Introduction
Hepatoid adenocarcinoma (HAC) is a rare form of
adenocarcinoma that is defined by morphological and functional
hepatic differentiation (1). To the
best of our knowledge, few cases have been reported in the
literature that have shown the prognosis of this subtype of lung
adenocarcinoma (Table I). The
present study reports an extremely rare case of HAC of the lung
(HAL) presenting with α-fetoprotein (AFP) production.
 | Table IClinical pathological data of cases of
hepatoid adenocarcinoma of the lung reported in the literature. |
Table I
Clinical pathological data of cases of
hepatoid adenocarcinoma of the lung reported in the literature.
| | | | | | | Prior to
treatment | | | |
|---|
| | | | | | |
| | | |
|---|
| First author, Year
(ref) | Gender | Age, years | Diagnosis | Stage | Location | Size, cm | Metastases | Serum AFP, ng/ml | Tissue AFP | Therapy | Time for AFP levels
to return to normal | Prognosis |
|---|
| Yoshino I, 1996
(13) | M | 54 | ADSCC | I | LU | 2.0 | No | 696 | | Surgery | 2 weeks post
surgery | 24 months, A |
| Arnould L, 1997
(14) | M | 36 | SCC | - | LU | 10.0 | No | 11,600 | + | Surgery | 19 weeks post
surgery | 7 months, S |
| Nasu, 1998 (15) | M | 63 | LCNEC | - | RU | 8.0 | No | 14,000 | + | - | - | 11 months, S |
| Hayashi Y, 2002
(16) | M | 55 | AD | IB | RU | 6.0 | No | / | + | Surgery | Postoperative | 32 months, A |
| Hiroshima K, 2002
(17) | M | 71 | SCC | IIIA | RL | 10.5 | No | 7,417 | + | Surgery +
radiotherapy | >2 year | >2 years, S |
| M | 70 | SCC | IIIB | RU | 6.0 | No | 24.3 | / | Surgery | - | >2 years, S |
| F | 64 | LCNEC | IIIB | RL | - | No | 74.4 | / | Chemotherapy +
surgery | - | >2 years, S |
| Kitada et al,
2011 (6) | M | 69 | AD | IIIA | RL | 7.0 | Lymph node | 4,620 | + | Surgery | 2 weeks post
surgery | A |
During embryonic development, the lung, liver and
stomach are derived from the primitive foregut. Due to certain
abnormalities in differentiation, the adenocarcinoma cells from
specific organs, including the lungs, differentiate into liver
cells. Studies have shown that this type of tumor is able to
generate products, including albumin, α-antitrypsin, prothrombin,
ferritin, transferrin and AFP, which are usually produced by normal
liver cells and liver cancer cells. Therefore, this type of tumor
is termed a HAC. The majority of HACs originate from the stomach
and HACs that originate from the lungs are extremely rare. A study
by Ishikura et al (2)
defined these lung cancers as lung liver adenocarcinoma. This type
of cancer exhibits a liver cell-like differentiation and
morphology, and is AFP(positive) in histopathological analysis.
The present study includes immunohistochemical
analyses, serum AFP levels, chest computed tomography (CT) images,
the clinical course of poor prognosis through concurrent
chemoradiation and adjuvant chemotherapy, and a relatively
long-term follow-up. The study was approved by the ethics committee
of the Cancer Hospital, Chinese Academy of Medical Sciences,
National GCP Center for Anticancer Drugs (Beijing, China). The
patient provided written informed consent.
Case report
A 48-year-old Chinese male was admitted to the
Cancer Institute and Hospital, Chinese Academy of Medical Sciences
and Peking Union Medical College (Beijing, China) due to the
primary complaint of back pain over a 6-month period, which became
progressively worse in the latter 3 months. The patient was 174-cm
tall and weighed 65 kg, resulting in a body surface area of 1.81
m2. The patient had a 70 pack-year smoking history of 30
years, however, did not report a dry or productive cough and
experienced no palpitations or chest tightness. The patient did not
have alcoholic hepatitis or a relevant family history. The
patient’s Karnofsky performance status (KPS) score was 90. There
was no abnormality in the abdomen and the pretreatment AFP levels
progressively increased from 1,926 to 6,283 ng/ml. A chest-CT scan
demonstrated a lobulated soft-tissue mass, extending from the left
lung apex to the middle and posterior mediastinum, and the longest
cross-sectional dimension of the mass was 7.9×10.0 cm (Fig. 1A). An abdominal-CT scan manifested a
1.5×1.5 cm contrast-enhancing nodule on the posterior lobe of the
liver, which was diagnosed as a hemangioma by the radiologist. No
enlarged lymph nodes were found on the retroperitoneal and
bilateral inguinal areas. A pretreatment ultrasound examination
revealed that no clear nodules or masses were observed elsewhere in
the liver, gallbladder, spleen or pancreas. No enlarged lymph nodes
were found in the abdominal cavity, retroperitoneal or inguinal
area. A bronchoscopy demonstrated a bloody discharge in the
subsegmental bronchus in the apex of the posterior segment of the
upper lobe of the left lung. Brain magnetic resonance imaging
showed that the bilateral ventricles were symmetrical and no
obvious enlargement or shift was observed. There were no abnormal
enhanced nodules or masses in the bilateral cerebral hemispheres,
cerebellum or pons. Complete blood counts, urinalysis, coagulation
studies and chemical analyses were normal. Except for the elevation
of AFP serum levels, the tumor markers, including carcinoembryonic
antigen, carbohydrate antigen 125, squamous cell carcinoma antigen,
neuron-specific enolase and cytokeratin (CK) 19 fragment, were
within the normal ranges. Hepatitis B virus (Hb) surface antigen,
Hb surface antibody, hepatitis C virus, treponema pallidum and
human immunodeficiency virus were all negative. The pathological
morphology of the biopsy from the mediastinal lymph node supported
the diagnosis of lung cancer. Immunohistochemical analyses
demonstrated positive staining for CK7 in a marginal number of
cells, and AFP(positive), pan-cytokeratin [AE1/AE3(positive)],
CK18(positive), vimentin(positive), hepatocyte(positive),
CK20(negative), renal cell carcinoma(negative) and thyroid
transcription factor-1(negative) (Fig.
