecules in breast cancer of
Chinese population. Asia Pac J Clin Oncol. 7:385–391. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou Q, Wang Y, Peng B, Liang L and Li J:
The roles of Notch1 expression in the migration of intrahepatic
cholangiocarcinoma. BMC Cancer. 13:2442013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ni X, Long J, Cen P, Chen L, Yang J and Li
M: Pancreatic cancer tumour initiating cells: the molecular
regulation and therapeutic values. J Cell Mol Med. 16:988–994.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Ahmad A, Li Y, Azmi AS, Miele L
and Sarkar FH: Targeting notch to eradicate pancreatic cancer stem
cells for cancer therapy. Anticancer Res. 31:1105–1113.
2011.PubMed/NCBI
|
|
25
|
Brzozowa M, Mielańczyk L, Michalski M,
Malinowski L, Kowalczyk-Ziomek G, Helewski K, Harabin-Słowińska M
and Wojnicz R: Role of Notch signaling pathway in gastric cancer
pathogenesis. Contemp Oncol (Pozn). 17:1–5. 2013.PubMed/NCBI
|
|
26
|
Mori M, Miyamoto T, Yakushiji H, Ohno S,
Miyake Y, Sakaguchi T, Hattori M, Hongo A, Nakaizumi A, Ueda M and
Ohno E: Effects of N-[N-(3,
5-difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester
(DAPT) on cell proliferation and apoptosis in Ishikawa endometrial
cancer cells. Hum Cell. 25:9–15. 2012.
|
|
27
|
Subramaniam D, Ponnurangam S, Ramamoorthy
P, Standing D, Battafarano RJ, Anant S and Sharma P: Curcumin
induces cell death in esophageal cancer cells through modulating
Notch signaling. PLoS One. 7:e305902012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Becker KF, Rosivatz E, Blechschmidt K,
Kremmer E, Sarbia M and Höfler H: Analysis of the E-cadherin
repressor Snail in primary human cancers. Cells Tissues Organs.
185:204–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saad S, Stanners SR, Yong R, Tang O and
Pollock CA: Notch mediated epithelial to mesenchymal transformation
is associated with increased expression of the Snail transcription
factor. Int J Biochem Cell Biol. 42:1115–1122. 2010. View Article : Google Scholar : PubMed/NCBI
|