Introduction
There are two types of stem cell in the human bone
marrow: Hematopoietic stem cells (HSCs) and mesenchymal stem cells
(MSCs). HSCs primarily maintain hematopoietic functions, while MSCs
have the ability to differentiate into various mesoderm- and
neuroectoderm-derived cells. MSCs are multipotent stromal cells
with high proliferative ability that can differentiate into a
variety of cell types, including vascular endothelial cells, islet
cells and hepatocytes, under certain conditions. Autologous MSCs
are considered ideal seed cells, since they can be conveniently
obtained without ethical issues and are characterized by limited
rejection and marked proliferative ability. With the development of
cell and tissue engineering, MSCs, as cell sources in cell and gene
therapy as well as tissue engineering, are a key area of study
(1,2). In 2002, bone marrow HSC
transplantation was applied in the treatment of peripheral
angiopathy for the first time (3,4). It
has been demonstrated in animal experiments that autologous bone
marrow mononuclear cells implanted in ischemic limbs can
differentiate into vascular endothelial cells to promote local
angiogenesis, suggesting that stem cell transplantation can be used
for the treatment of ischemic limb diseases and severe skin defects
in cases of diabetes mellitus (DM) (5–7).
Skin is an important part of the body in that it
maintains homeostasis and acts as a barrier to the invasion of
microorganisms and harmful substances. However, various injuries,
including trauma, war wounds, and burn and earthquake injuries
often cause extensive skin defects, resulting in bacterial invasion
and reproduction, which may also induce water and electrolyte
disturbance, septic shock, multiple organ dysfunction and
mortality. Currently, auto-skin grafting is the main approach to
treating extensive burns or skin defects following trauma (8). However, such a method inevitably
results in complications through causing trauma to the normal skin.
It is therefore urgent that the methods of repairing extensive skin
defects are improved.
DM is a chronic metabolic disorder. Difficulty in
skin wound healing and skin defects are the most common chronic
complications of diabetes. Diabetic foot, as a representative
complication, causes disability as a result of amputation. Although
numerous hypotheses have been presented, the mechanism of the
development of refractory skin wounds under hyperglycemic
conditions remains unclear. Oxidative stress (OS) is considered an
important common mechanism (9).
However, wound healing is a complicated process involving three
stages of inflammatory reactions, cell proliferation, tissue
maturity and reconstruction. In DM, nonenzymatic glycosylation
accelerates, and the level of glycoxidation end-product increases
at advanced stages, which interferes with endothelial
cell-leukocyte interactions, inhibits the functions of
monocytes/macrophages, reduces cytokine secretion capacity and
prolongs the duration of infiltration of these cells in the wound.
This in itself can lead to a refractory skin defect (10).
As comprehensive therapies, including conventional
anti-infection therapy, wound debridement and dressing changes, and
glucose-lowering therapy were ineffective for treatment of
postoperative wound infection in the hand in the present report, an
extended amputation, right hand amputation or local autologous skin
transplant for the skin defect was recommended. However, the
patient refused these recommendations being concerned that new skin
defects would arise as a result of harvesting skin grafts from
healthy and intact skin. The patient chose to undergo autologous
stem cell transplantation on the understanding that this option
would cause minimal injury and pain. Written, informed consent was
obtained from the patient prior to autologous bone marrow MSC
transplantation on the skin defect of the right hand in August,
2010. The clinical efficacy and complications, including
transplantation-associated rejection reactions and tumorigenicity
of transplanted autologous bone marrow MSCs, were investigated for
a period of 18 months. This program was approved by the Scientific
and Ethics Committee of Sichuan University (Chengdu, China).
