Introduction
Prostate cancer is the most common cancer in males
in the UK and in the majority of Western countries, accounting for
40,975 cases of malignancies diagnosed in the UK and 10,721
mortalities, according to the cancer incidence and mortality rates
of 2010 outlined by Cancer Research UK statistics (1). Although the majority of prostate
cancers may remain indolent for years, 20–30% of affected patients
are diagnosed with metastases in the UK. The distant metastases and
local invasion remain major causes for the mortality and morbidity
of the disease. Intensive research is being carried out to identify
molecules and pathways for targeting cancer metastases. Adhesion
molecules are among those molecules that play pivotal roles during
the dissemination of cancerous cells (2). The transmembrane adhesion molecules,
such as cluster of differentiation 44, and the intracellular
adhesion associated molecules, such as focal adhesion kinase, have
been indicated in the multiple steps of metastasis, particularly
from prostate cancer (3,4). The present study aimed to examine
capillary morphogenesis gene 2 (CMG2) in prostate cancer. This is a
transmembrane protein that exhibits multiple functions, including
adhesion and migration.
CMG2, also known as anthrax toxin receptor 2, has
been identified as a gene that is upregulated in endothelial cells
during tubule formation (5).
Together with tumour endothelial marker-8 (TEM-8), this gene is a
receptor of anthrax toxin that is able to mediate the
internalisation of the toxin (6,7).
Compared with TEM-8, which is selectively overexpressed during
tumour angiogenesis, CMG2 is more widely expressed in normal
tissues, including the heart, lung, liver, skeletal muscle,
peripheral blood leukocytes, placenta, small intestine, kidney,
colon and spleen (6). The discovery
of TEM-8 and the importance of the anthrax toxin receptor,
highlighted by the events of 9/11 in 2001, have fuelled the
investigation of TEM-8 and CMG2 since the beginning of this
century.
The CMG2 gene, located on chromosome 4q, encodes a
protein of 489 amino acids with a putative signal peptide and
extracellular, transmembrane and cytoplasmic domains (6). Mutations of the CMG2 gene have been
identified in hyaline fibromatosis syndrome (HFS), including
juvenile hyaline fibromatosis and infantile systemic hyalinosis,
which are autosomal recessive syndromes characterized by multiple,
recurring subcutaneous tumours, gingival hypertrophy, joint
contractures, osteolysis and osteoporosis (8,9).
Different natural variants encoded by alternatively spliced mRNA
transcripts have been reported. The full-length protein product of
this gene is CMG2489. In comparison to
CMG2489, CMG2488 has 12 different amino acids
at the cytoplasmic tail of the protein. CMG2386 lacks
amino acids 213–233 of the full-length protein, and
CMG2322 is predicted to be a secreted isoform of CMG2
lacking the transmembrane domain (6). The von Willebrand factor type
A/inserted (vWA/I) domain with a metal ion-dependent adhesion site
region in the extracellular section of the protein allows binding
to the protective antigen (PA) subunit of the anthrax toxin to
mediate the internalisation of the toxin. In addition to binding
with PA, the extracellular domain also interacts with collagen IV,
laminin and fibronectin (5).
CMG2 has been implicated in tumour-related
angiogenesis (10) and to date,
little is known about its role in cancer. The present study aimed
to examine the expression of CMG2 in prostate cancer and the effect
on cellular functions of prostate cancer cells.
Materials and methods
PC-3 (European Collection of Animal Cell Culture,
Salisbury, UK), DU-145, LNCapFGC, CA-HPV10 and PZ-HPV-7 (American
Type Culture Collection, Mannasas, VA, USA), PNT1A and PNT2C2
(Professor Normal Maitland, University of York, York, UK) were
routinely cultured in Dulbecco’s modified Eagle’s medium-F12
supplemented with 10% fetal calf serum and antibiotics (penicillin
and streptomycin). Other reagents or kits were obtained from
Sigma-Aldrich (Poole, UK).
