Introduction
Hepatocellular carcinoma (HCC) is the most common
tumor that is highly aggressive and has a high recurrence (1). It is estimated that the majority of
HCCs in China develop as a consequence of chronic infection with
hepatitis B virus and arise in fibrotic or cirrhotic livers
(2). However, early diagnosis of
HCC remains a challenge, as the majority of patients have no
symptoms in the early stage. Thus, there is a prominent requirement
for oncology imaging modalities or biomarkers capable of
identifying early-stage tumors, and signs of tumor progression and
recurrence of HCC (3).
It is known that Toll-like receptors (TLRs) play
prominent roles in inflammatory responses against pathogen
infection. These receptors are primarily expressed on innate immune
cells and recognize conserved pathogen-associated molecular
patterns (4). TLR-expressing cells
represent the first line of defense sensing pathogen invasion,
triggering innate immune responses and subsequently initiating
antigen-specific adaptive immunity. In addition to microbial
molecules, TLRs can also recognize specific endogenous ligands,
including heat shock proteins or fragments of extracellular matrix
proteins (5,6). Current advancement in cancer
immunobiology highlights these receptors as crucial actors involved
in tumor growth and progression (7), while it was found that various TLRs
exhibit either antitumor or protumor activities (8,9).
Previously, much attention has been paid to
investigating the role of TLR5 in cancer progression and metastasis
(10). Current studies show that
TLR5 is expressed in multiple epithelial tissues, but also by
several cancer cells. For example, the majority of human breast
cancer samples also express TLR5 and there is an elevated
expression of TLR5 in certain subtypes of breast carcinomas
(11). It has also been shown that
TLR5 is overexpressed in gastric carcinoma cells, and activation of
TLR5 by flagellin provokes potent antitumor activity and thus
inhibits the growth of colon tumors in vivo (12). By contrast, a study by Sfondrini
et al (13) demonstrated
that the early administration of flagellin simultaneous to
implanting mouse mammary cells induced an increase in tumor growth.
Currently, the particular function and exact mechanism of TLR5
signaling pathways in cancer cells remains poorly understood, and
the abnormal expression of TLR5 has been noted as a potential
biomarker for tumors. Therefore, TLR5 presents as an enticing
target for molecular imaging of metastases and the metastatic
potential of the primary tumor that expresses TLR5.
Due to its anatomical site, the liver is constantly
exposed to gut-derived bacterial products, viral infection, alcohol
or other products, which may be the reason for chronic liver
damage, thus increasing the risk for HCC. Possibly as a consequence
of this, TLRs play a key role in liver physiology and
pathophysiology, due to their role in the immune system and their
significant contribution to several biological processes, including
promotion of epithelial regeneration and carcinogenesis (14). It has been demonstrated that there
is a strong association between TLR3, TLR4 and TLR9 expression and
tumor aggressiveness and poor prognosis in HCC (15). In addition, it was recently reported
that the liver was a major target for TLR5 agonists and a key
mediator of TLR5-dependent effects in vivo (16).
The main obstacle in the diagnosis of HCC is the low
sensitivity for the detection of tumors <2 cm in size. The
traditional imaging modalities indicated for small-HCC detection
are contrast-enhanced ultrasound and contrast-enhanced magnetic
resonance imaging (MRI) that have shown a high false-negative
detection rate. Thus, a novel and more sensitive detection method
is urgently required for the diagnosis of small HCC without a
biopsy. Nuclear molecular imaging is such an emerging and promising
science that has been applied in a broad range of clinical
diagnoses and therapy. 11C-acetate and
18F-fluorodeoxyglucose (FDG) are complementary tracers
in the role of a functional and biochemical probe for detecting
both primary and secondary HCC through the degree of tumor cell
differentiation. Although increasing evidence has shown that TLR5
plays a prominent role in cancer progression, its expression and
role in HCC remain unclassified.
As aforementioned, we hypothesize that TLR5 may be a
good biomarker for the detection of HCC, and therefore a
radioiodinated anti-TRL5 monoclonal antibody (mAb) was prepared and
its tumor-targeting potential was evaluated using the H22
hepatocarcinoma-bearing mice model.
