Introduction
Primary thyroid cancers account for 1% of all
malignant tumors. Although the life expectancy is generally high,
these cancers cause more fatalities than all endocrine organ
cancers (1). Medullary thyroid
carcinomas (MTCs) are the third most common of all the thyroid
cancers and are responsible for ~3–4% of all thyroid cancer cases
(2). A common discovery in MTCs
that are diagnosed late is lymph node metastasis in the upper
mediastinum and neck. Metastases occur in ~70% of patients with MTC
who have a palpable thyroid nodule (>1.0 cm diameter) (3). At this stage of the disease, patients
cannot undergo surgical resection and do not concentrate
radioactive iodine (RAI131), and biochemical ‘cure’
rates drop to ≤30% (4,5). Therefore, it is important to develop
novel therapies for the treatment of MTC.
Doxorubicin (DOX), a broad-spectrum anthracylin, is
isolated from Streptomyces peucetius and has been used for
the treatment of several cancers, including ovarian, breast and
prostate cancer (6). Notably, DOX
is the most widely used anticancer drug that is approved by the
Food and Drug Administration (7).
However, studies have shown that specific cancer cells, including
those of the thyroid, are resistant to the apoptotic effects of DOX
(8). Non-tumorous tissues,
including those of the liver, heart and kidney, develop severe
side-effects following DOX-based chemotherapy, which limits its
clinical applications (9,10). In addition, the severe
dose-dependent side-effects, including stomatitis, neurological
disturbances, acute nausea and vomiting, myocardial toxicity,
alopecia and bone marrow aplasia, also limit the use of DOX
(7). Thus, improved therapeutic
regimens that potentiate DOX effects, allowing dose reduction and
protection of non-tumorous tissues, are required to improve the
treatment of patients with MTC.
The mechanisms involved in DOX-mediated cytotoxicity
differ between normal tissues and cancer cells (11). DOX toxicity in cancer cells
primarily occurs due to its ability to intercalate between the DNA
strands to act as a topoisomerase II inhibitor and/or bind
covalently to proteins involved in DNA replication and
transcription (7). In normal
tissue, however, the DOX-induced side-effects, including
hepatotoxicity or cardiotoxicity, are mainly due to the generation
of oxygen free radicals, which are inhibited by free radical
scavengers (12). This difference
in DOX-mediated toxicity in cancer and normal cells can be analyzed
to improve the antitumor effects of DOX in combination with other
antitumor drugs, thus allowing a dose reduction of DOX to protect
the normal cells. Combination therapy with DOX has recently gained
much attention (13,14). A study by Dayton et al
(14) found that combining DOX with
HO-3867 could reduce myocardial toxicity and enhance cell death
through the use of DOX at lower doses. Therefore, combination
therapy has been shown to be a productive method of lessening the
side-effects associated with DOX, while retaining the therapeutic
function of the drug.
Celecoxib (CXB) is a selective cyclooxygenase
(COX)-2 inhibitor that has been promoted as an anti-inflammatory
drug with improved safety and lower toxicity compared with other
non-steroidal anti-inflammatory drugs. Recently, a study has shown
that CXB in combination with DOX could increase growth inhibition
and apoptosis in acute myeloid leukemia cells compared with
treatment of DOX or CXB alone (15). The combination of CXB with DOX may
induce significant growth inhibition of neuroblastoma tumors, and
prevent and treat neuroblastoma (16). However, the combined effect of DOX
and CXB has not been reported in MTC, a mechanism of action has not
been determined and a combination treatment has not been tested
in vivo for the suppression of tumor growth. Thus, the
present study aimed to evaluate the effects of the combination of
the DOX chemotherapeutic agent and COX on MTC and normal cells.
Furthermore, the study examined the effect that a combination
treatment in vivo had on tumor growth, and the possible
mechanism was examined by assessing the expression of
multidrug-resistance protein 1 (MDR1) and COX-2 using xenograft
tumors produced by injecting thyroid carcinoma TT cells into nude
mice.
Materials and methods
Reagents
CXB and DOX were obtained from Pfizer, Inc., (New
York, NY, USA). Stock solutions of 1 mM CXB (Sigma Aldrich, St.
Louis, MO, USA) were dissolved in dimethyl sulfoxide (Sigma
Aldrich), stored at −20°C and diluted in fresh medium prior to use.