Prostate tissue samples were available following
collection from the patients at the Department of Urology,
University Hospital of Wales (Cardiff, UK). The samples were
snap-frozen in liquid nitrogen immediately after the surgery or
biopsy. All protocols were reviewed and approved by the local
ethical committee (Bro Taf Health Authority, Cardiff, UK) and all
patients gave written informed consent.
RNA extraction, reverse transcription
polymerase chain reaction (RT-PCR) and quantitative PCR
Total RNA was isolated from frozen tissues and
culture cells using TRI reagent (Sigma-Aldrich). RT-PCR was carried
out using an RT kit with an anchored oligo (dT) primer supplied by
AbGene (Thermo Scientific, Rockford, IL, USA), using 0.5 μg total
RNA for each 20 μl RT reaction. The quality of cDNA was verified
using GAPDH primers (sense, 5′-CAGGAGGTTGAAGGACTAAA-3′ and
antisense, 5′-GGGATCAGTTTTCTTTGTCA-3′). Conventional PCR was
performed with specific primers for CMG2 (sense,
5′-CAAAATCAGTAAAGGCTTGG-3′, and antisense,
5′-CAAAGGTTCTTCTTCCTCCT-3′). The conditions for the amplification
were: 94°C for 5 min, followed by 35 cycles of 94°C for 30 sec,
55°C for 30 sec and 72°C for 1 min, and the final extension for 7
min at 72°C. The products were separated on 1.5% agarose gel
followed by staining with ethidium bromide.
Western blot analysis
The cells were lysed in a lysis buffer containing 1%
Triton, 0.1% SDS, 2 mM CaCl2, 100 μg/ml
phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin and 1 μg/ml
aprotinin for 60 min. Equal amounts of each protein sample (20
μg/lane) were separated in a 10% polyacrylamide gel. Following
electrophoresis, the proteins were blotted onto nitrocellulose
sheets. Subsequent to blocking in 10% skimmed milk for 60 min, the
blots were probed with the polyclonal goat anti-human CMG2 (R&D
Systems, Minneapolis, MN, USA) and peroxidase-conjugated secondary
antibody. Protein bands were visualised using the
SupersignalTM West Dura system (Pierce Biotechnology,
Inc., Rockford, IL, USA) and an UVITech imager (UVITech, Inc.,
Cambridge, UK).
Altering the expression of CMG2 in
prostate cancer cells
The mammalian CMG2 expression vectors were
constructed using a pEF6/V5/His vector (Invitrogen, Paisley, UK)
and used to transfect PC-3 cells by electroporation. Following
transfection, the cells were selected using blasticidin (5 μg/ml).
A subline named PC-3CMG2exp, which overexpressed CMG2,
and a subline named PC-3pEF, which carried empty plasmid
vectors, were also established. PC-3WT was designated
for the wild-type cells. Constructed vectors carrying anti-CMG2
ribozymes were cloned into the same plasmid vectors and used to
knock down the expression of CMG2 in the PC-3 cells
(PC-3ΔCMG2).
In vitro cell growth assay
The cells were plated onto a 96-well plate (3,000
cells/well). Cell growth was assessed after one, three and five
days. Crystal violet was used to stain the cells, and absorbance
was determined at a wavelength of 540 nm using a spectrophotometer
(Elx800; BioTek, Bedfordshire, UK).
In vitro invasion assay
According to a standard procedure, Transwell inserts
with an 8-μm pore size were coated with 50 μg Matrigel (BD
Matrigel™ Basement Membrane Matrix; BD Bioscience, Oxford, UK) and
air-dried. Following rehydration, 30,000 cells were added to each
well. After 96 h, the cells that had migrated through the matrix to
the other side of the insert were fixed, stained and then counted
under a microscope.
Cell-matrix adhesion assay
The cell-matrix adhesion assay was performed as
previously described (3). In total,
40,000 cells were added to each well of the 96-well plate, which
was pre-coated with Matrigel (5 μg/well). Subsequent to 40 min of
incubation, the non-adherent cells were removed using a balanced
salt solution buffer. The adhered cells were fixed, stained and
then counted.