Case report
Case summary
A 64-year-old male farmer was admitted to the
Department of Hand and Foot Surgery at the People’s Liberation Army
520 Hospital (Mianyang, China) on July 22nd, 2010 complaining of
elevated blood glucose levels for three years and right hand pain
and swelling following trauma for longer than half a month. The
patient was diagnosed with DM at a local hospital three years
previously, but had not adhered to regular treatment due to
personal financial problems. Therefore, blood glucose levels had
remained largely unchecked. Notes from a previous hospital visit
indicated that the random blood glucose level was 16.08 mmol/l and
percentage glycosylated hemoglobin was 12.7%. Approximately half a
month previously, the patient had accidentally cut his right hand
at work, producing a non-healing wound resulting in the back of the
right hand becoming swollen and painful, together with infection,
necrosis and a large defect in the skin of the root of the right
forefinger. Debridement and sutures at a local hospital achieved
unsatisfactory results, and the case was transferred to the
People’s Liberation Army 520 Hospital. On admission, a 5.0×3.0
cm-deep skin defect in the metacarpophalangeal joint of the
forefinger and back of the right hand back with flavescent fishy
effusions was observed. The bone was exposed and visible through
the defect, the tendon was ruptured and the distal tendon was pale.
Poor local blood supply was observed, and the patient was suffering
from local numbness. The defect was irregular and the contusion was
severely contaminated. The case was preliminarily diagnosed as
wound infection following trauma, vascular, nerve and tendon
injuries and complex tissue defects in the right hand, and DM and
diabetic nephropathy. Following intensive insulin pump therapy,
anti-infection therapy and local wound debridement and dressing
changes for one week, blood glucose became well controlled
(Table I) but the local wound did
not improve. Therefore, amputation of the right forefinger was
performed on August 7th, 2010. Following surgery, the surgical
wound in the right hand developed suppuration and infection and was
poorly healed, with a size of ~5.0×3.0 cm. As the condition had not
improved following comprehensive therapy, including anti-infection
therapy, wound dressing changes and glucose-lowering therapy, the
case was transferred the Diabetic Centre of Control and Prevention
for further treatment. After group consultation and discussion, a
choice of extended amputation, right hand amputation or local
autologous skin grafting was recommended. However, as discussed
previously, the patient refused these recommendations and instead
opted for autologous MSC transplantation. On August 5th, 2010,
patient bone marrow was harvested under local anesthesia and MSCs
were separated, cultured and amplified in vitro for 14 days.
The third generation of MSCs was prepared as a stem cell suspension
with a concentration of 6×106 cells/ml. On August 19th,
2010, autologous bone marrow MSC transplantation was performed
after the patient provided written, informed consent. No common
complications or adverse reactions associated with transplantation
were observed. On day 10 after surgery, the patient was completely
healed and was discharged from hospital. Before and after
autologous stem cell transplantation, blood glucose levels had
almost reached the standard range (Table I).
 | Table IBlood glucose levels during two
hospital admissions. |
Table I
Blood glucose levels during two
hospital admissions.
| | Blood glucose level,
mmol/l |
|---|
| |
|
|---|
| Date | Diabetes mellitus
treatment protocol | Prior to
breakfast | 2 h after
breakfast | 2 h after lunch | 2 h after supper | 2:00 AM |
|---|
| 2010-07-29a | Intensive insulin
pump therapy (Humalog at 63 U/day) | 5.2 | 8.5 | 8.7 | 7.1 | 5.2 |
| 2010-08-12a | Humalog Mix 50 (16,
12 and 14 units) | 6.1 | 5.2 | 10.5 | 7.8 | 8.8 |
| 2010-08-19b | Humalog Mix 50 (16,
13 and 14 units) | 4.8 | 10.4 | 8.8 | 8.7 | 8.8 |
| 2010-08-20c | Humalog Mix 50 (16,
12 and 14 units) | 5.7 | 6.7 | 8.1 | 9.3 | 5.5 |
| 2010-09-02d | Humalog Mix 50 (16,
14 and 14 units) | 5.7 | 8.1 | 7.9 | 7.9 | 7.1 |
| 2012-02-29e | Metformin (500 mg, 3
times/day) | 7.0 | 16.4 | 12.7 | 11.8 | Unmeasured |
| 2012-03-05e | Metformin (850 mg, 3
times/day) | 6.1 | 10.0 | 8.1 | 9.1 | 8.9 |
Reagents
Lymphocyte separation medium was purchased from
Shanghai Qcbio Science & Technologies Co., Ltd. (Shanghai,
China). Xylene and hematoxylin and eosin (H&E) stain were
purchased from Shanghai Source Leaf Biological Technology Co., Ltd.
(Shanghai, China). Complete Dulbecco’s modified Eagle’s medium and
fetal bovine serum were purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). Penicillin and streptomycin were purchased
from Nanjing KeyGEN Biotech Co., Ltd. (Nanjing, China).