For the western blot analysis, the following antibodies were used:
Rabbit monoclonal anti-COX-2 and anti-MDR1 (Cell Signaling
Technology, Beverly, MA, USA), mouse monoclonal anti-β-Actin (Sigma
Aldrich) and horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G (IgG; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA). All other reagents were obtained from Sigma Aldrich
unless otherwise stated.
Cell culture
The human MTC cell line, TT, was obtained from the
cell bank of the Chinese Academy of Sciences (Beijing, China) and
the cells were grown in RPMI 1640 medium (Invitrogen, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS), 100 M
non-essential amino acids and 100 mM L-glutamine (Invitrogen) at
37°C in a 5% CO2 atmosphere and at 95% humidity.
Cell viability analysis
The TT cells were incubated at a concentration of
5×103 cells/well in a 96-well plate, and grown at 37°C,
with 5% CO2 until cell adherence. The cells on the
culture plate were divided into groups on the basis of parallel
well lines following an overnight incubation in fresh RPMI 1640
containing 0.5% FBS, and each group had four wells in one line.
Following the 24-h attachment period, the cells were treated with
DOX and CXB either alone or in combination. A total of 20 μl MTT (5
mg/ml) was added and the cells were incubated for another 4 h at
the end of the treatment. Subsequent to the removal of the
supernatant, 200 μl DMSO was added to each well. The plate was then
agitated for 5 min. In the shaking board, cell viability was
obtained by measuring the absorbance at 490 nm using an
enzyme-labeling instrument (Bio-Tek ELX800; Bio-Tek, Vermont, VT,
USA), and this assay was performed in triplicate. The inhibition
rate was calculated according to the following formula (17): Inhibition rate (%) = [1 - (average
absorbance of experimental group / average absorbance of blank
control group)] × 100.
Apoptosis analysis
The cells were cultured in six-well plates in RPMI
1640 supplemented with 10% FBS medium, and were treated with DOX
and CXB alone or with a combination of CXB/DOX for 24, 48 and 72 h.
The cover slips were washed three times with phosphate-buffered
saline (PBS) and single cell suspensions were fixed in 1% PBS. The
cells were stained with 100 μg/ml acridine orange and 100 μg/ml
ethidium bromide for 1 min. The cells were then observed under a
fluorescence microscope (CKX41-F32FL, Olympus, Tokyo, Japan). At
least 200 cells were counted and the percentage of apoptotic cells
was determined. Triplicates were performed in all experiments and
each experiment was performed three times.
Human MTC xenograft experiment
For the human MTC xenograft experiment, 4–6-week-old
female BALB mice were maintained under specific pathogen-free
conditions and provided with food and water ad libitum. All
the animals were fed with a normal pellet diet one week prior to
experimentation. In vitro cultured human MTC TT cells were
injected subcutaneously into the right supra scapula region. The
tumor volume was calculated by the following formula: Volume =
(length × width2 ) / 2. When tumors grew to an average
volume of 75 mm3, the mice were randomly divided into
four groups (30 mice per group) and treated intragastrically with
300 mg/kg CXB three times a week (CXB group), by intraperitoneal
(i.p.) injection with 4 mg/kg DOX once a week (DOX group), by i.p
injection of a combination of 2 mg/kg DOX and 150 mg/kg CXB once a
week (COX plus DOX group) or injected with the same volume of
saline once a week (Control group) for three weeks. The tumor
volumes were determined by caliper measurement twice a week. When
the control mice started to succumb to their tumors, the mice in
all treatment groups were euthanized and the tumors were weighed to
determine treatment efficacy. The tumor tissue and liver samples of
the mice were isolated for histopathological evaluation. The
present animal study was performed following approval of the
protocol by the Jilin University Animal Care and Use Committee
(Changchun, Jilin, China).