Tumour growth in an athymic mouse
model
Female athymic nude mice (4–8 weeks old; CD-1;
Charles River Laboratories, Inc., Wilmington, MA, USA) were
subcutaneously injected with PC-3 cells (1×106) in
Matrigel (2.5 mg/ml). The mice were kept in sterilised, filtered
cages in 12-h dark/12-h light standardised environmental conditions
approved by the local ethical committee. Tumours were measured
twice a week using digital callipers and calculated as tumour
volume = 0.512 × width2 × length (mm3).
Statistical analysis
Two sample t-test was performed using the SPSS
statistical software (version 18; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of CMG2 in prostate cancer
tissues and cell lines
CMG2 has been detected in various cell types,
including vascular endothelial cells, epithelial cells lining the
lumen of the intestine and respiratory system and cells of the
epidermis (5–7). In the present study, CMG2 mRNA was
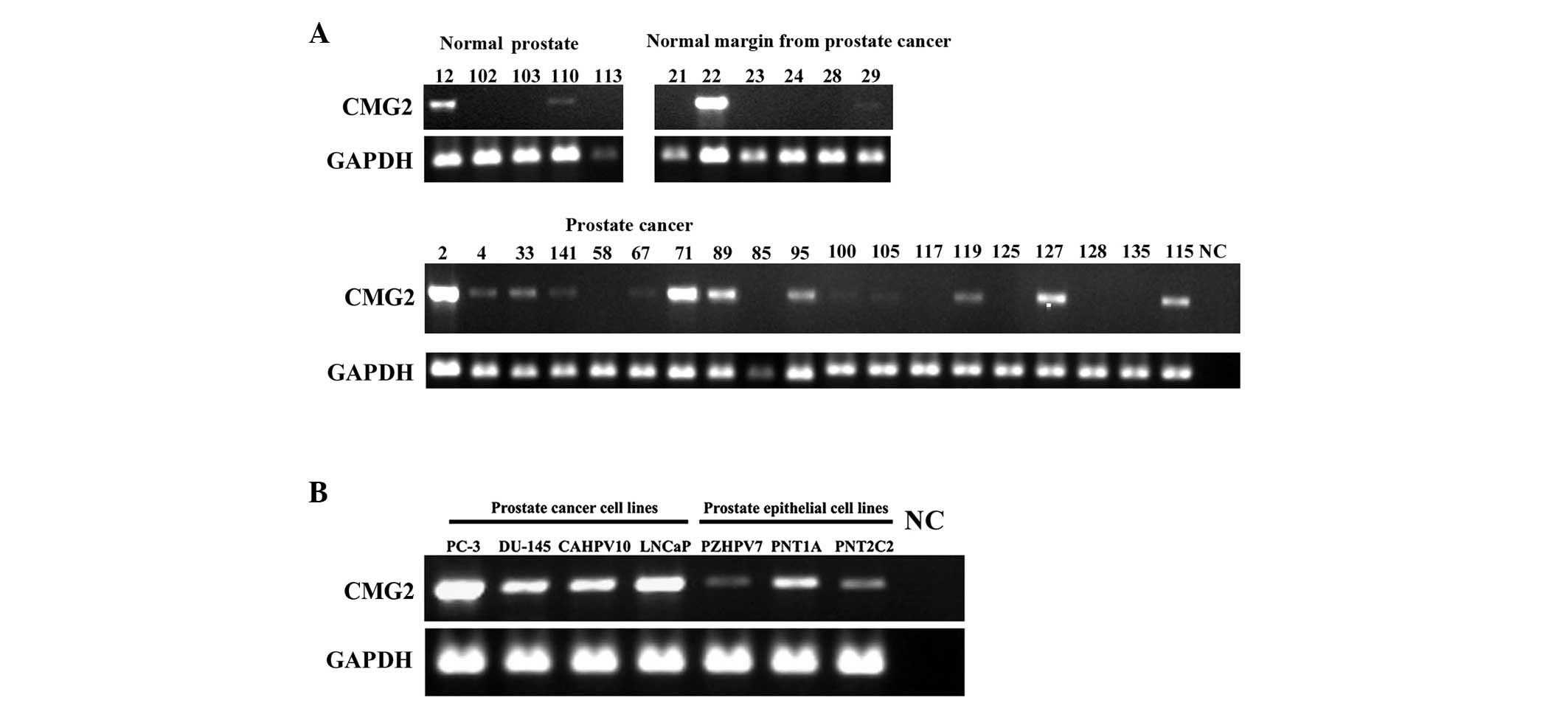
examined in the human prostate tumours and cell lines (Fig. 1). CMG2 transcripts were detected in
two of five normal prostate tissues (40.0%), and also in two of the
six background tissues of prostate cancer (33.3%). CMG2 appeared to
be detectable in the majority of the prostate tumours (13/19,
68.4%). In line with the observations in the prostate tissues, CMG2
was expressed at relatively high levels in the four prostate cancer
cell lines (PC-3, DU-145, CA-HPV-10 and LNCaP) compared with its
expression in the three immortalised prostate epithelial cell lines
(PZHPV-7, PNT1A and PNT2C2).