Collection of autologous bone marrow
Following completion of pre-operative examinations,
the patient fasted on the morning of the surgery. The patient was
placed in a prone position and conventional disinfection and
sterilization procedures were followed. Blood bags and storage
solution were prepared. Bone marrow was collected from the
bilateral posterior superior iliac spine under local anesthesia.
Approximately 10 ml bone marrow was collected from each of three
sites at 1-cm intervals along the lateral superior edge of the
ilium (a total of 30 ml bone marrow).
Separation of bone marrow MSCs
The collected bone marrow samples were diluted with
twice the volume of physiological saline on a laminar flow cabinet,
and the resulting suspension was transferred to a lymphocyte
separation medium (at a ratio of 2:1). The obtained solution was
centrifuged at 640–800 × g for 20 min at 20°C. The white membrane
layer in the 15 ml centrifuge tube was gently transferred to a
clean Eppendorf tube, washed twice with double the volume of
phosphate buffer solution (pH 7.2–7.4), and centrifuged at 450 × g
for 10 min. Mononuclear cells were separated, amplified in
vitro for 14 days and passaged to the third generation. The
precipitate was diluted with 30 ml physiological saline to produce
a stem cell suspension containing 6×106 cells/ml. Of
this suspension, 1 μl was used to count cells and observe
the cell morphology under a microscope.
Autologous bone marrow MSC
transplantation
Autologous bone marrow MSC transplantation was
performed under strictly aseptic conditions in the operating room.
The infection site on the right hand was routinely debrided and the
MSC suspension was transplanted into the wound by injection of
0.3–0.5 ml in each site at 1.0-cm intervals. The injection depth
was 0.5–1.0 cm. Following injection, the wound was protected with
sterile gauzes and bandages.
Observation of wound healing