2), indicating mediastinal lymph node involvement of HAL. The
patient received concurrent chemoradiation between August 2, 2011
and September 13, 2011. Radiation therapy of 60 Gy/2 Gy/30 F was
delivered through intensity-modulated radiation therapy using 6-MV
X-rays, concomitant with two cycles of paclitaxel plus cisplatin. A
CT scan demonstrated a partial response following the concurrent
chemoradiation, with a maximal cross-sectional area of 5.3×4.6 cm
and the hilar lymph was ~0.5×0.9 cm (Fig. 1B). Subsequent to four cycles of
paclitaxel plus cisplatin chemotherapy and concurrent
chemoradiation, the area of the largest cross-section of the left
lung apex to the middle and posterior mediastinum was 3.3×4.2 cm
(Fig. 1C). There was a decrease in
the AFP serum levels to 23.11 ng/ml observed in the period between
October 22, 2011 and March 6, 2012 (Fig. 3). Subsequently, the patient
continued to receive consolidative chemotherapy. However, following
five cycles of consolidative chemotherapy, the AFP serum level
increased to 386.8 ng/ml again and correspondingly the CT scan
showed new pulmonary lesions (Fig.
1D), indicating disease progression. Therefore, the patient
received a different chemotherapy regimen (docetaxel and
nedaplatin) and the serum level of AFP increased to 2,070 ng/ml two
months after five cycles of the regimen (Fig. 3). A repeated CT scan did not
demonstrate metastasis in the liver, however, the size of the lung
lesions continued to increase (Fig.
1E), an additional imaging examination showed tumor recurrence.
The patient received regular follow-up (Table II). The left lung lump of the
largest cross-section was 5.0×4.0 cm, and the nodules of metastasis
increased and enlarged, with the largest being ~2.1 cm (Fig. 1F–H). On March 17, 2013, the patient
succumbed to a lung infection.
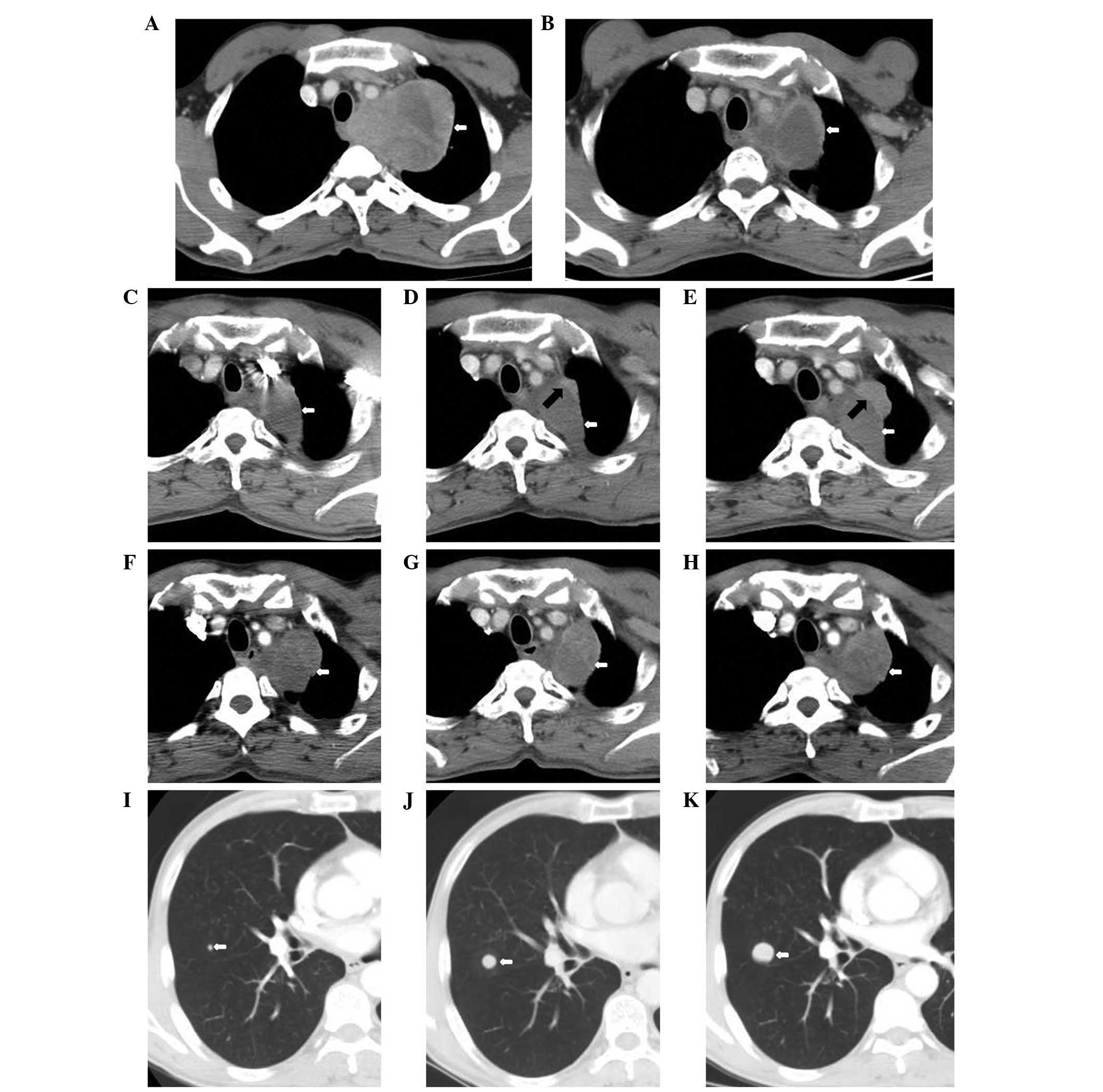 | Figure 1Chest computed tomography (CT) during
the clinical course. (A) Pre-treatment on July 29, 2011 of the left