Quantitative polymerase chain reaction
(qPCR) for COX-2 and MDR1 expression
The tumor tissue samples were isolated from
sacrificed mice and total RNA was extracted using TRIzol reagent
following the manufacturer’s instructions (Invitrogen Life
Technologies). RNA was reverse-transcribed into complementary DNA
(cDNA) using a Primescript™ RT reagent kit (Takara Bio, Inc.,
Dalian, China) according to the manufacturer’s instructions. qPCR
was performed with the SYBR green fluorescent dye method and a
Rotor-Gene 3000 Real-Time PCR machine (Qiagen, Duesseldorf,
Germany). COX-2, MDR1 and β-actin primer sequences are listed in
Table I. β-actin was used as an
internal control to evaluate the relative expressions of COX-2 and
MDR1. The PCR conditions were as follows: Pre-denaturing at 95°C
for 2 min followed by 45 cycles of denaturation at 95°C for 10 sec
and annealing/extension at 59°C for 20 sec. The amplification
specificity was checked by melting curve analysis. The PCR products
were visualized by gel electrophoresis to confirm the presence of a
single product with the correct size. The 2−ΔΔCT method
was used to calculate the relative abundance of the target gene
expression generated by Rotor-Gene Real-Time Analysis Software
6.1.81 (Qiagen). For each cDNA sample, the target gene mRNA level
was normalized to the β-actin mRNA level. The experiments were
performed three times.
 | Table IPCR primers for the objective
gene. |
Table I
PCR primers for the objective
gene.
| Gene | Primer sequences
(5′-3′) | Fragment |
|---|
| MDR1 |
| Forward |
ACCGCAAACGCTTTATGCTG | 158 |
| Reverse |
ACGAGCTATGGCAATGCGTT | |
| COX-2 |
| Forward |
ACCGCAAACGCTTTATGCTG | 179 |
| Reverse |
AAAGATGGCATCTGGCGGA | |
| β-actin |
| Forward |
GTTGCGTTACACCCTTTCTTG | 142 |
| Reverse |
TGCTGTCACCTTCACCGTTC | |
Western blot analysis
Protein from the tumor tissue was extracted by the
Mammalian Protein Extraction kit (Kangwei Century Co., Ltd.,
Beijing, China) following the manufacturer’s instructions. The
protein concentration was determined by the bicinchoninic acid
assay with bovine serum albumin (Sigma Aldrich) as the standard.
Western blotting was performed. Briefly, an equal amount of the
total cell lysate (50 μg) was solubilized in the sample buffer and
boiled for 5 min. A total of 25 μl of this lysate was
electrophoresed on an 8% SDS-PAGE gel and then the proteins were
transferred to polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA) by transfer buffer at 400 mA for 1 h.
Non-specific binding was blocked with 5% skimmed milk powder for 1