The wound surface of the skin defect prior to
surgery and during recovery was recorded using a digital camera,
and any presence of rejection reactions, hand inflammation and
swelling was recorded.
H&E staining
Skin tissues were surgically collected, fixed in 4%
paraformaldehyde overnight, dehydrated with a series of ethanol
solutions, embedded in paraffin and cut into sections. The sections
were deparaffinized with xylene, rehydrated, stained with H&E
stain and enveloped. The pathological changes of the skin were
observed using the Olympus BX41 microscope (Olympus Corporation,
Tokyo, Japan).
Observations following
transplantation
The pain, coldness and numbness in the right hand
improved 24 h after transplantation. After 48 h, the purulent
secretions from the wound had clearly decreased, local swelling
alleviated, local ischemia gradually improved and the wound shrank
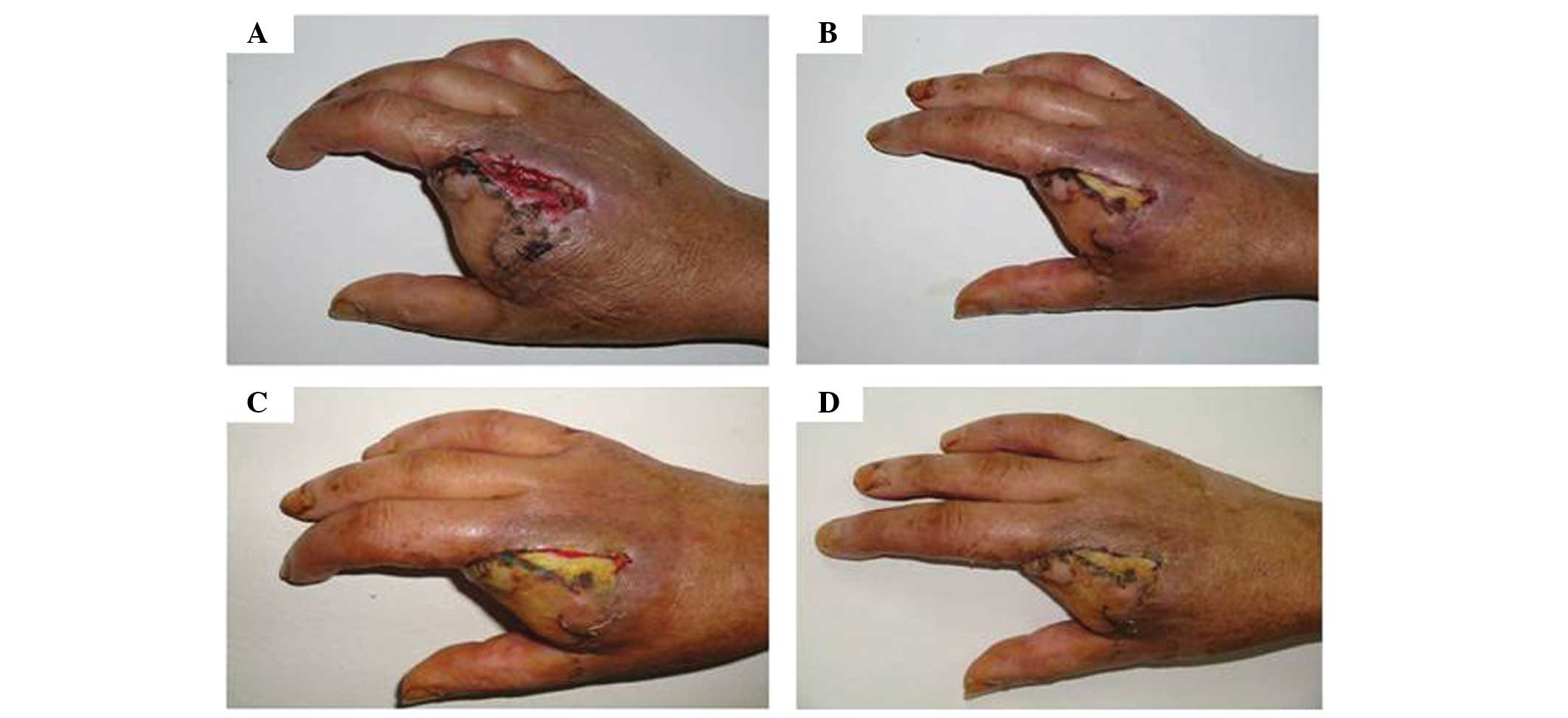
from 5.0×3.0 cm to 4.0×1.0 cm (Fig. 1A
and B). After 96 h, the wound was 4.0×0.5 cm in size (Fig. 1C) and local ischemia gradually
improved. After 192 h, the wound had completely healed (Fig. 1D). Therefore, following autologous
bone marrow MSC transplantation, the injection puncture wounds
healed rapidly. No local abnormal symptoms or signs were observed,
and blood, urine and stool tests, and biochemical examinations
(including liver and renal function tests) revealed no systemic
abnormalities.
The patient was readmitted to the People’s
Liberation Army 520 Hospital for examination 18 months after
surgery. The local condition of the hand that underwent autologous
bone marrow MSC transplantation was stable and no
transplantation-associated rejection reactions had occurred. A
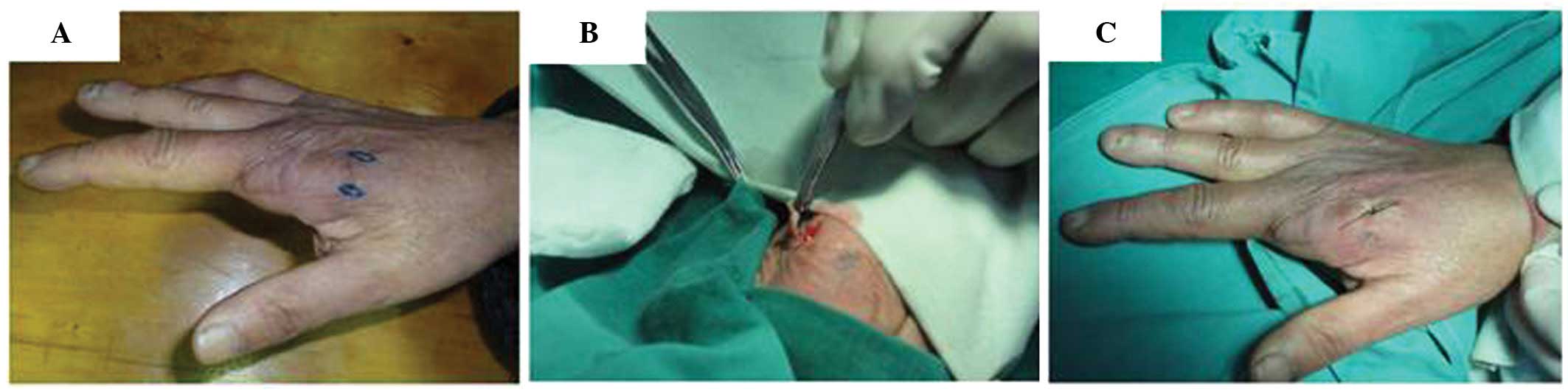
sample of local full-thickness skin tissue, at a size of
0.7×0.3×0.2 cm, was cut from the healed wound (Fig. 2) for pathological examination.