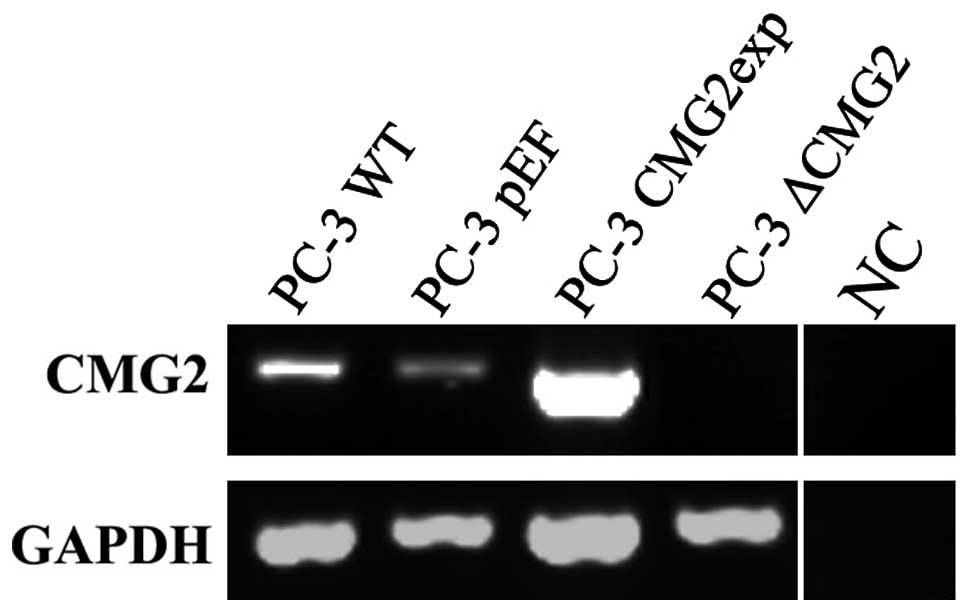
Overexpression and knockdown of CMG2 in
prostate cancer cells
For assessing the effect of CMG2 on cellular
functions, constructed plasmid vectors carrying either the
full-length CMG2 coding sequence or anti-CMG2 ribozymes were
transfected into the prostate cancer PC-3 cells. Following the
selection with blasticidin, RNA was extracted and the CMG2
transcripts in the transfected cells were determined using RT-PCR.
The overexpression and knockdown of CMG2 were verified in the
respective transfectants (Fig.
2).