h at room temperature. The membranes were incubated with the
specific primary antibody overnight at 4°C. The primary antibodies
used were the polyclonal rabbit anti-human MDR1 (1:10,000) and
rabbit anti-human COX-2 (1:1,000) antibodies. Subsequent to washing
three times with Tris-buffered saline plus Tween 20 solution and
incubation with the horseradish peroxidase-conjugated goat
anti-rabbit IgG as the secondary antibody (1:5000 dilution) for 1 h
at room temperature, the bands were visualized with the enhanced
chemiluminescence system (GE Healthcare, Little Chalfont,
Buckinghamshire, UK). The membranes were then re-blotted with
anti-β-actin antibody for normalization and confirmation of equal
protein loading.
Statistical analysis
All the statistical analyses were performed by
GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego,
CA, USA). Data are presented as the mean ± standard deviation. The
statistical significance was determined using one-way analysis of
variance and Student’s t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
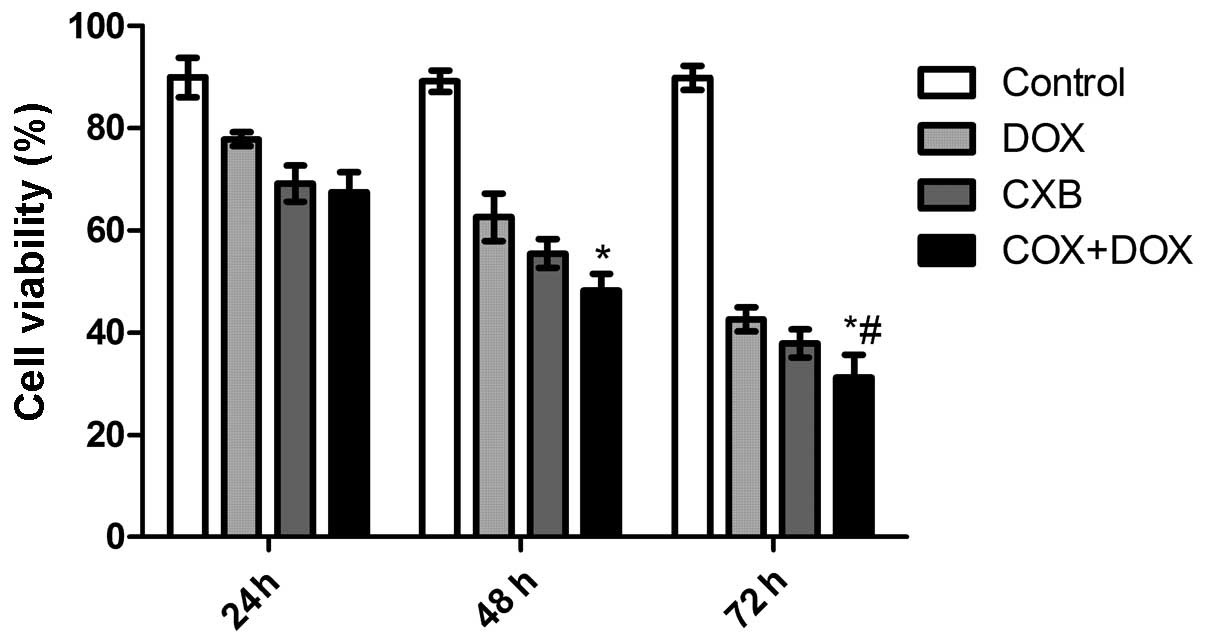
CXB plus DOX reduces cell viability
To investigate whether DOX combined with CXB
inhibits thyroid cancer cell proliferation, TT cells derived from
poorly-differentiated human medullary carcinoma cells were treated
with CXB, DOX or CXB combined with DOX for 24, 48, and 72 h. The
anti-proliferative effect of DOX, CXB and DOX plus CXB on the TT
cells was examined by MTT assay. It was found that CXB, DOX or CXB
plus DOX could significantly inhibit the proliferation of the TT
cells in a time-dependent manner (P=0.011). As shown in Fig. 1, the inhibitory rates of the CXB
plus DOX group was higher compared with the COX and DOX groups
(P=0.006 and 0.007, respectively). There was no significance
difference between the COX and DOX groups (P=0.678).
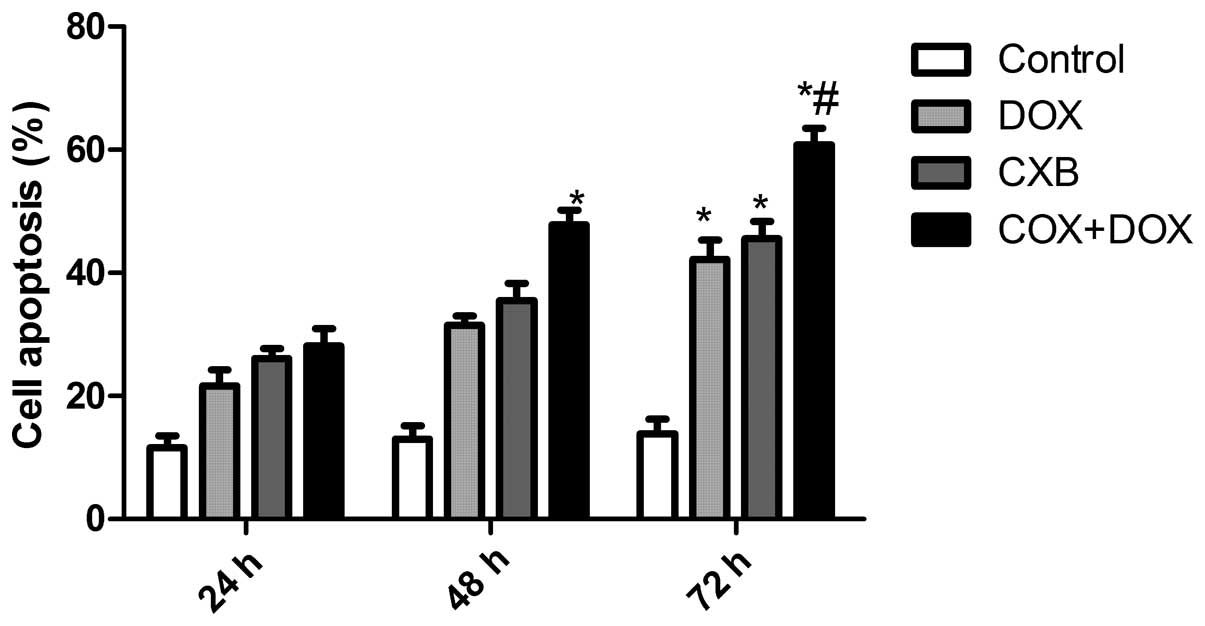
CXB plus DOX synergistically induces
apoptosis
The effects of DOX and CXB on the cell cycle of the
TT cells were analyzed. The TT cells treated with DOX or CXB had an
increased percentage of apoptotic cells compared with untreated
cells (Fig. 2). The DOX plus CXB
combination resulted in an even greater percentage of apoptotic
cells compared with the higher doses of either drug alone (P=0.004
and 0.006, respectively). These data are consistent with the
results from the MTT assay. Taken together, these results indicate
an additive mechanism for DOX and CXB in inducing cell death
through apoptosis.