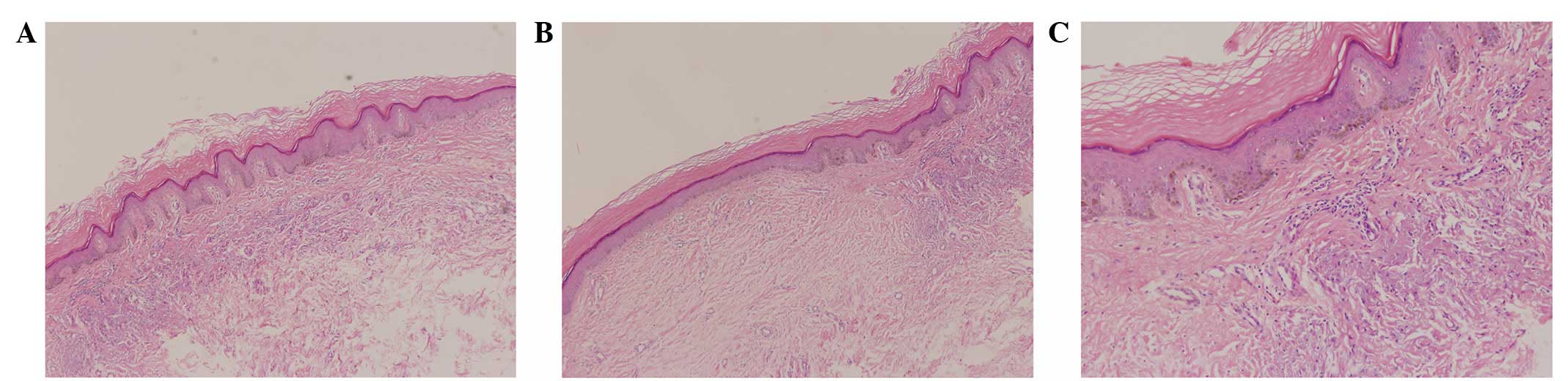
H&E staining revealed mild epidermal hyperplasia with
hyperkeratosis, increased pigment in local basal cells, hyperplasia
and degeneration of collagen fibers, and minor infiltration of
lymphocytes in the small perivascular regions of the superficial
dermis. These observations are not significantly different from the
pathology of normal skin tissues. In addition, there was no
evidence of tumor formation (Fig.
3).
Discussion
It has been demonstrated that skin constructed by
MSCs used as seed cells can significantly promote healing of skin
defects. Such wounds possess full-thickness skin structures
following repair (11–13). During the process of wound healing,
MSCs are closely involved in the formation of small blood vessels
in the granulation tissue (14).
MSCs can differentiate into vascular endothelial cells and are
involved in repair of the skin defect in the wound
micro-environment. It has been reported that MSCs may also be used
for treatment of ischemic diseases (15–18).
In a study by Subrammaniyan et al (19), injection of bone marrow-derived
mononuclear cells had satisfactory efficacy in the treatment of six
cases of DM with critical limb ischemia and skin defects, and
amputation was avoided in all patients undergoing autologous bone
marrow-derived mononuclear cell injection.
However, it is debated whether MSCs transplanted
into the body may induce various gene mutations resulting in
infinite cell proliferation and growth similar to tumor cells or
even induce tumorigenesis (20–22).
