Introduction
Primary testicular lymphoma (PTL) is a rare disease
accounting for ~1% of all non-Hodgkin’s lymphomas (NHLs) and 5% of
all testicular malignant tumors (1–4). PTL
has a marked tendency to involve additional extranodal sites,
including the central nervous system (CNS), contralateral testicles
and Waldeyer’s ring, however, the CNS is the most common site of
involvement. Due to its rarity, the treatment of PTL has not been
standardized. Despite adequate treatment, the majority of PTL
patients relapse within the first two years of treatment and
therefore, the prognosis of PTL is poor.
The current study presents an extremely rare case of
PTL presenting initially as stage I extranodal (IE) disease with
the successive recurrence of the subcutaneous soft tissue and CNS
lymphoma. Patient provided written informed consent.
Case report
In September 2004, a 65-year-old male with no
significant past medical history presented to the Huashan Hospital
(Shanghai, China) with a painless testicular swelling. Physical
examination of the patient revealed a right testicular mass and a
system-by-system review of the body functions was unremarkable,
including the absence of B symptoms. In addition, the Eastern
Cooperative Oncology Group performance status was 0. The patient
underwent a right orchiectomy, and pathological examination
revealed a diffuse large B-cell lymphoma (DLBCL) of non-germinal
center B-cell (GCB) subtype. The results of the patient’s
immunohistochemical analysis were as follows: Leukocyte common
antigen+, cluster of differentiation (CD)20+,
CD10−, B-cell lymphoma (BCL)6−, multiple
myeloma oncogene-1+, BCL2+, CD3−,
CD56−, S100−, epithelial membrane
antigen−, CD30− and anaplastic lymphoma
kinase−, with a Ki67 score of 80%. A further staging
work-up included a bone marrow biopsy with negative observations.
Computed tomography (CT) scans and ultrasound examinations also
showed no evidence of enlarged lymph nodes or extranodal
involvement. Analysis of the cerebrospinal fluid (CSF) was normal
and the serology for human immunodeficiency virus (HIV) was
negative. The clinical Ann Arbor stage was determined as stage I
extranodal (IE) with an age-adjusted International Prognostic Index
score of 0.
The patient completed four cycles of
cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP)
chemotherapy between September 2004 and December 2004. During the
treatment and follow-up, CT scans and ultrasound examinations
showed no evidence of lymphoma progression.
In July 2012, the patient visited the clinic due to
the appearance of three subcutaneous masses above the right knee.
The physical examination was unremarkable, with the exception of
three mobile and rock-hard subcutaneous masses, which were ~3–4 cm
in diameter. The masses were not swollen or painful and the local
temperature was within the normal range. A biopsy was performed on
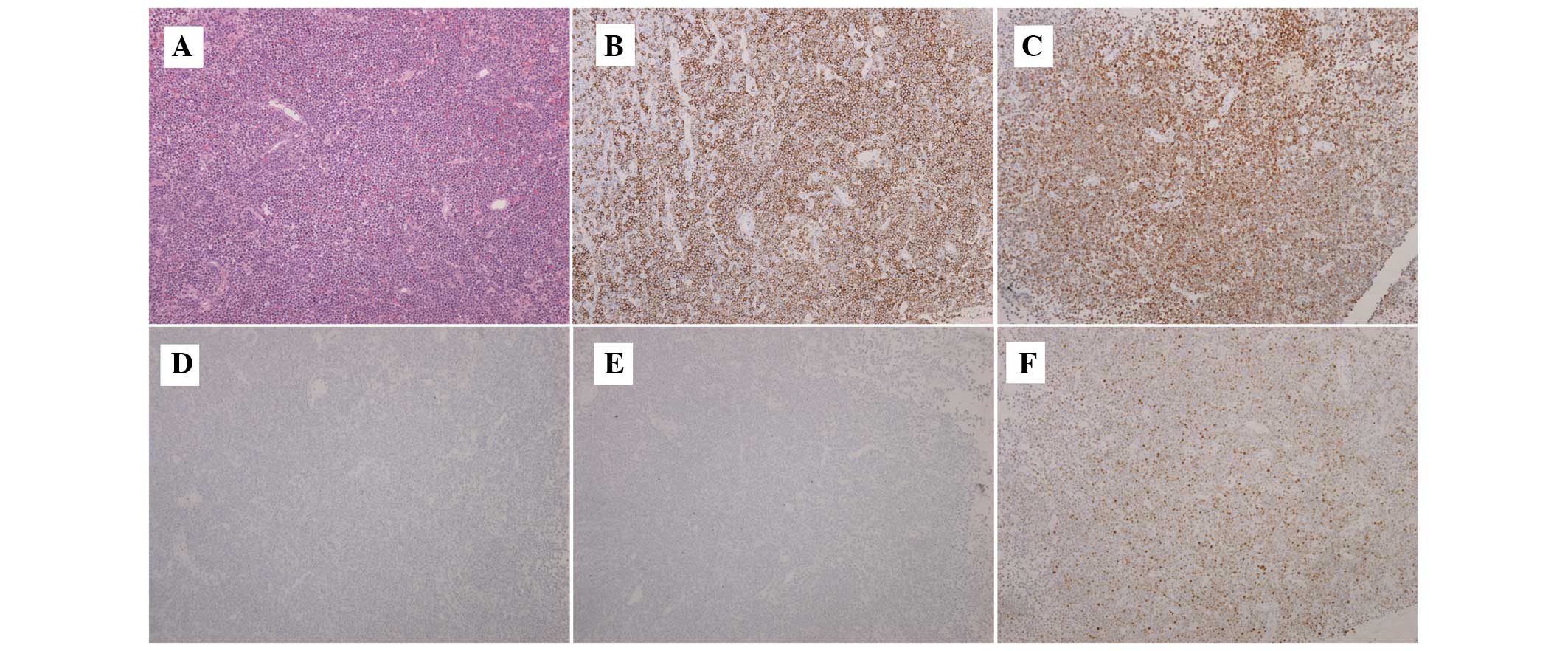
the new lesions and the pathological observations revealed a DLBCL
of non-GCB subtype, with identical morphological and
immunohistochemical features to those of the original testicular