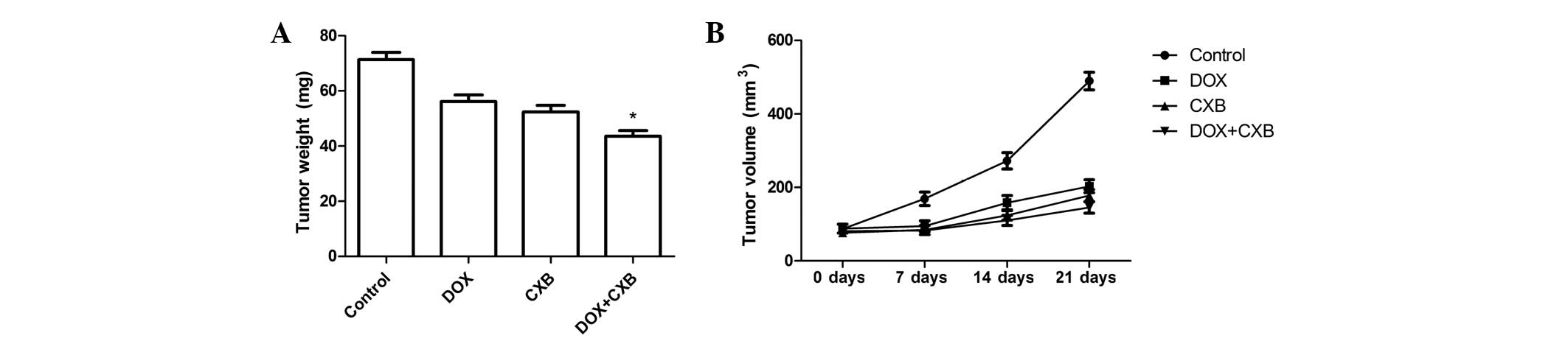
DOX plus CXB causes significant
inhibition of tumor growth
The in vivo therapeutic efficacy of DOX and
CXB was assessed in female BALB mice bearing medullary thyroid
tumors. The mice were sacrificed and the tumor tissue was removed
21 days after treatment. The tumor weight of the animals was then
measured. It was found that the tumor weight of the treatment group
was lower compared with the untreated group. The DOX plus CXB group
was lower compared with the single CXB or DOX groups (P=0.02 and
0.03, respectively) (Fig. 3A).
There were no significant differences between the COX and DOX
groups (P>0.05). Furthermore, the in vivo activity of DOX
combined with CXB was examined, and it was identified that the
growth of the established medullary thyroid tumor xenografts in
terms of tumor volume was inhibited when treated with a single or
combination of the drugs during days 7, 14 and 21 (Fig. 3B). CXB, DOX and the combination of
drugs showed a 45.37, 49.71 and 69.68% decrease in the mean tumor
volume at day 21, respectively, compared with tumors from the
untreated controls (Fig. 3B). As
shown in Fig. 3, DOX combined with
CXB resulted in an even greater percentage of tumor inhibition
rates compared with either drug alone at the various times (all
P<0.05). These results showed that DOX and CXB, but particularly
DOX combined with CXB, induced tumor regression and slowed tumor
growth in the mice of the treatment groups compared with the
untreated group.