Materials and methods
Cells and animals
The H22 hepatoma cell line was stored in our
laboratory (Institute of Experimental Nuclear Medicine, School of
Medicine, Shandong University, Shandong, China). The cells were
cultured in Dulbecco’s modified Eagle’s medium (Gibco, Invitrogen
Life Technologies, Grand Island, NY, USA) supplemented with 10%
(v/v) fetal bovine serum (Gibco), 100 U/ml penicillin and 100 mg/l
streptomycin (Beyotime Biotech, Ltd., Shanghai, China) in
humidified air containing 5% CO2 at 37°C.
Female BALB/c mice, 6 and 8 weeks of age, were
purchased from the Experimental Animal Center of Shandong
University (Shangdong, China). The mice were inoculated
subcutaneously on the rear flanks with 4×106 H22 cells
in 100 μl normal saline. The animals were used for biodistribution
and autoradiography experiments when the tumor size reached 6–8 mm
in diameter. All experimental protocols described in the present
study were under the approval of the Ethics Review Committee for
Animal Experimentation of Shandong University (Jinan, China).
Semi-quantitative reverse transcription
polymerase chain reaction (RT-PCR)
The TLR5 mRNA expression level in the H22
tumor-bearing mice was measured using RT-PCR. Briefly, total RNA
was extracted in accordance with the manufacturer’s instructions
and then reverse transcribed to cDNA using the Gene Amp RNA PCR kit
in a DNA thermal cycler (Bio-Rad, Hercules, CA, USA). A
non-template control was included in all experiments. Primer
sequences were as follows: TLR5 forward,
5′-GCAGGATCATGGCATGTCAAC-3′ and reverse,
5′-AATGGTCAAGTTAGCATACTGGG-3′; GAPDH forward,
5′-AGGCCGGTGCTGAGTATGTC-3′ and reverse, 5′-TGCCTGCTTCACCACCTTCT-3′.
The amplification products were separated on an agarose gel (Gene
Ltd., Hong Kong, China) and visualized with ethidium bromide. The
predicted size for TLR5 and GAPDH was 269 and 530 bp,
respectively.
Immunohistochemistry analysis
Sections (4 μm thick) cut from the archived paraffin
blocks, were attached to slides and deparaffinized with toluene,
and gradually dehydrated through a descending alcohol series. To
block non-specific binding of the antibodies, the sections were
incubated with 2% goat serum in phosphate-buffered saline (PBS;
blocking buffer) for 2 h at room temperature. Subsequently, the
slides were stained using the rabbit anti-mouse anti-TLR5 mAb
(1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or
immunoglobulin G (IgG; Beijing Biosynthesis Biotechnology Co.,
Ltd., Beijing, China) as the negative control. Positive expression
was indicated by brownish-yellow granules in the plasma membrane of
hepatoma cells for TLR5. The sections were analyzed using an
Olympus DP72 digital camera (Olympus Co., Centre Valley, PA, USA)
at a magnification of ×400, and images were captured.
Radiopharmaceutical preparation
Sodium [131I] iodide was obtained from
the China Institute of Atomic Energy (Beijing, China). Anti-TLR5
mAb and control IgG were labeled with 131I-NaI by the
Iodogen method. Briefly, 100 μl 0.05 M phosphate buffer (PB) and
22.8 MBq 131I-NaI were added into the prepared
Iodogen-coated tubes (Pierce Biotechnology Ltd., Rockford, IL, USA)
and then 20 μg of anti-TLR5 mAb or IgG were added respectively.
Subsequently, the mixture was incubated at room temperature for 15
min with occasional shaking. The reaction was quenched by
incubation with 150 μl 0.05 M PB for 15 min at room temperature.
Radiolabeled antibodies were then purified by size-exclusion
chromatography using a PD-10 Sephadex G-25 column (GE-Healthcare,
Diegem, Belgium). The radiochemical purity was measured by a Wipe
Test/Well Counter (Caprac; Capintec, Inc., Ramsey, NJ, USA). The
in vitro stability of the radiotracer was determined in a
serial time in human serum (Fame Ltd., Beijing, China) or PBS (0.05
mol/l; pH 7.4) at 37°C, and analyzed by radio-thin-layer
chromatography (TLC) Strip Scanner (Mini-Scan radio-TLC Strip
Scanner; Bioscan, Inc., Washington, DC, USA).