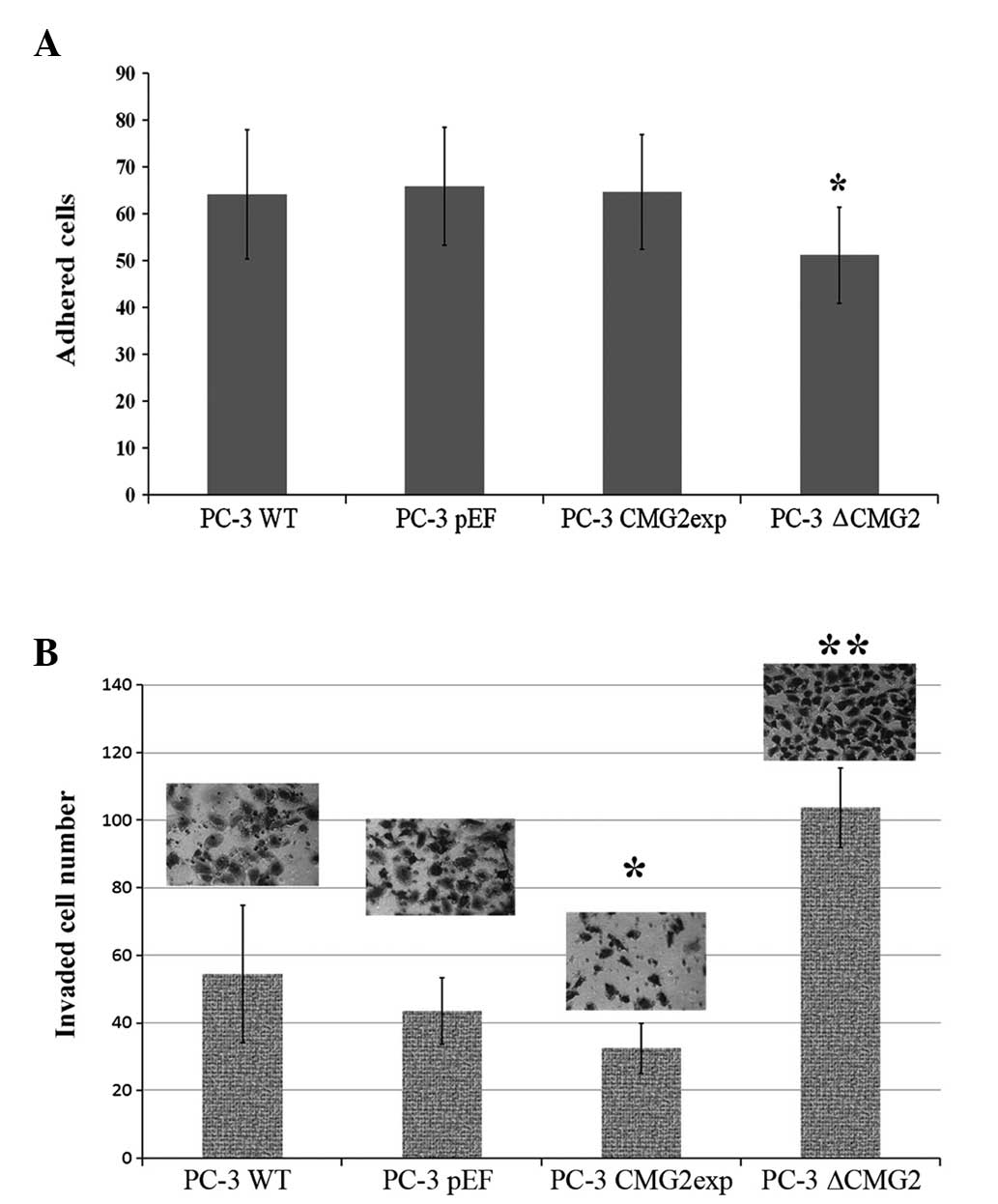
Altered CMG2 expression and the effect on
adhesion and invasion of prostate cancer cells
CMG2 has been shown to mediate the binding of
prostate cancer cells to the extracellular matrix, mainly via
interactions with laminin and collagen IV (5). In the present study, the adherence of
prostate cancer cells to Matrigel was examined first (Fig. 3A). Overexpression of CMG2 did not
enhance the adhesion of the prostate cancer cells to the Matrigel
in comparison with the control cells, while the knockdown of CMG2
resulted in a significant decrease in matrix adherence.
Although reduced adhesion was observed in the
CMG2-knockdown cells, a further examination of the invasiveness
showed a significantly increased invasive capacity of the cells. In
contrast to the increased invasion, an impaired invasive capacity
was observed in the cells with overexpression of CMG2 (Fig. 3B).