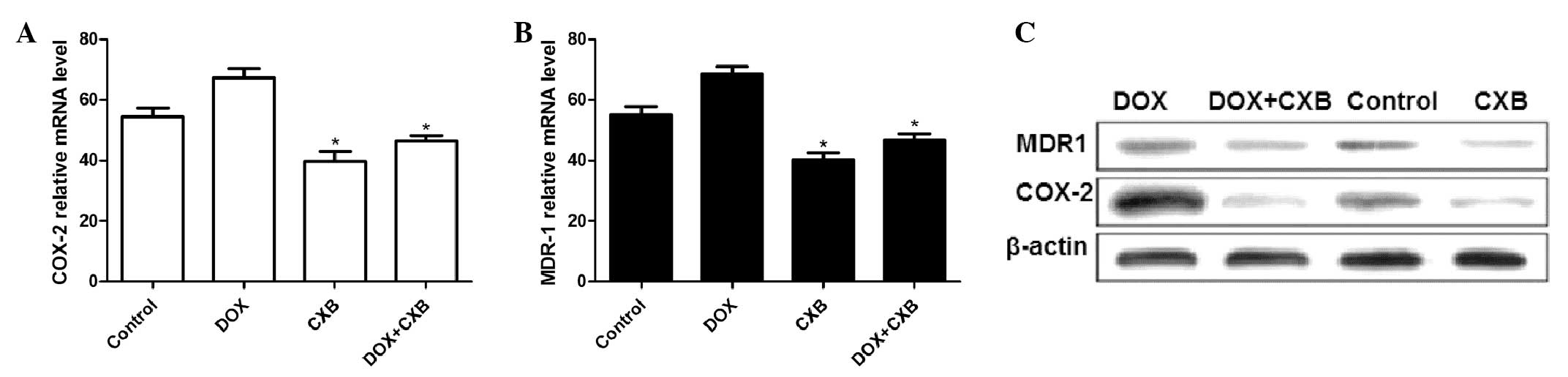
DOX plus CXB effects COX-2 and MDR1
production in MTC tumors
The COX-2 and MDR1 mRNA and protein expression
levels were examined by qPCR and western blot analysis,
respectively. The COX-2 and MDR1 expression at the mRNA level
decreased following treatment with CXB or the combination of DOX
and CXB compared with the untreated and DOX groups (all P<0.05)
(Fig. 4A and B). Additionally,
COX-2 and MDR1 expression of the mRNA in the DOX group was higher
compared with the untreated group. DOX alone considerably
upregulated the MDR1 and COX-2 protein expression level and the
combination of CXB and DOX, or CXB alone notably decreased the
protein expression levels compared with no treatment (Fig. 4C).
Discussion
The resistance of MTC to conventional chemotherapy
drugs is the major reason for the high mortality rate of patients
with MTC who fail to respond to surgery. Novel target therapies
have been under evaluation for the treatment of advanced MTC cases
in the last 10 years. Although certain promising antitumor drugs
have been used in phase II/III clinical trials previously (18–21),
certain patients cannot be enrolled due to the extremely
restrictive inclusion criteria or they drop out of the trial due to
severe side-effects. DOX is the most widely used anticancer drug,
and despite being previously used for the treatment of several
cancers, including ovarian, breast and prostate cancer (6), its clinical use is limited by its
toxicity to normal tissues, such as those of the heart and liver.
Another limitation of its effectiveness is the development of
multidrug resistance by cancer cells. Thus, combination therapy has
proven to be a useful method in reducing the side-effects
associated with DOX for the treatment of patients with MTC.
A previous study has shown that the combination of
DOX with rofecoxib, as a selective COX-2 inhibitor, could reduce TT
cell growth (22). This is in
agreement with the results of the present study, which showed that
DOX plus CXB could reduce cell viability with a significant
increase in apoptosis compared with a single-drug treatment.
Additionally, a study by Vivaldi et al (23) identified that a combination of CXB
and vinorelbine, but not DOX, induced a significant reduction in
cell viability and an increase in apoptosis in vitro, which
is also in agreement with the results of the present study. These
studies and the results of the present study further show that
combination therapy may be a useful method in treating patients
with cancer.
DOX treatment has led to partial biochemical and
tumor responses in a few patients, ranging from 10–20% of treated
cases (24), which showed that the
chemotherapy for metastatic MTC has a limited efficacy. Multidrug
resistance is one of the mechanisms for the resistance of MTC to
conventional chemotherapy (25).