Whole-body autoradiography
The H22 tumor-bearing mice were injected via the
tail vein with a PBS solution (100 μl) of 131I-anti-TLR5
mAb or 131I-IgG (0.74 MBq) respectively. To block the
uptake in the thyroid gland, 5% potassium iodide was fed to the
mice for three days before injection. Serial images were performed
at 12, 24, 36 and 48 h post-injection. The anesthetized groups of
mice (n=4, per group) were placed in the supine position on the
storage phosphor screen plate for 15 min. Subsequently, the plate
was scanned by the Cyclone Plus Storage Phosphor system
(Perkin-Elmer, Waltham, MA, USA) and analyzed using the OptiQuant
Acquisition software (Perkin-Elmer).
Biodistribution studies
To validate the imaging studies and further quantify
the 131I-mAb uptake, biodistribution studies were
performed at various times in the H22 tumor-bearing mice model. The
mice were administered 0.37 MBq of 131I-mAb (100 μl) via
the lateral tail vein. Subsequently, groups of four mice were
sacrificed by cervical dislocation at 12, 24, 36 and 48 h after
injection, respectively. Blood was collected and the selected
tissues were rapidly harvested, weighed and analyzed for total
γ-counts by the Wipe Test/Well Counter. Data were corrected for
radioactive decay and the radioactivity values were expressed as
percentage of the injected dose [ID (%)] per organ, per gram of
tissue and as T/NT (target/non-target) ratio.
Data analysis
Data are expressed as the mean ± standard deviation
and P<0.05 was considered to indicate a statistically