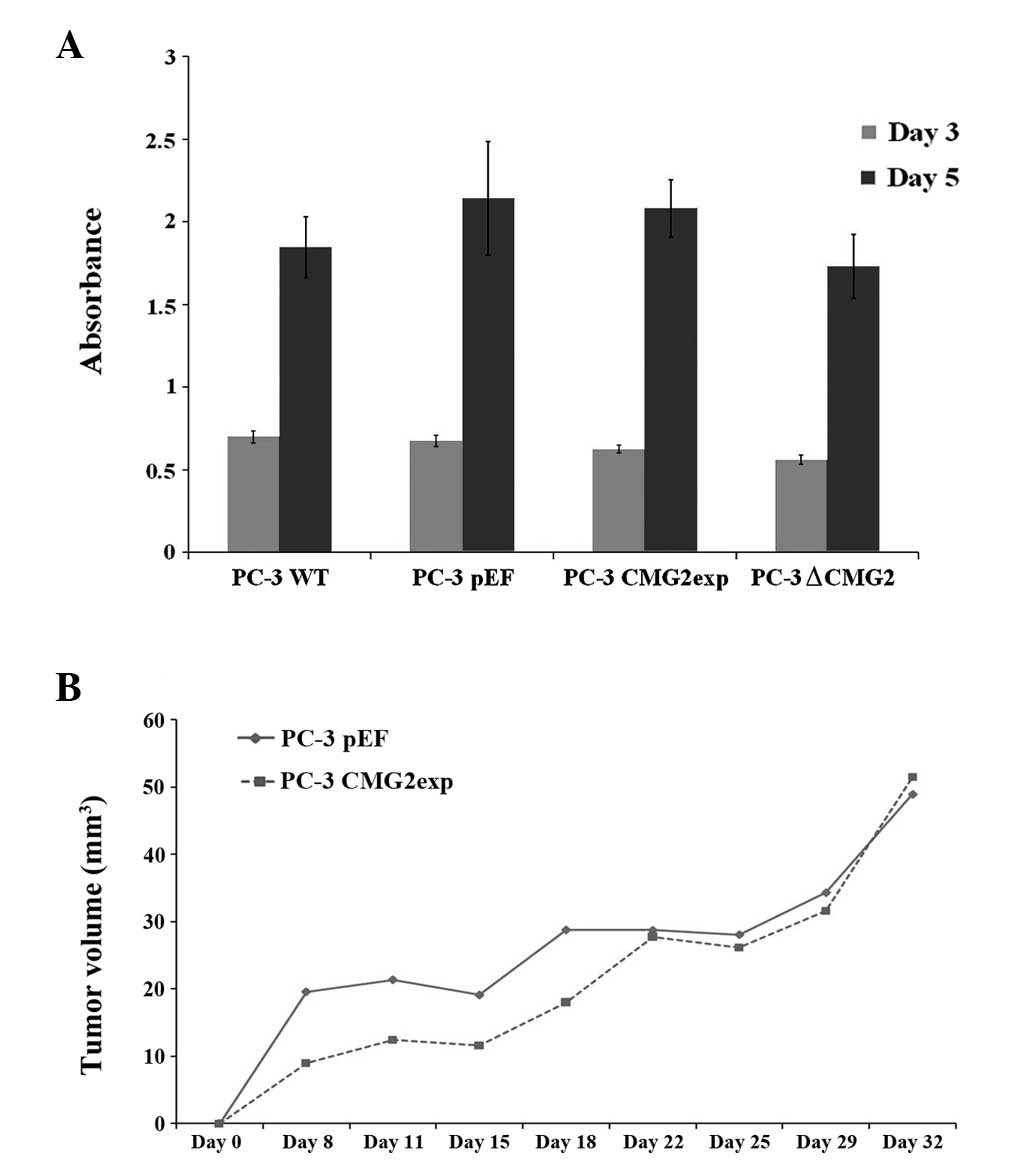
CMG2 and the growth of prostate cancer
cells
The effect on cell growth was determined using an
in vitro growth assay (Fig.