DLBCL of non-GCB subtype (Fig. 1).
A whole body PET-CT was subsequently performed and showed no other
sites of lymphoma involvement. The patient declined chemotherapy,
but underwent 20 fractions of regional irradiation with a total
dose of 40 cGy and consequently experienced resolution of the right
leg masses. The post-treatment CT scan and ultrasound examination
showed no additional evidence of lymphoma.
In January 2013, the patient was admitted to the
Department of Neurosurgery due to progressive weakness in the right
extremities. A neurological examination upon admission showed
right-sided hemiparesis and brain magnetic resonance imaging
revealed a lesion in the left frontal lobe. In addition, the CSF
and ocular slit lamp examinations were normal and the serology for
HIV was negative. A biopsy of this lesion was performed and the
histopathological and immunohistochemical examinations revealed
DLBCL of non-GCB subtype. The patient was then transferred to the
Department of Hematology and the CT scans of the chest, abdomen and
pelvis, and the ultrasound examination of the lymph nodes and left
testicle were normal. The bone marrow biopsy and aspiration showed
no involvement. The patient subsequently received two cycles of
methotrexate-based combined chemotherapy (2 g/m2
methotrexate intravenously on day one, 100 g/m2
teniposide intravenously on days two and three, and 15 mg
dexamthasone on days one to three; for one cycle of 28 days). Brain
magenetic resonance imaging showed that the lesion had evidently
contracted and the patient remained symptom-free. Subsequently, the
patient refused chemotherapy. The patient was closely followed for
three months, but was then lost to follow-up.
Discussion
The current study presents a rare case of PTL with
subcutaneous masses as the sole manifestation of first relapse and
CNS lymphoma as the sole manifestation of second relapse.
PTL is a rare entity, with a reported annual
incidence of ~0.26 per 100,000 individuals in Denmark (5). However, it is also the most common
testicular neoplasm in males over 50 years old (6). One retrospective study that was
reported by the International Extranodal Lymphoma Study Group
(IELSG) included 10 countries and 373 patients with primary
testicular DLBCL and found that the median age at diagnosis was 66
years. Testicular DLBCL is characterized by a particularly high
risk of extranodal relapse even in cases with localized disease at
diagnosis. In this study, the median follow-up was 7.6 years and
195 patients (52%) relapsed; CNS relapse was detected in 56
patients (15%). In addition, the median overall survival (OS) time
was 4.8 years (7). In a
population-based study utilizing the Surveillance, Epidemiology and
End Results database to identify primary testicular DLBCL patients
diagnosed between 1980 and 2005, 769 patients were identified and
the median age at diagnosis was 68.0 years. The incidence of DLBCL
was found to increase over time and the median OS time was 4.6
years. The study confirmed a short overall survival time and a
higher risk of continuous relapse of primary testicular DLBCL
(4).
