Introduction
Chronic lymphocytic leukemia (CLL) is one of the
chronic lymphoproliferative disorders that is predominantly
diagnosed in the elderly. Annually, >100 cases of CLL are
diagnosed in Taiwan, R.O.C. (1),
with ~25% of patients initially asymptomatic at diagnosis and
referred to the clinic due to an abnormal white blood cell count
(WBC). The common clinical presentations of CLL are B symptoms,
lymphadenopathy, splenomegaly, hepatomegaly, skin lesions and
membranoproliferative glomerulonephritis. CLL presenting with
ascites is a rare complication, however, it has previously been
reported in the literature (2–4).
The diagnosis of CLL presenting with ascites is
determined when other etiologies of ascites are excluded. In a
previous study, morphological and immunophenotypic characteristics
were used for diagnosis (5);
however, a large number of clonal cells are required in order to
identify these characteristics. When the ascites contain a large
number of inflammatory cells, diagnosis of CLL is difficult. In the
present study, polymerase chain reaction (PCR)-based gene
rearrangement was used to identify CLL presenting with ascites. The
current findings indicate that this technique serves as a more
powerful approach and an effective method for diagnosing ascites in
patients with CLL. Patient provided written informed consent.
Case report
The current case report presents an 80-year-old male
with a history of hypertension who underwent medical treatment for
15 years. Leukocytosis was identified three years ago during an
annual health examination and the patient was referred to Chang
Gung Memorial Hospital (Linkou, Taiwan, R.O.C.). The WBC count was
71.2×109 cells/l with lymphocyte predominance
(lymphocytes, 78.7%; atypical lymphocytes, 5.7%; segments, 12.7%;
monocytes, 2.2%; and eosinophils, 0.7%). The patient’s hemoglobin
level and platelet count was 12.1 g/dl and 199×109
cells/l, respectively. Whole body computed tomography (CT) revealed
multiple small lymphadenopathy bilaterally in the neck, in the lung
hilum, celiac trunk, para-aortic area, as well as splenomegaly with
an enlarged splenic node. Immunophenotyping of lymphocytes in the
peripheral blood revealed cluster of differentiation (CD)5(+),
CD20(+) and CD23(+), which was compatible with B-cell CLL. A bone
marrow biopsy revealed diffuse interstitial infiltrates of small
lymphocytes, which accounted for 72.5% of the bone marrow smear.
Trisomy 12 and del (17p) were detected by fluorescent in
situ hybridization and the patient was diagnosed with CLL, Rai
stage II and Binet stage B.
One year after diagnosis, 2 mg chlorambucil was
administered twice daily due to progressive lymphocytosis
(163.5×109 cells/l with 90% lymphocytes). The WBC count
and differentials had returned to the normal range following 11
months of chlorambucil treatment. However, 18 months after
chlorambucil treatment, the patient developed progressive abdominal
distention, which was painless, without B symptoms. Complete blood
counts were as follows: Hemoglobin, 11.5 g/dl; platelet count,
106×109 cells/l; WBCs, 7.8×109 cells/l;
segments, 63%; lymphocytes, 30.8%; monocytes, 5.3%; eosinophils,
0.6%; and basophils, 0.3%. The level of creatinine and albumin was
0.94 mg/dl and 3.57 g/dl, respectively. The electrocardiogram was
normal and the cardiac sonography revealed adequate left
ventricular function. Liver cirrhosis was excluded by abdominal
sonography and the viral markers of hepatitis B and C were
negative. The cells in ascites were predominantly lymphocytes (red
blood cells, 1.285×109 cells/l; WBCs,
0.710×109 cells/l; neutrophils, 17%; and lymphocytes,
83%). The serum-ascites albumin gradient (SAAG) was 1.7, indicating
transudative ascites. The ascites culture was negative for bacteria
and tuberculosis. An abdominal CT scan showed enlarged mesenteric
nodes with a progressive change of mesenteric inflammatory disease
compared with the CT results at diagnosis. These findings did not
exclude peritonitis.