significant difference. An unpaired two-tailed t-test was used, and
statistical analysis was performed using PRIZM SPSS 15.0 software
(SPSS, Inc., Chicago, IL, USA).
Results
Expression of TLR5 in H22 cell lines and
tumor tissue
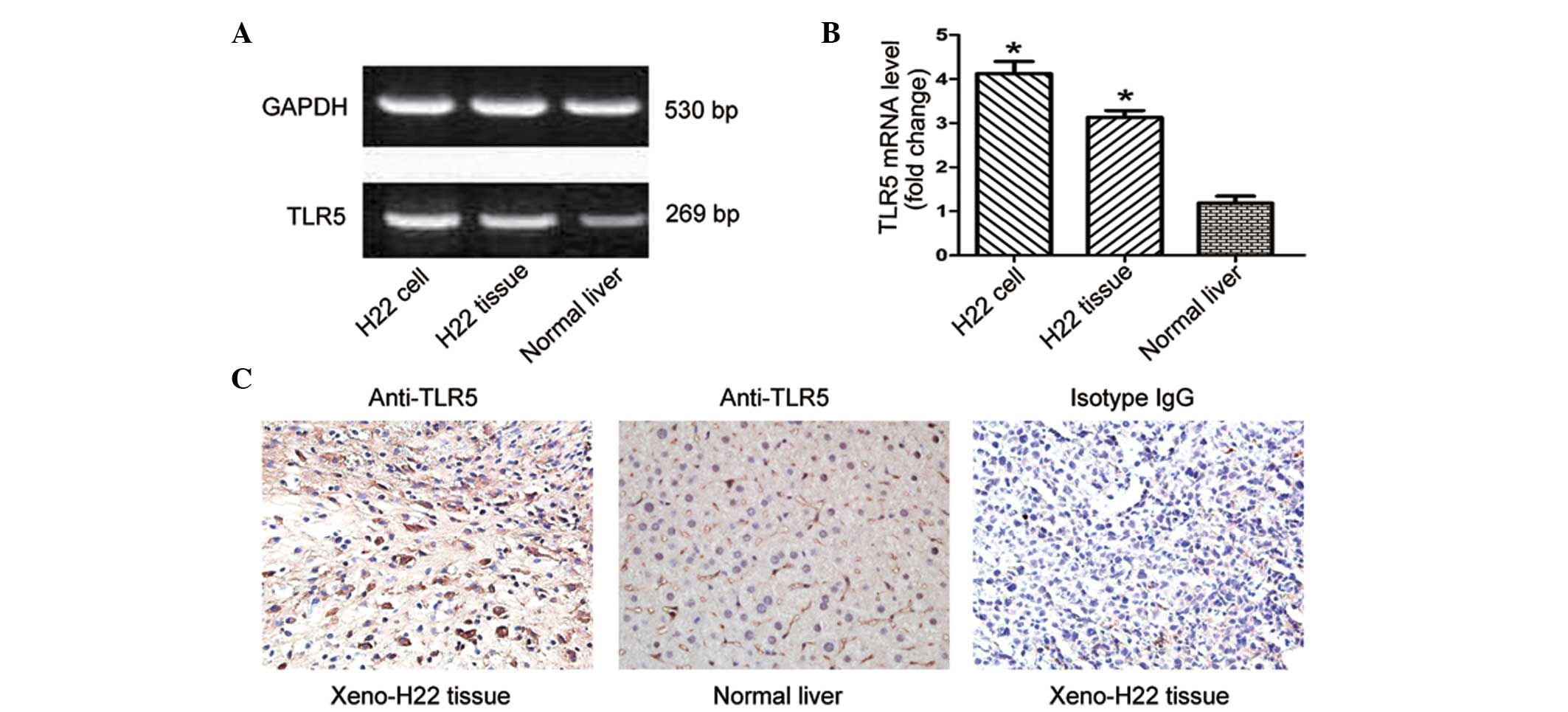
The expression of TLR5 mRNA in the H22 cell line,
H22 xenograft tumor tissue and normal liver tissue are shown in
Fig. 1A. Compared with the normal
liver group, the groups of the H22 cell line and H22 xenograft
tumor tissue exhibited higher levels of TLR5 mRNA expression
(P<0.05) (Fig. 1B). Consistent
with the RT-PCR results, immunohistochemistry staining (Fig. 1C) showed that the expression of TLR5
was strongly localized among the tumor cells than the normal liver
cells.
Radiolabeling and stability
assessment
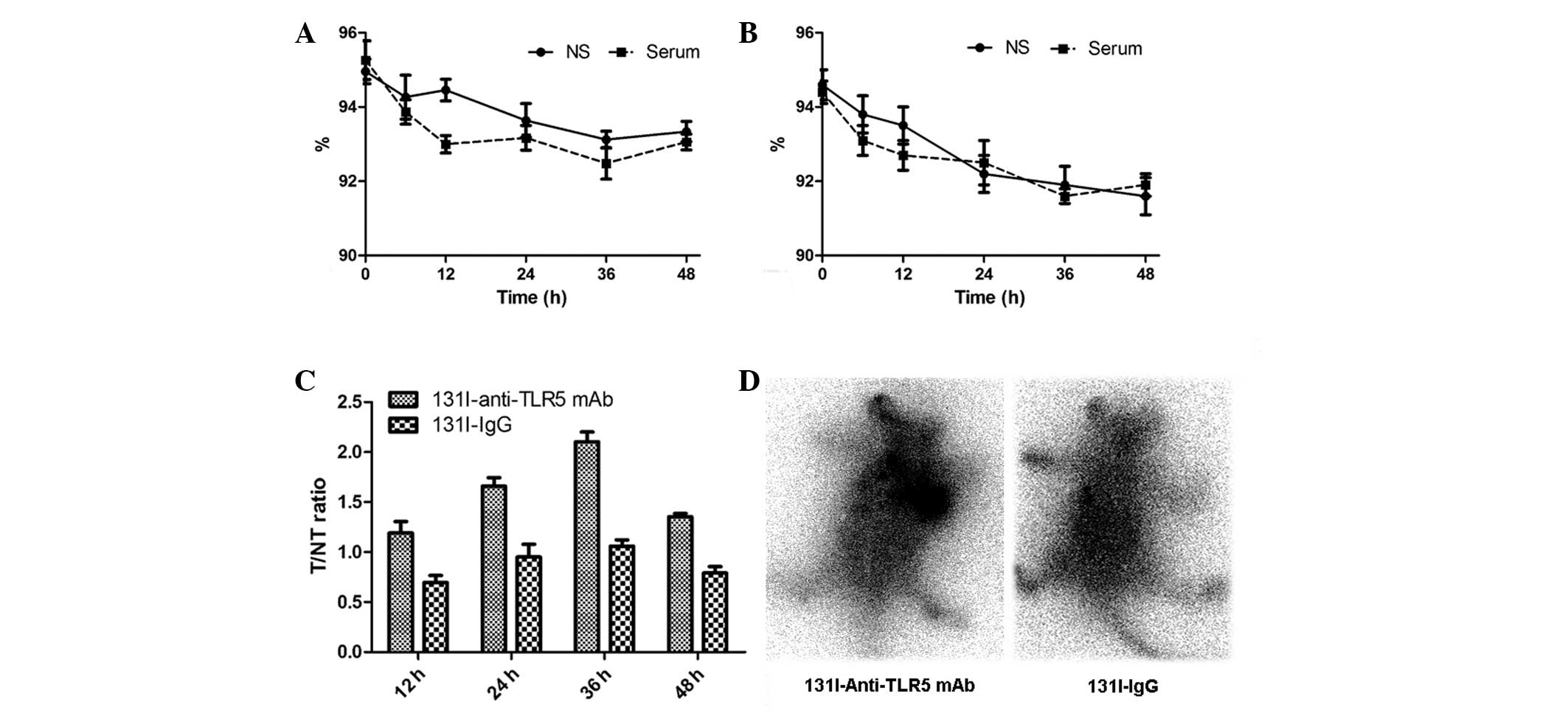
131I-anti-TLR5 mAb and its control
131I-IgG were successfully radioiodinated. The
radiochemical purity of 131I-anti-TLR5 mAb and
131I-IgG were both >95%. The specific activity of
131I-anti-TLR5 mAb and 131I-IgG was 29.56 and
25.43 GBq/μmol, respectively. 131I-anti-TLR5 mAb
(Fig. 2A) and 131I-IgG
(Fig. 2B) were stable in
vitro for 48 h without an obvious decrease of radiochemical
purity (>90%).
Biodistribution studies
The tissue distributions of radioactivity at 12, 24,
36 and 48 h after injection are illustrated in Table I and II. During the first 12 h, a relatively
high uptake of 131I-anti-TLR5 mAb was observed in the
tumor site and in the blood, liver, kidney, spleen and lung. It is
apparent that 131I-anti-TLR5 mAb localized at the site