In total, 68–77.8% of PTL cases are DLBCL (8,9) and
gene expression profiling can further subdivide DLBCL into GCB and
activated B-cell [ABC (non-GCB)] subtypes, which have different
clinical outcomes. In addition, the ABC subtype has a more
aggressive clinical behavior than the GCB subtype (10–12).
Previously, two studies have shown that almost all testicular
DLBCLs belong to the non-GCB subtype. Al-Abbadi et al
(13) studied 18 cases of
testicular DLBCL and found that 89% of them belonged to the non-GCB
subtype and all exhibited high proliferative activity. Furthermore,
Li et al (14) also showed
that 16 out of 17 testicular DLBCL cases belonged to the ABC
subtype. However, two other studies have shown contrasting results.
Kemmerling et al (15)
presented the immunohistochemical analysis of 18 cases of PTL and
found that 15 cases were classified as DLBCL, showing no
significant prevalence of the ABC subtype (9/15, 60%) compared with
the GCB subtype (6/15, 40%). The survival analysis showed that
patients with GCB subtype DLBCL exhibited a trend towards a longer
OS time than the patients with ABC subtype DLBCL, however, no
statistically significant difference was observed. In addition,
Hasselblom et al (16)
reported that 29 testicular DLBCL patients could be classified into
nine (31%) cases of GCB phenotype and 20 (69%) cases of non-GCB
phenotype, however, no difference was identified in the event-free
survival or OS time between the two groups. The statistical results
may be affected by the small sample size and the different single
center studies. Therefore, PTL cases from multiple institutes must
be enrolled and analyzed in future studies.
PTL is an immune-privileged site-associated
lymphoma, and in order to escape immunological surveillance, the
lymphoma cells must develop an immune escape phenotype (17,18). A
common aberration leading to immune escape is the loss of human
leukocyte antigen expression, while an additional common aberration
is a high level of somatic hypermutation. Primary central nervous
system lymphoma (PCNSL) is also an immune-privileged
site-associated lymphoma and may exhibit the same immune escape
ability as PTL. However, these two types of rare lymphoma have
significantly different presentations. PTL has a marked tendency to
involve additional extranodal sites, including other
immune-privileged sites. The CNS is the most commonly involved
site. However, the relapse of PCNSL is almost (90–95%) confined to
the CNS and few studies have reported the testis involvement of
PCNSL. These phenomena indicate that PTL is much more aggressive
than PCNSL and has unique mechanisms of invasion, but the exact
mechanism of this aggressive behavior remains unknown. The aberrant
expression of adhesion molecules may be an important factor, as the
adhesion molecules mediate the cell-cell and cell-matrix
interactions, affecting the homing and migration of lymphoma cells,
which are important in metastatic processes. One previous study
(19) detected the expression of
integrins and other adhesion molecules in the testicular lymphoma
cells and matrix. A few adhesion molecules, including CD49f/very
late activation antigen (VLA)-6, CD49d/VLA-4, CD54 and CD62L, were
detectable in a small number of lymphomas, however, the expression
of other adhesion molecules was lacking. This expression pattern
was indicative of high metastatic potential. Furthermore, Kawakami
et al (20) reported that
testicular lymphoma tissues showed hypermethylation of the
tumor-suppressor genes, including E-cadherin, Ras association
(RalGDS/AF-6) domain family member 1 and retinoic acid receptor β,
which have been implicated in the pathogenesis of human cancer. The
study partially explained the mechanism of the dysexpression of
adhesion molecules. Dysadherin is a recently described cell
membrane glycoprotein, which exhibits an anti-cell-cell adhesion
function and downregulates E-cadherin. A study that detected the
expression of E-cadherin and dysadherin in eight primary testicular
B-cell lymphomas by immunohistochemistry identified that dysadherin
was highly expressed in all cases and found to correlate with
aberrant E-cadherin expression (21).