4A). No marked change was observed in the cells with altered
CMG2 expression over the periods of three and five days. A further
experiment using the mouse xenograft tumour model showed slower
tumour growth of the cells with CMG2 overexpression at an earlier
stage following the inoculation (Fig.
4B). The tumours grew to sizes similar to the control group
over the 32-day period.
Discussion
CMG2 has been identified as an upregulated gene in
endothelial cells during angiogenesis, and as a receptor for the
anthrax toxin (5,7). Mutations of this gene have been
observed in HFS (9,11,12).
Compared with the other receptor of the anthrax toxin, TEM-8, CMG2
exhibits a wider expression pattern that is observed in more tissue
types (6). In contrast to the
investigations of its roles in angiogenesis and Bacillus
anthracis infection, little is known about the role of CMG2 in
malignancies. Our recent study examined the expression of CMG2 in
breast cancer and reduced expression was found to be associated
with a shorter patient survival rate (unpublished data). In the
present study, the expression of CMG2 in human prostate cancer
tissues was first assessed using RT-PCR. The existence of the CMG2
transcripts was evident in the prostate cancer specimens, in which
68.4% of the samples appeared to be positive. CMG2 was found to be
less frequently expressed in the normal prostate tissues (40.0%)
and also the background tissues of prostate cancer (33.3%). To
clarify the heterogeneity of CMG2 expression in the prostate
tissues, the study further examined the expression of CMG2 in
prostate cancer and prostatic epithelial cells using RT-PCR. CMG2
transcripts were highly expressed in the prostate cancer cell lines
compared with the relatively low expression observed in the
immortalised prostatic epithelial cell lines. This indicates that
CMG2 may play a positive role in prostate cancer that may be
different from its function in breast cancer.
Following the assessment of CMG2 expression in human
prostate cancer tissues and cell lines, the study assessed the
expression of CMG2 in one prostate cancer cell line, PC-3, which is
one of the prostate cancer cell lines most commonly used in
research. Due to the relatively low expression levels observed in
the PC-3 cells in comparison with the other prostate cancer cell
lines, the overexpression and knockdown of CMG2 were performed to
provide double evidence for its functions in the prostate cancer
cells. The extracellular section of CMG2 has a vWA domain, also
called the I-domain, which is a well-characterised protein-protein
interaction domain involved in cell adhesion that exists in
extracellular matrix proteins and integrins (13). The homology shared in the vWA domain
between CMG2 and integrins indicates a potential involvement of
CMG2 in cell adhesion. An earlier study indicated specific possible
binding proteins for CMG2 in the extracellular matrix, including
collagen IV and laminin (5). In the
present study, following the verification of the overexpression and
knockdown of CMG2, the effect on cell adhesion was first
determined. Reduced cell-matrix adhesion was observed in the cells
with reduced CMG2 expression, and contrasting results were not
observed in the cells with CMG2 overexpression. This indicates that
CMG2 is involved in cell adhesion, but that above a certain
threshold it makes no further contribution to the cell adhesion in
the prostate cancer cells.
Mutations of CMG2 that are evident in HFS indicate
that CMG2 plays a role in the maintenance of extracellular matrix
homeostasis. A recent study has shown that CMG2 and TEM-8 can
regulate the extracellular matrix via regulation of membrane type
1-matrix metalloproteinase (MMP) and MMP2 (14). All these results indicate a
potential involvement of CMG2 in the invasion of prostate cancer
cells. However, in the present study, it was observed that a
different role may be played by CMG2 in the regulation of invasion
of the prostate cancer cells. Following the overexpression of CMG2,
inhibition of the invasion of the PC-3 cells was observed, while an
enhanced level of invasion was observed in the CMG2-knockdown
cells. This indicates that CMG2 may participate in the regulation
of the invasion of prostate cancer cells via a different mechanism
that is yet to be elucidated.
In addition to the effect on cell adhesion and
invasion, the effects on cell growth were also examined. There was
no obvious effect observed in the in vitro growth assays.