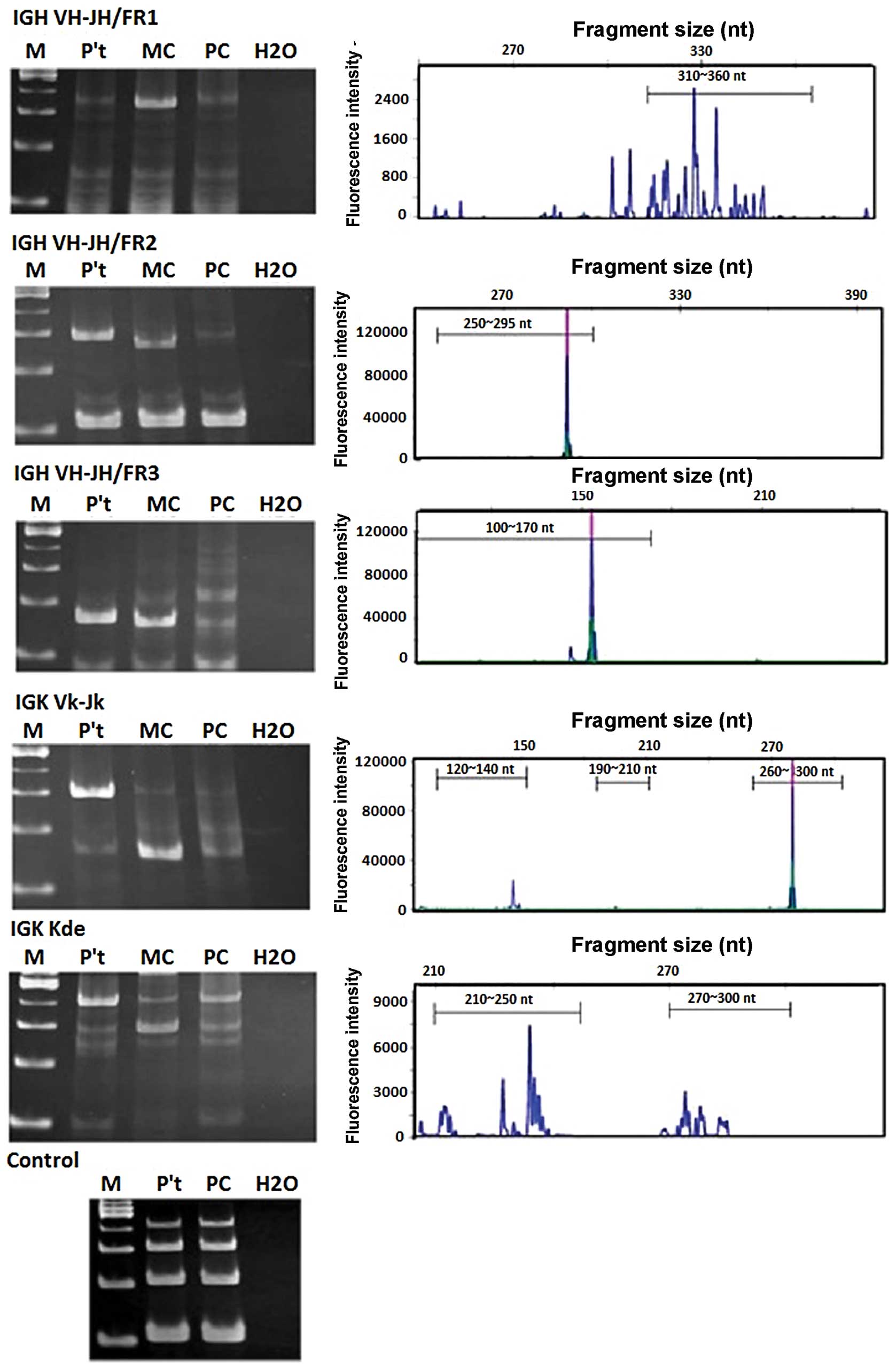
Immunophenotypic analysis of the cells in ascites
showed that 80% of the cells were lymphocytes, and T and B cells
accounted for <5%. The immunoglobulin (Ig) gene rearrangements
analysis using the BIOMED-2 PCR protocol (6) to determine the clonality status of B
cells revealed positive monoclonal B cells in the ascites. The
presence of a clonal band for IGH VH-JH/FR2, IGH VH-JH/FR3 and the
Igκ Vκ-Jκ genes were positive for monoclonal B cells in the ascites
(Fig. 1). An explorative
laparoscopy was performed to exclude peritonitis, second malignancy
or large cell transformation, and large ascites with multiple white
small lymph nodes over the peritoneum were identified. Biopsy of
the peritoneal lymph node revealed lymphoproliferation by
hematoxylin and eosin staining. Further immunohistochemical
staining was positive for CD20, CD5, CD23 and negative for CD10 and
cyclin D1. These findings were compatible with CLL involving the
peritoneum. The patient subsequently received prednisolone and
chlorambucil therapy, and the ascites rapidly regressed and
disappeared after one month.