Due to the rarity of PTL, the optimal strategy for
treatment remains unclear, however, an almost universal agreement
on the treatment of PTL has been reached. According to the
different stages of the disease, the treatment varies; for the
early stage (stages I/II), the standard treatment has not yet been
established, but orchiectomy is required. Post-orchiectomy systemic
treatment decreases the risk of relapse, and CHOP and CHOP-like
regimens are the mainstay of chemotherapy. One previous study of
373 cases of testicular DLBCL showed that anthracycline-based
chemotherapy, CNS prophylaxis and contralateral testicular
irradiation may improve the outcome of PTL (7). As CNS relapse continues to be a major
problem for PTL patients, routine CNS prophylaxis is recommended by
the majority of physicians, however, the best strategy remains
debatable; radiation therapy and intrathecal chemotherapy may be
favorable. A retrospective study by Mazloom et al (22) showed that scrotal radiation therapy
is associated with an improved OS. Furthermore, the addition of the
anti-CD20 monoclonal antibody, rituximab, to the chemotherapy has
led to a marked improvement in the treatment of B-cell lymphoma.
The study also showed that rituximab CHOP-based chemotherapy as
intrathecal chemotherapy may improve OS. However, the
population-based study did not achieve the anticipated improvement
in the survival of testicular DLBCL patients in the rituximab era,
although, it does not imply that rituximab can not add value to the
treatment of PTL. An additional study also showed that patients
with testicular DLBCL exhibited significantly worse survival times
in the rituximab era (23). Further
studies are therefore required. For advanced disease, patients must
be treated according to the guidelines for the treatment of
advanced-stage DLBCL. The standard therapeutic option for patients
with stage III/IV disease is conventional anthracycline-containing
combined chemotherapy, however, chemotherapy plus rituximab with
the addition of prophylactic scrotal radiotherapy and intrathecal
chemotherapy is the most positive option. The study by the IELSG
showed an apparently improved outcome in advanced-stage DLBCL
patients following anthracycline-based chemotherapy, CNS
prophylaxis and contralateral testicular irradiation (7). The optimal therapy for patients with
relapsed PTL has not yet been defined in prospective trials,
however, the therapeutic strategy should be similar to other
relapsed NHLs. In addition, the treatment should be decided
according to age, performance status, organ function and previous
treatments.
PTL has a predilection for spreading to unusual
extranodal sites, including the CNS, contralateral testicles and
Waldeyer’s ring. However, the localized subcutaneous relapse and
subsequent brain relapse observed in the present case is an
extremely rare sign of PTL dissemination.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. NSFC81170491) and
the Shanghai Municipal Health Bureau (grant no. 2010079).
References
|
1
|
Gospodarowicz MK, Sutcliffe SB, Brown TC,
Chua T and Bush RS: Patterns of disease in localized extranodal
lymphomas. J Clin Oncol. 5:875–880. 1987.PubMed/NCBI
|
|
2
|
Zouhair A, Herrmann E, Ugurluer G, Gaye
PM, Mirimanoff RO and Ozsahin M: Primary testicular lymphoma. Swiss
Med Wkly. 140:w130762010.
|
|
3
|
Eskey CJ, Whitman GJ and Chew FS:
Malignant lymphoma of the testis. AJR Am J Roentgenol. 169:8221997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gundrum JD, Mathiason MA, Moore DB and Go
RS: Primary testicular diffuse large B-cell lymphoma: a
population-based study on the incidence, natural history, and
survival comparison with primary nodal counterpart before and after
the introduction of rituximab. J Clin Oncol. 27:5227–5232. 2009.
View Article : Google Scholar
|
|
5
|
Møller MB, d’Amore F and Christensen BE:
Testicular lymphoma: a population-based study of incidence,
clinicopathological correlations and prognosis. The Danish Lymphoma
Study Group, LYFO. Eur J Cancer. 30A:1760–1764. 1994.PubMed/NCBI
|
|
6
|
Horne MJ and Adeniran AJ: Primary diffuse
large B-cell lymphoma of the testis. Arch Pathol Lab Med.
135:1363–1367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zucca E, Conconi A, Mughal TI, Sarris AH,
Seymour JF, Vitolo U, Klasa R, Ozsahin M, Mead GM, Gianni MA, et
al; International Extranodal Lymphoma Study Group. Patterns of
outcome and prognostic factors in primary large-cell lymphoma of
the testis in a survey by the International Extranodal Lymphoma
Study Group. J Clin Oncol. 21:20–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tepperman BS, Gospodarowicz MK, Bush RS
and Brown TC: Non-Hodgkin lymphoma of the testis. Radiology.