For the in vivo tumour growth, a slower growth rate was
observed at an earlier stage of tumour development following the
subcutaneous inoculation for which the effect on cell invasion may
account. However, the tumours all grew to a similar size at the end
of the 32-day experimental period. This indicates that little
impact was made by CMG2 on the in vivo tumour growth of
prostate cancer cells.
In conclusion, CMG2 is expressed by prostate cancer
cells and can regulate the adhesion and invasion of these cells.
CMG2 may play a certain role during the dissemination of prostate
cancer cells and has little impact on the in vitro and in
vivo growth of these cells. However, the exact role played by
CMG2 in prostate cancer and the possible relevance to sexual
hormones are yet to be investigated in future studies.
Acknowledgements
The authors would like to thank Cancer Research
Wales for their support.
References
|
1
|
Cancer Research UK. Numbers of cases and
deaths. http://www.cancerresearchuk.org/cancer-info/cancerstats/mort-ality/uk-cancer-mortality-statistics.
Accessed August 6, 2013
|
|
2
|
Mason MD, Davies G and Jiang WG: Cell
adhesion molecules and adhesion abnormalities in prostate cancer.
Crit Rev Oncol Hematol. 41:11–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harrison GM, Davies G, Martin TA, Jiang WG
and Mason MD: Distribution and expression of CD44 isoforms and
Ezrin during prostate cancer-endothelium interaction. Int J Oncol.
21:935–940. 2002.PubMed/NCBI
|
|
4
|
Johnson TR, Khandrika L, Kumar B, et al:
Focal adhesion kinase controls aggressive phenotype of
androgen-independent prostate cancer. Mol Cancer Res. 6:1639–1648.
2008. View Article : Google Scholar
|
|
5
|
Bell SE, Mavila A, Salazar R, et al:
Differential gene expression during capillary morphogenesis in 3D
collagen matrices: regulated expression of genes involved in
basement membrane matrix assembly, cell cycle progression, cellular
differentiation and G-protein signaling. J Cell Sci. 114:2755–2773.
2001.
|
|
6
|
Scobie HM, Rainey GJ, Bradley KA and Young
JA: Human capillary morphogenesis protein 2 functions as an anthrax
toxin receptor. Proc Natl Acad Sci USA. 100:5170–5174. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradley KA, Mogridge J, Mourez M, Collier
RJ and Young JA: Identification of the cellular receptor for
anthrax toxin. Nature. 414:225–229. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dowling O, Difeo A, Ramirez MC, et al:
Mutations in capillary morphogenesis gene-2 result in the allelic
disorders juvenile hyaline fibromatosis and infantile systemic
hyalinosis. Am J Hum Genet. 73:957–966. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanks S, Adams S, Douglas J, et al:
Mutations in the gene encoding capillary morphogenesis protein 2
cause juvenile hyaline fibromatosis and infantile systemic
hyalinosis. Am J Hum Genet. 73:791–800. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reeves CV, Dufraine J, Young JA and
Kitajewski J: Anthrax toxin receptor 2 is expressed in murine and
tumor vasculature and functions in endothelial proliferation and
morphogenesis. Oncogene. 29:789–801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Antaya RJ, Cajaiba MM, Madri J, et al:
Juvenile hyaline fibromatosis and infantile systemic hyalinosis
overlap associated with a novel mutation in capillary morphogenesis
protein-2 gene. Am J Dermatopathol. 29:99–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hatamochi A, Sasaki T, Kawaguchi T, Suzuki
H and Yamazaki S: A novel point mutation in the gene encoding
capillary morphogenesis protein 2 in a Japanese patient with
juvenile hyaline fibromatosis. Br J Dermatol. 157:1037–1039. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Whittaker CA and Hynes RO: Distribution
and evolution of von Willebrand/integrin A domains: widely
dispersed domains with roles in cell adhesion and elsewhere. Mol
Biol Cell. 13:3369–3387. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reeves CV, Wang X, Charles-Horvath PC, et
al: Anthrax toxin receptor 2 functions in ECM homeostasis of the
murine reproductive tract and promotes MMP activity. PLoS One.
7:e348622012. View Article : Google Scholar : PubMed/NCBI
|