of the tumor to a significant extent at 24 h (ID/g reached
≤8.26±0.91%), and was retained there for >48 h (ID/g at 48 h:
2.17±0.53 %). However, there was no significant radioactivity in
the tumor at all time points for 131I-IgG. The T/NT
ratio is provided in Fig. 2C. It
shows that the T/NT ratio for 131I-anti-TLR5 mAb was
1.19±0.20 at 12 h, and increased continually, eventually reaching
≤2.11±0.17 at 36 h. These ratios were significantly higher than
that of the 131I-IgG group (P<0.05).
 | Table IDistribution of
131I-anti-TLR5-mAb in the H22 tumor-bearing mice. |
Table I
Distribution of
131I-anti-TLR5-mAb in the H22 tumor-bearing mice.
| Tissue | 12 h | 24 h | 36 h | 48 h |
|---|
| Blood | 6.37±0.48 | 4.26±0.35 | 2.30±0.22 | 1.57±0.20 |
| Heart | 2.98±0.16 | 2.36±0.06 | 1.51±0.05 | 0.86±0.04 |
| Liver | 6.28±0.51 | 4.64±0.31 | 3.21±0.10 | 2.04±0.21 |
| Spleen | 2.83±0.14 | 2.95±0.17 | 1.39±0.04 | 1.22±0.06 |
| Kidney | 11.60±0.92 | 9.56±0.12 | 7.23±0.38 | 4.91±0.73 |
| Stomach | 1.70±0.27 | 1.04±0.06 | 0.86±0.03 | 0.41±0.02 |
| Intestine | 1.69±0.13 | 0.79±0.08 | 1.00±0.04 | 0.64±0.10 |
| Bone | 0.87±0.10 | 0.74±0.03 | 0.74±0.11 | 0.34±0.01 |
| Muscle | 0.98±0.05 | 0.99±0.03 | 0.48±0.07 | 0.23±0.02 |
| Lung | 2.79±0.40 | 1.69±0.10 | 1.08±0.14 | 0.94±0.08 |
| Thyroid gland | 1.62±0.04 | 1.24±0.07 | 0.77±0.04 | 0.58±0.01 |
| Tumor | 6.81±0.73 | 8.26±0.91 | 4.98±0.17 | 2.17±0.53 |
 | Table IIDistribution of 131I-IgG
in the H22 tumor-bearing mice. |
Table II
Distribution of 131I-IgG
in the H22 tumor-bearing mice.