MDR in cancer cells has been attributed to the overexpression of
several plasma membrane adenosine triphosphate (ATP)-dependent
efflux pumps, including P-glycoprotein (P-gp), which is encoded by
the ATP-binding cassette, sub-family B, member 1 gene, also named
MDR1 (26), breast
cancer-resistance protein (BCRP), which is encoded by the BCRP gene
(27) or multidrug-resistance
proteins (MRP1–3), which are encoded by the MRP genes (28). A previous study has shown that the
COX-2 gene is able to regulate MDR1 expression in rat mesangial
cells (29), and that the use of a
COX-2 inhibitor, rofecoxib, was able to sensitize MTC cells to DOX
in the treatment of an MTC-derived cell line in vitro
(22). It has been found that COX-2
expression is able to induce the expression of the MDR1 gene, which
codes for the P-gp efflux pump, which pumps numerous drugs out of
cells (29). This association
indicates that the inhibition of COX-2 by specific inhibitors,
including CXB, rofecoxib or others, may improve the sensitivity of
cancer cells to chemotherapy. In the present study, the data
confirmed that CXB, a COX-2 inhibitor, could improve the
sensitivity of the MTC cells to DOX and decrease the effective dose
of DOX that must be used.
In the present study, the effect of CXB on the mRNA
and protein expression of MDR1 and COX-2 was examined by qPCR and
immunohistochemistry, respectively. It was found that CXB alone or
in combination with DOX could decrease MDR1 and COX-2 expression,
which complies with previous results (23). As MDR1 codes for the P-gp efflux
pump, it is accepted that the action of CXB on drug efflux is
exerted through the inhibition of MDR1 expression. In addition, the
present results showed that DOX alone was able to increase MDR1 and
COX-2 expression, which showed that DOX has multidrug resistance
for metastatic MTC, and therefore, treatment of metastatic MTC by a
combination of DOX with COX-2 inhibitors is an effective method.
Despite the anti-tumor activity of COX-2 inhibitors, particularly
CXB, they are effective against a wide variety of human epithelial
tumor types, including colorectal, non-small cell lung, breast and
prostate cancers (30). To the best
of our knowledge, the present study is the first to show that DOX
plus CXB is able to decrease tumor growth in vitro and in
vivo.
In conclusion, in the present study it has been
shown that CXB, a COX-2 inhibitor, in combination with DOX, a
chemotherapeutic drug, is able to induce MTC cell apoptosis and
reduce tumor growth in vivo. Furthermore, CXB could enhance
the chemotherapeutic effect of this drug by the inhibition of COX-2
and MDR1 expression.
Acknowledgements
The authors gratefully acknowledge the financial
support provided by the Health Bureau of Jilin (2008Z017).
References
|
1
|
Zhang J, Wang Y, Li D and Jing S: Notch
and TGF-β/Smad3 pathways are involved in the interaction between
cancer cells and cancer-associated fibroblasts in papillary thyroid
carcinoma. Tumour Biol. 35:379–385. 2014.
|
|
2
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006.
|
|
3
|
Tavares MR, Toledo SP, Montenegro FL, et
al: Surgical approach to medullary thyroid carcinoma associated
with multiple endocrine neoplasia type 2. Clinics (Sao Paulo).
67(Suppl 1): 149–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tavares MR, Michaluart P Jr, Montenegro F,
et al: Skip metastases in medullary thyroid carcinoma: a
single-center experience. Surg Today. 38:499–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Machens A, Lorenz K and Dralle H:
Individualization of lymph node dissection in RET (rearranged
during transfection) carriers at risk for medullary thyroid cancer:
value of pre-therapeutic calcitonin levels. Ann Surg. 250:305–310.
2009. View Article : Google Scholar
|
|
6
|
Singal PK, Li T, Kumar D, et al:
Adriamycin-induced heart failure: mechanism and modulation. Mol
Cell Biochem. 207:77–86. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carvalho C, Santos RX, Cardoso S, et al:
Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem.