142:203–208. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shahab N and Doll DC: Testicular lymphoma.
Semin Oncol. 26:259–269. 1999.
|
|
10
|
Rosenwald A, Wright G, Chan WC, Connors
JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland
EB, Giltnane JM, et al; Lymphoma/Leukemia Molecular Profiling
Project. The use of molecular profiling to predict survival after
chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med.
346:1937–1947. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosenwald A and Staudt LM: Gene expression
profiling of diffuse large B-cell lymphoma. Leuk Lymphoma. 44(Suppl
3): S41–S47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Z, Du Z, Chen J, Chen Z, Bao Y and
Tang F: Prognostic evaluation of immunohistochemical profiles in
diffuse large B-cell lymphoma: a Chinese study. Med Oncol.
28:241–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Abbadi MA, Hattab EM, Tarawneh MS, Amr
SS, Orazi A and Ulbright TM: Primary testicular diffuse large
B-cell lymphoma belongs to the nongerminal center B-cell-like
subgroup: A study of 18 cases. Mod Pathol. 19:1521–1527. 2006.
View Article : Google Scholar
|
|
14
|
Li D, Xie P and Mi C: Primary testicular
diffuse large B-cell lymphoma shows an activated B-cell-like
phenotype. Pathol Res Pract. 15:611–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kemmerling R, Stintzing S, Mühlmann J,
Dietze O and Neureiter D: Primary testicular lymphoma: a strictly
homogeneous hematological disease? Oncol Rep. 23:1261–1267.
2010.PubMed/NCBI
|
|
16
|
Hasselblom S, Ridell B, Wedel H, Norrby K,
Sender Baum M and Ekman T: Testicular lymphoma - a retrospective,
population-based, clinical and immunohistochemical study. Acta
Oncol. 43:758–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Booman M, Douwes J, Glas AM, Riemersma SA,
Jordanova ES, Kok K, Rosenwald A, de Jong D, Schuuring E and Kluin
PM: Mechanisms and effects of loss of human leukocyte antigen class
II expression in immune-privileged site-associated B-cell lymphoma.
Clin Cancer Res. 12:2698–2705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riemersma SA, Jordanova ES, Schop RF,
Philippo K, Looijenga LH, Schuuring E and Kluin PM: Extensive
genetic alterations of the HLA region, including homozygous
deletions of HLA class II genes in B-cell lymphomas arising in
immune-privileged sites. Blood. 96:3569–3577. 2000.
|
|
19
|
Horstmann WG and Timens W: Lack of
adhesion molecules in testicular diffuse centroblastic and
immunoblastic B cell lymphomas as a contributory factor in
malignant behaviour. Virchows Arch. 429:83–90. 1996.PubMed/NCBI
|
|
20
|
Kawakami T, Okamoto K, Kataoka A, Koizumi
S, Iwaki H, Sugihara H, Reeve AE, Ogawa O and Okada Y: Multipoint
methylation analysis indicates a distinctive epigenetic phenotype
among testicular germ cell tumors and testicular malignant
lymphomas. Genes Chromosomes Cancer. 38:97–101. 2003. View Article : Google Scholar
|
|
21
|
Batistatou A, Scopa CD, Ravazoula P,
Nakanishi Y, Peschos D, Agnantis NJ, Hirohashi S and
Charalabopoulos KA: Involvement of dysadherin and E-cadherin in the
development of testicular tumours. Br J Cancer. 93:1382–1387. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mazloom A, Fowler N, Medeiros LJ, Iyengar
P, Horace P and Dabaja BS: Outcome of patients with diffuse large
B-cell lymphoma of the testis by era of treatment: the M. D.
Anderson Cancer Center experience. Leuk Lymphoma. 51:1217–1224.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahashi H, Tomita N, Yokoyama M, Tsunoda
S, Yano T, Murayama K, Hashimoto C, Tamura K, Sato K and
Ishigatsubo Y: Prognostic impact of extranodal involvement in
diffuse large B-cell lymphoma in the rituximab era. Cancer.
118:4166–4172. 2012. View Article : Google Scholar : PubMed/NCBI
|