| Tissue | 12 h | 24 h | 36 h | 48 h |
|---|
| Blood | 6.16±0.43 | 3.69±0.12 | 2.09±0.07 | 1.12±0.24 |
| Heart | 3.43±0.19 | 2.01±0.09 | 1.40±0.25 | 0.42±0.08 |
| Liver | 5.79±0.41 | 4.15±0.07 | 3.73±0.13 | 1.03±0.08 |
| Spleen | 2.61±0.12 | 2.15±0.10 | 0.79±0.03 | 0.55±0.12 |
| Kidney | 10.51±1.08 | 8.20±0.80 | 5.38±0.33 | 3.71±0.29 |
| Stomach | 1.69±0.13 | 0.92±0.13 | 0.74±0.02 | 0.48±0.13 |
| Intestine | 1.48±0.25 | 1.02±0.09 | 0.87±0.14 | 0.45±0.11 |
| Bone | 1.17±0.19 | 0.95±0.27 | 1.02±0.06 | 0.36±0.05 |
| Muscle | 1.21±0.31 | 0.67±0.11 | 0.59±0.08 | 0.32±0.07 |
| Lung | 3.01±0.68 | 1.77±0.12 | 1.33±0.12 | 0.62±0.03 |
| Thyroid gland | 1.63±0.07 | 0.97±0.05 | 0.80±0.06 | 0.50±0.01 |
| Tumor | 3.83±0.26 | 3.27±0.34 | 2.68±0.06 | 1.13±0.18 |
Whole-body autoradiography
As shown in the imaging of autoradiography, it was
found that 131I-anti-TLR5 became preferentially
accumulated in the xenografted H22 tumor at 24 h (Fig. 2D), and then showed a gradual decline
of uptake. Even 48 h after injection, H22 tumors were clearly
visible in mice injected with 131I-anti-TLR5 mAb. While
there was no clearly visualized accumulation in the tumor site in
the group of 131I-IgG at all time points. Other organs
with obvious uptake were the liver and kidneys. These results were
consistent with the results detailed in Tables I and II, demonstrating that
131I-anti-TLR5 mAb appears to be more specifically
retained in hepatocarcinoma than 131I-IgG.
Discussion
Novel diagnostic imaging approaches for HCC have
been developed during the past decades. Ultrasound scanning is
non-invasive and widely used in clinical diagnosis of hepatoma,
however the false-negative detection rate is >50% (17). Compared with the anatomical imaging
strategies, including computed tomography and MRI, nuclear medicine
imaging with radioisotope has the major advantage of high
sensitivity. 99mTc-methoxyisobutyl isonitrile and
18F-FDG have been commonly used for detection of HCC in
the clinic (18), however, they are
not specificity radiotracers for tumors. Therefore, solving the
deficiency of specific-targeting probes in clinic is urgently
required.
TLRs are extraordinarily notable in cancer research
due to their role in a number of biological processes, including
induction of innate and adaptive immune responses, carcinogenesis
and regulation of inflammation. Previously, intense links have
emerged between inflammation and the initiation and progression of
several cancer types, including stomach, breast, ovary and liver
(19). Chronic inflammation
elicited by certain bacteria, for instance Helicobacter
pylori, has been found to promote carcinogenesis (20). Activation of TLRs may favor a
contribution for cancer progression and development, however,
activation of various TLRs may exhibit contradictory results
(21). It was reported that the
activation of TLR4 signaling by lipopolysaccharide protects tumor
cells from immune attack and therefore induces tumor growth
(22,23). The activation of TLR9 on cancer
cells could prevent apoptosis in cancer cells and stimulate
proliferation of tumor cells (24).
However, TLR3 exhibited an antiproliferative role in human breast
cancer and melanoma (25). Thus,
the function and biological significance of TLRs expressed on
various tumor cells appears to be complicated.