It has been demonstrated that embryonic stem cells (ESCs) isolated
from rodents and humans are very similar to embryonal carcinoma
cells, and their potent tumorigenicity has the potential to lead to
teratomas (23). Stem cell
tumorigenicity is the key obstacle to the safe use of stem
cell-based regenerative therapies. Although certain adult stem cell
therapies appear to be safe, they have only a narrow range of
application in human disease. Human induced pluripotent stem cells
are predicted to possess tumorigenic potential equal to or greater
than that of ESCs (23).
Based on the aforementioned issues surrounding stem
cell tumorigenicity, further follow-up observation and clinical
study were conducted for the present patient. Pathological
examinations of the full-thickness skin tissues from the healed
wound revealed no significant difference from the pathology of the
normal skin tissue, and no tumor formation was identified (Fig. 3). Thus, we hypothesize that
autologous transplantation of MSCs amplified in vitro may be
a novel, simple and effective approach to the treatment of severe
skin defects and infection. In addition, such therapy presents a
solution to the problems of severe wound infection and poor local
blood supply in DM without transplantation-associated rejection and
tumor formation, thereby achieving the goals of treatment. In
addition, the present study provides a novel method for the
treatment of other skin defects caused by various traumas,
including burns, knife wounds and earthquake injuries, using
autologous MSC transplantation in clinical practice.
The identification and study of stem cells is a
promising field in biomedicine. However, it has been reported that
the tissues grown from skin-derived autologous stem cells may still
be rejected by the immune system (24). Consequently, the application of
autologous MSC transplantation in clinical treatment requires
further studies, and there is a great need to investigate
transplant-associated rejection reactions, tumorigenicity and the
long-term efficacy of autologous MSC transplantation. The
methodology of autologous MSC transplantation in the treatment of
skin defects induced by various traumas should be improved, and
high-quality, multicenter, randomized, double-blind and
placebo-controlled trials are required to demonstrate the clinical
efficacy of this novel therapy. In addition, certain aspects in
particular should be noted. Firstly, the dose of MSCs, observation
duration and data units should be standardized and unified, and a
widely recognized criteria for assessment of therapeutic efficacy
should be employed as far as possible. Additionally, besides the
observation of the size of the wound, it is also important that the
expanded MSCs be labeled with bromodeoxyuridine (BrdU) prior to
transplantation. Full-thickness skin from the wound and the healed
skin should be incised between two and 12 weeks after surgery for
H&E staining and BrdU immunohistochemistry to pathologically
compare the two. Furthermore, observation reports of adverse
reactions and tumorigenesis should be normalized and standardized,
and the follow-up period should be extended so that the long-term
efficacy may be assessed. In addition, the negative results of the
clinical trials should be emphasized and more attention to the
ethical issues concerning stem cell therapy should be paid.
Finally, the differentiation mechanism and induction conditions of
MSCs, and the mechanism underlying their efficacy in the treatment
of skin defects, remain unclear (13). It is speculated that MSCs can
differentiate into vascular endothelial cells in a wound
micro-environment and be further involved in wound repair and
promote angiogenesis (25).
Additional animal experiments and basic studies are required to
observe the transition of MSCs to endothelial cells in local
transplantation regions, the release of multiple cytokines in the
local region and signal transduction (26).
The novel therapy presented in the current study may
solve the current problems of severe wound infection and poor local
blood supply in DM, without transplantation-related rejection
reactions and tumor formation, thereby achieving the goals of
treatment.
References
|
1
|
Gu YQ: Determination of amputation level
in ischaemic lower limbs. ANZ J Surg. 74:31–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamamoto K, Kondo T, Suzuki S, et al:
Molecular evaluation of endothelial progenitor cells in patients
with ischemic limbs: therapeutic effect by stem cell
transplantation. Arterioscler Thromb Vasc Biol. 24:e192–e196. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inaba S, Egashira K and Komori K:
Peripheral-blood or bone marrow mononuclear cells for therapeutic
angiogenesis? Lancet. 360:2083author reply 2084–2002.
|
|
4
|
Tateishi-Yuyama E, Matsubara H, Murohara
T, et al: Therapeutic angiogenesis for patients with limb ischaemia
by autologous transplantation of bone marrow cells: a pilot study
and a randomised controlled trial. Lancet. 360:427–435. 2002.