Discussion
The first study to describe CLL presenting with
ascites was in 1965 (7). Patients
with CLL presenting with ascites have a short survival time
(2,8). In addition, CLL presenting with
chylous (9,10) and hemorrhagic (8) ascites have also been reported, as well
as portal hypertension, which was identified in four cases
(2,8,11,12) in
which lymphocytic infiltration was considered as the etiology and
resulted in transudative ascites. Additional studies have also
reported exudative ascites (3,5). The
difference in albumin gradient between the studies may be
attributed to the various types of pathophysiology. Lymphocytic
infiltration and portal hypertension may cause transudative
ascites. Furthermore, peritoneal CLL involvement, which affects
absorption of lymphatic ascites, increases net capillary
fluid-production and may result in exudative ascites (5). However, the number of available
studies are limited and it is difficult to determine the etiology
of CLL using the albumin gradient.
In the present case, the patient’s SAAG was 1.7,
which classified the ascites as transudates (13). The etiology of transudative ascites
(SAAG ≥1.1 g/dl), including cirrhosis, alcoholic hepatitis, heart
failure, massive hepatic metastases, constrictive pericarditis,
Budd-Chiari syndrome and infection, were excluded by serial
examinations. The cultures for bacteria and tuberculosis showed
negative results, and the cytology identified mesothelial cells,
macrophages, neutrophils and abundant small lymphocytes (>70%).
However, the majority of the lymphocytes in ascites were T and B
cells that accounted for <5%, and exhibited no evidence of
light-chain restriction. In a patient with decompensated liver
cirrhosis without CLL, the ascitic lymphocytes were predominantly T
rather than B cells (14), and were
distributed in the peripheral blood. For patients with peritoneal
malignancy and ascites, tumor-infiltrating lymphocytes and T
regulatory cells may contribute to the majority of T cells in
ascites. The cytological findings of our patient did not support a
diagnosis of CLL involvement. The traditional method used to
diagnose the etiology of ascites is explorative laparotomy.
Multiplex PCR assays have been developed and
standardized for the detection of clonal Ig and T cell receptor
genes (15). PCR assessment of
clonal Ig gene rearrangement was adopted as an important diagnostic
tool in mature B-cell neoplasms (16). In the study, 56 patients exhibiting
B-cell CLL were enrolled, of these, 54 (96%) cases showed a Vκ-Jκ
rearrangement and 34 (61%) cases showed either a Vκ-κde or intron
RSS-κde rearrangement (16). Igκ
rearrangements were detectable in all of the cases studied.
Therefore, this is considered to be a powerful tool for the
diagnosis of B-cell CLL. The EuroClonality (BIOMED-2) consortium
summarized the important pre- and post-analytical aspects of
clonality testing, providing a guideline for the interpretation of
clonality testing results (6).
According to this protocol, the findings of our case were positive
regarding clonality (generally multiple clonal results). Compared
with a laparotomy, the PCR-based gene rearrangements analysis is a
less invasive diagnostic approach.