Predominantly, TLRs may operate in two ways, which
is dependent on the cell type. Cancer cells are more aggressive in
response to TLRs activation, whilst immune cells usually respond to
TLRs agonist by applying antitumor effects. Higher expression
levels of TLR and the structural aberrations that characterize
malignant epithelia, including the loss of cell polarity and
abnormal intercellular junctions, may allow bacteria and their
components to induce TLRs, therefore contributing to the disease
progression (26).
As a pattern recognition receptor, TLR5 can
recognize flagellin, which is a component of bacterial flagella. In
malignant cells, TLR5 activates inflammatory responses and also
induces invasion, migration and chemokine secretion (27). The significance of TLR5 in oral
carcinoma has been demonstrated by assessing TLR5 expression in a
cohort of 119 patients with oral tongue squamous cell carcinoma
(28). Besides, TLR5 was identified
as highly expressed and activated in breast carcinomas (11). Furthermore, activation of TLR5 by
flagellin in breast cancer cells has been shown to alter the
production of proinflammatory cytokines, and this creates potent
antitumor activity in breast cancer, which may serve as a novel
therapeutic target for human breast cancer therapy (29).
Therefore, the present study investigated the
expression of TLR5 in the hepatocarcinoma cells. It was found that
H22 cells and H22-xenografted tumor tissue exhibited higher levels
of TLR5 expression than normal liver tissue, indicating that TLR5
may be a novel biomarker of hepatocarcinoma, although the
mechanisms underlying remain far from understood.
The 131I-labeled anti-TLR5 mAb was also
evaluated as a specific targeted radiotracer in H22
xenograft-bearing mice models. The biodistribution data showed that
131I-anti-TLR5 mAb had a high tumor uptake and T/NT
ratio. In addition, the result of autoradiography showed that the
radioactive accumulation in the tumor site became visible from 12 h
post injection, and increased continually.
These results showed the potential of
131I-anti-TLR5 mAb as a promising molecular imaging
agent for HCC diagnosis and encouraged further investigation.
Nevertheless, since TLR5 mAb has a large molecular weight and an
immunogenicity that may hinder its application in the clinic, it
remains a great challenge to explore a novel small fragment of mAb
or a small molecule with improved TLR5 targeting. In addition, to
analyze the association between the expression of TLR5 in HCC and
clinical stage, and to evaluate its significance in early-stage
diagnosis, will be extremely helpful for the prognosis of patients
suffering from HCC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (81071172) and the Natural
Science Foundation of Shandong Province (ZR2010CM025).
References
|
1
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: a global and regional perspective. Oncologist. 15:5–13.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Bruix J and Sherman M; the American
Association for the Study of Liver Diseases. Management of
hepatocellular carcinoma: an update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kutikhin AG: Association of polymorphisms
in TLR genes and in genes of the Toll-like receptor signaling
pathway with cancer risk. Hum Immunol. 72:1095–1116. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roelofs MF, Boelens WC, Joosten LA,
Abdollahi-Roodsaz S, Geurts J, Wunderink LU, et al: Identification
of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and
potential involvement in the pathogenesis of rheumatoid arthritis.
J Immunol. 176:7021–7027. 2006. View Article : Google Scholar
|
|
6
|
Jiang D, Liang J, Fan J, Yu S, Chen S, Luo
Y, et al: Regulation of lung injury and repair by Toll-like
receptors and hyaluronan. Nat Med. 11:1173–1179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cook DN, Pisetsky DS and Schwartz DA:
Toll-like receptors in the pathogenesis of human disease. Nat
Immunol. 5:975–979. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Killeen SD, Wang JH, Andrews EJ and
Redmond HP: Exploitation of the Toll-like receptor system in
cancer: a doubled-edged sword? Br J Cancer. 95:247–252. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kelly MG, Alvero AB, Chen R, Silasi DA,
Abrahams VM, Chan S, et al: TLR-4 signaling promotes tumor growth
and paclitaxel chemoresistance in ovarian cancer. Cancer Res.
66:3859–3868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kluwe J, Mencin A and Schwabe RF:
Toll-like receptors, wound healing, and carcinogenesis. J Mol Med
(Berl). 87:125–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai Z, Sanchez A, Shi Z, Zhang T, Liu M
and Zhang D: Activation of Toll-like receptor 5 on breast cancer
cells by flagellin suppresses cell proliferation and tumor growth.