View Article : Google Scholar
|
|
5
|
Hershey JC, Baskin EP, Glass JD, Hartman
HA, Gilberto DB, Rogers IT and Cook JJ: Revascularization in the
rabbit hindlimb: dissociation between capillary sprouting and
arteriogenesis. Cardiovasc Res. 49:618–625. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heil M, Ziegelhoeffer T, Mees B and
Schaper W: A different outlook on the role of bone marrow stem
cells in vascular growth: bone marrow delivers software not
hardware. Circ Res. 94:573–574. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chappell DC, Varner SE, Nerem RM, Medford
RM and Alexander RW: Oscillatory shear stress stimulates adhesion
molecule expression in cultured human endothelium. Circ Res.
82:532–539. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coruh A and Yontar Y: Application of
split-thickness dermal grafts in deep partial- and full-thickness
burns: a new source of auto-skin grafting. J Burn Care Res.
33:e94–e100. 2012.PubMed/NCBI
|
|
9
|
Wlaschek M and Scharffetter-Kochanek K:
Oxidative stress in chronic venous leg ulcers. Wound Repair Regen.
13:452–461. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishida S, Segawa T, Murai I and Nakagawa
S: Long-term melatonin administration reduces hyperinsulinemia and
improves the altered fatty-acid compositions in type 2 diabetic
rats via the restoration of Delta-5 desaturase activity. J Pineal
Res. 32:26–33. 2002. View Article : Google Scholar
|
|
11
|
Bickenbach JR and Chism E: Selection and
extended growth of murine epidermal stem cells in culture. Exp Cell
Res. 244:184–195. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oshima H, Rochat A, Kedzia C, Kobayashi K
and Barrandon Y: Morphogenesis and renewal of hair follicles from
adult multipotent stem cells. Cell. 104:233–245. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taylor G, Lehrer MS, Jensen PJ, Sun TT and
Lavker RM: Involvement of follicular stem cells in forming not only
the follicle but also the epidermis. Cell. 102:451–461. 2000.
View Article : Google Scholar
|
|
14
|
Fang LJ, Fu XB, Sun TZ, et al: An
experimental study on the differentiation of bone marrow
mesenchymal stem cells into vascular endothelial cells. Zhonghua
Shao Shang Za Zhi. 19:22–24. 2003.(In Chinese).
|
|
15
|
Bjornson CR, Rietze RL, Reynolds BA, Magli
MC and Vescovi AL: Turning brain into blood: a hematopoietic fate
adopted by adult neural stem cells in vivo. Science.
283:534–537. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mezey E, Chandross KJ, Harta G, Maki RA
and McKercher SR: Turning blood into brain: cells bearing neuronal
antigens generated in vivo from bone marrow. Science.
290:1779–1782. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Isner JM and Asahara T: Angiogenesis and
vasculogenesis as therapeutic strategies for postnatal
neovascularization. J Clin Invest. 103:1231–1236. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Subrammaniyan R, Amalorpavanathan J,
Shankar R, et al: Application of autologous bone marrow mononuclear
cells in six patients with advanced chronic critical limb ischemia
as a result of diabetes: our experience. Cytotherapy.
13:993–999
|
|
20
|
Yamanaka S: Strategies and new
developments in the generation of patient-specific pluripotent stem
cells. Cell Stem Cell. 1:39–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Huso DL, Harrington J, Kellner J,
Jeong DK, Turney J and McNiece IK: Outgrowth of a transformed cell
population derived from normal human BM mesenchymal stem cell
culture. Cytotherapy. 7:509–519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Shi L, Zhang L, Li R, Liang J, Yu
W, Sun L, Yang X, Wang Y, Zhang Y and Shang Y: The molecular
mechanism governing the oncogenic potential of SOX2 in breast
cancer. J Biol Chem. 283:17969–17978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knoepfler PS: Deconstructing stem cell
tumorigenicity: a roadmap to safe regenerative medicine. Stem
Cells. 27:1050–1056. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hayden EC: Stem cells: The growing pains
of pluripotency. Nature. 473:272–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan J, Tie G, Xu TY, Cecchini K and
Messina LM: Mesenchymal stem cells as a treatment for peripheral
arterialdisease: current status and potential impact of type
IIdiabetes on their therapeuticefficacy. Stem Cell Rev. 9:360–372.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams AR and Hare JM: Mesenchymal stem
cells: biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar
|