In conclusion, the detection of leukemic cells in
ascites may explain the formation of large ascites found in the
patient in the present study. However, the interpretation of
clonality of leukemic cells in ascites should be correlated with
the clinical condition. To the best of our knowledge, this is the
first study to detect CLL cells in ascites by clonality analysis
using a gene rearrangement assay. Further clinical evidence of the
application of this non-invasive technique is required.
References
|
1
|
Department of Health, Taiwan, Republic of
China. Cancer Registration Report. 2009, https://cris.bhp.doh.gov.tw/.
Accessed March 20, 2013
|
|
2
|
May JT and Costanzi JJ: Ascites in chronic
leukemia: a case report and review of the literature. Oncology.
39:55–58. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimoni A, Shvidel L, Shtalrid M, Klepfish
A and Berrebi A: Prolymphocytic transformation of B-chronic
lymphocytic leukemia presenting as malignant ascites and pleural
effusion. Am J Hematol. 59:316–318. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mamode C, Beauregard P, Langevin S and
Mongeau CJ: Chronic lymphoid leukemia complicated with ascites. Can
J Gastroenterol. 14(Suppl D): 181D–184D. 2000.(In French).
|
|
5
|
Siddiqui N, Al-Amoudi S, Aleem A, Arafah M
and Al-Gwaiz L: Massive ascites as a presenting manifestation of
chronic lymphocytic leukemia. World J Gastroenterol. 14:3594–3597.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Langerak AW, Groenen PJ, Brüggemann M, et
al: EuroClonality/BIOMED-2 guidelines for interpretation and
reporting of Ig/TCR clonality testing in suspected
lymphoproliferations. Leukemia. 26:2159–2171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palleschi M: Findings on a case of chronic
lymphatic leukosis with ascites and its therapy. Clin Ter.
33:350–359. 1965.(In Italian).
|
|
8
|
Masana L, Blanch MD, Vila J and
Rubiés-Prat J: Portal hypertension in chronic lymphatic leukemia
(author’s transl). Med Clin (Barc). 73:186–189. 1979.(In
Spanish).
|
|
9
|
Davis MN, Alloy AM, Chiesa JC and Pecora
AA: Chronic lymphocytic leukemia presenting with massive chylous
ascites. Am J Gastroenterol. 85:593–596. 1990.PubMed/NCBI
|
|
10
|
Sivakumaran M, Qureshi H and Chapman CS:
Chylous effusions in CLL. Leuk Lymphoma. 18:365–366. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mouly S, Cochand-Priollet B, Halimi C and
Bergmann JF: Portal hypertension caused by intra-hepatic block in
chronic lymphoid leukemia. Presse Med. 25:497–498. 1996.(In
French).
|
|
12
|
Pauwels M, Pauwels S, Capron JP, Sevestre
H and Desablens B: Portal hypertension caused by intra-hepatic
block during chronic lymphoid leukemia. Gastroenterol Clin Biol.
24:221–224. 2000.(In French).
|
|
13
|
Runyon BA, Montano AA, Akriviadis EA,
Antillon MR, Irving MA and McHutchison JG: The serum-ascites
albumin gradient is superior to the exudate-transudate concept in
the differential diagnosis of ascites. Ann Intern Med. 117:215–220.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kiyici M, Nak SG, Budak F, et al:
Lymphocyte subsets and cytokines in ascitic fluid of decompensated
cirrhotic patients with and without spontaneous ascites infection.
J Gastroenterol Hepatol. 21:963–969. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Dongen JJ, Langerak AW, Brüggemann M,
et al: Design and standardization of PCR primers and protocols for
detection of clonal immunoglobulin and T-cell receptor gene
recombinations in suspect lymphoproliferations: report of the
BIOMED-2 Concerted Action BMH4-CT98–3936. Leukemia. 17:2257–2317.
2003.PubMed/NCBI
|
|
16
|
Evans PA, Pott C, Groenen PJ, et al:
Significantly improved PCR-based clonality testing in B-cell
malignancies by use of multiple immunoglobulin gene targets. Report
of the BIOMED-2 Concerted Action BHM4-CT98–3936. Leukemia.
21:207–214. 2007.PubMed/NCBI
|