Cancer Res. 71:2466–2475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rhee SH, Im E and Pothoulakis C: Toll-like
receptor 5 engagement modulates tumor development and growth in a
mouse xenograft model of human colon cancer. Gastroenterology.
135:518–528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sfondrini L, Rossini A, Besusso D, Merlo
A, Tagliabue E, Mènard S and Balsari A: Antitumor activity of the
TLR-5 ligand flagellin in mouse models of cancer. J Immunol.
176:6624–6630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawai T and Akira S: TLR signaling. Cell
Death Differ. 13:816–825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eiró N, Altadill A, Juárez LM, et al:
Toll-like receptors 3, 4 and 9 in hepatocellular carcinoma:
Relationship with clinicopathological characteristics and
prognosis. Hepatol Res. Jun 6–2013.(Epub ahead of print).
View Article : Google Scholar
|
|
16
|
Burdelya LG, Brackett CM, Kojouharov B,
Gitlin II, Leonova KI, Gleiberman AS, et al: Central role of liver
in anticancer and radioprotective activities of Toll-like receptor
5 agonist. Proc Natl Acad Sci USA. 110:E1857–E1866. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu K, Dai Z and Zhou J: Biomarkers for
hepatocellular carcinoma: progression in early diagnosis,
prognosis, and personalized therapy. Biomark Res. 1:102013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pant V, Sen IB and Soin AS: Role of
18F-FDG PET CT as an independent prognostic indicator in
patients with hepatocellular carcinoma. Nucl Med Commun.
34:749–757. 2013.
|
|
19
|
DeNardo DG, Johansson M and Coussens LM:
Immune cells as mediators of solid tumor metastasis. Cancer
Metastasis Rev. 27:11–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song EJ, Kang MJ, Kim YS, Kim SM, Lee SE,
Kim CH, et al: Flagellin promotes the proliferation of gastric
cancer cells via the Toll-like receptor 5. Int J Mol Med.
28:115–119. 2011.PubMed/NCBI
|
|
21
|
Rakoff-Nahoum S and Medzhitov R: Toll-like
receptors and cancer. Nat Rev Cancer. 9:57–63. 2009. View Article : Google Scholar
|
|
22
|
Huang B, Zhao J, Li H, He KL, Chen Y, Chen
SH, et al: Toll-like receptors on tumor cells facilitate evasion of
immune surveillance. Cancer Res. 65:5009–5014. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Szczepanski MJ, Czystowska M, Szajnik M,
et al: Triggering of Toll-like receptor 4 expressed on human head
and neck squamous cell carcinoma promotes tumor development and
protects the tumor from immune attack. Cancer Res. 69:3105–3113.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiron D, Pellat-Deceunynck C, Maillasson
M, Bataille R and Jego G: Phosphorothioate-modified TLR9 ligands
protect cancer cells against TRAIL-induced apoptosis. J Immunol.
183:4371–4377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salaun B, Coste I, Rissoan MC, Lebecque SJ
and Renno T: TLR3 can directly trigger apoptosis in human cancer
cells. J Immunol. 176:4894–4901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kauppila JH, Mattila AE, Karttunen TJ and
Salo T: Toll-like receptor 5 and the emerging role of bacteria in
carcinogenesis. Oncoimmunology. 2:e236202013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park JH, Yoon HE, Kim DJ, Kim SA, Ahn SG
and Yoon JH: Toll-like receptor 5 activation promotes migration and
invasion of salivary gland adenocarcinoma. J Oral Pathol Med.
40:187–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kauppila JH, Mattila AE, Karttunen TJ and
Salo T: Toll-like receptor 5 (TLR5) expression is a novel
predictive marker for recurrence and survival in squamous cell
carcinoma of the tongue. Br J Cancer. 108:638–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai Z, Sanchez A, Shi Z, Zhang T, Liu M
and Zhang D: Activation of Toll-like receptor 5 on breast cancer
cells by flagellin suppresses cell proliferation and tumor growth.
Cancer Res. 71:2466–2475. 2011. View Article : Google Scholar : PubMed/NCBI
|