16:3267–3285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silber JH and Barber G:
Doxorubicin-induced cardiotoxicity. N Engl J Med. 333:1359–1360.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
King PD and Perry MC: Hepatotoxicity of
chemotherapy. Oncologist. 6:162–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Konorev EA, Kotamraju S, et al:
Doxorubicin induces apoptosis in normal and tumor cells via
distinctly different mechanisms. Intermediacy of H(2)O(2)- and
p53-dependent pathways. J Biol Chem. 279:25535–25543. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukhopadhyay P, Rajesh M, Bátkai S, et al:
Role of superoxide, nitric oxide, and peroxynitrite in
doxorubicin-induced cell death in vivo and in vitro. Am J Physiol
Heart Circ Physiol. 296:1466–1483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Das A, Durrant D, Mitchell C, Mayton E, et
al: Sildenafil increases chemotherapeutic efficacy of doxorubicin
in prostate cancer and ameliorates cardiac dysfunction. Proc Natl
Acad Sci USA. 107:18202–18207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dayton A, Selvendiran K, Meduru S, Khan M,
et al: Amelioration of doxorubicin-induced cardiotoxicity by an
anticancer-antioxidant dual-function compound, HO-3867. J Pharmacol
Exp Ther. 339:350–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen C, Xu W and Wang CM: Combination of
celecoxib and doxorubicin increases growth inhibition and apoptosis
in acute myeloid leukemia cells. Leuk Lymphoma. 54:2517–2522. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ponthan F, Wickström M, Gleissman H, et
al: Celecoxib prevents neuroblastoma tumor development and
potentiates the effect of chemotherapeutic drugs in vitro and in
vivo. Clin Cancer Res. 13:1036–1044. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai ZJ, Gao J, Li ZF, et al: In vitro and
in vivo antitumor activity of Scutellaria barbate extract on murine
liver cancer. Molecules. 16:4389–4400. 2001.PubMed/NCBI
|
|
18
|
Cohen EE, Rosen LS, Vokes EE, et al:
Axitinib is an active treatment for all histologic subtypes of
advanced thyroid cancer: results from a phase II study. J Clin
Oncol. 26:4708–4713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salgia R, Sherman S, Hong DS, Ng CS, et
al: A phase I study of XL184, a RET, VEGFR2, and MET kinase
inhibitor, in patients (pts) with advanced malignancies, including
pts with medullary thyroid cancer (MTC). J Clin Oncol. 26(15S):
35222008.
|
|
20
|
Schlumberger MJ, Elisei R, Bastholt L, et
al: Phase II study of safety and efficacy of motesanib in patients
with progressive or symptomatic, advanced or metastatic medullary
thyroid cancer. J Clin Oncol. 27:3794–3801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wells SA Jr, Gosnell JE, Gagel RF, et al:
Vandetanib for the treatment of patients with locally advanced or
metastatic hereditary medullary thyroid cancer. J Clin Oncol.
28:767–772. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zatelli MC, Luchin A, Piccin D, et al:
Cyclooxygenase-2 Inhibitors Reverse Chemoresistance Phenotype in
Medullary Thyroid Carcinoma by a Permeability Glycoprotein-Mediated
Mechanism. J Clin Endocrinol Metab. 90:5754–5760. 2005. View Article : Google Scholar
|
|
23
|
Vivaldi A, Ciampi R, Tacito A, et al:
Celecoxib, a cyclooxygenase-2 inhibitor, potentiates the
chemotherapic effect of vinorelbine in the medullary thyroid cancer
TT cell line. Mol Cell Endocrinol. 355:41–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoff AO and Hoff PM: Medullary thyroid
carcinoma. Hematol Oncol Clin North Am. 21:475–488. 2008.
View Article : Google Scholar
|
|
25
|
Massart C, Gibassier J, Lucas C, et al:
Expression of the MDR1 gene in five human cell lines of medullary
thyroid cancer and reversion of the resistance to doxorubicine by
ciclosporin A and verapamil. Bull Cancer. 83:39–45. 1996.(In
French).
|
|
26
|
Breier A, Barancík M, Sulová Z and Uhrík
B: P-glycoprotein - implications of metabolism of neoplastic cells
and cancer therapy. Curr Cancer Drug Targets. 5:457–468. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Doyle L and Ross DD: Multidrug resistance
mediated by the breast cancer resistance protein BCRP (ABCG2).
Oncogene. 22:7340–7358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnstone RW, Ruefli AA and Smyth MJ:
Multiple physiological functions for multidrug transporter
P-glycoprotein? Trends Biochem Sci. 25:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patel VA, Dunn MJ and Sorokin A:
Regulation of MDR-1 (P-glycoprotein) by cyclooxygenase-2. J Biol
Chem. 277:38915–38920. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jendrossek V: Targeting apoptosis pathways
by Celecoxib in cancer. Cancer Lett. 322:313–324. 2013. View Article : Google Scholar
|