lung apex to the middle and posterior mediastinum. The area of the
largest cross-section is 7.9×10.0 cm (white arrow). (B)
Post-concurrent chemoradiation on September 16, 2011. CT of the
soft-tissue mass shows a maximal cross-sectional area of 5.3×4.6 cm
(white arrow). (C) November 22, 2011, the left lung apex to the
middle and posterior mediastinum following four cycles of
paclitaxel + cisplatin chemotherapy and concurrent chemoradiation.
The area of the largest cross-section is 3.3×4.2 cm (white arrow).
(D) March 7, 2012, pulmonary primary lesions of the anterior and
lateral to the adjacent irregular nodules following five cycles of
docetaxel + nedaplatin chemotherapy. The area of the largest
cross-section is 0.7×2.1 cm (black arrow). (E) May 14, 2012,
pulmonary primary lesions of the anterior and lateral to the
adjacent irregular nodules. The area of the largest cross-section
is 1.6×3.1 cm (black arrow). (F) July 26, 2012 and (G) September
13, 2012, the left lung lump of the largest cross-section is
4.9×4.8 cm (white arrow). (H) October 25, 2012, the left lung lump
of the largest cross-section is 5.0×4.0 cm (white arrow). (I) July
26, 2012, the nodes in the image demonstrate nodules of metastatic
carcinoma and the largest is ~0.4 cm. (J) September 13, 2012 and
(K) October 25, 2012, the nodules of metastasis increased and
enlarged. The largest were ~1.2 and ~2.1 cm, respectively. |
 | Table IICT/MRI and tumor marker examinations
of the patient’s clinical course. |
Table II
CT/MRI and tumor marker examinations
of the patient’s clinical course.
| Date | Clinical course | AFP-ng/ml
(07)a | CEA ng/ml
(0–5)a | CA125 U/ml
(0–35)a | SCC ng/ml
(0–1.5)a | Cyfra21-1 ng/ml
(0–3.3)a | NSE ng/ml
(0–18)a | LDH U/l
(135–225)a | CT/MRI |
|---|
| July 29, 2011 | Prior to
treatment | 6,283 | 4.16 | 8.05 | 0.5 | 2.33 | 14.34 | 327 | The left lung apex to
the middle and posterior mediastinum, the area of the largest
cross-section was 7.8×7.9×10.0 cm |
| August 15, 2011 | Concurrent | 4,241 | 4.11 | 8.54 | 0.5 | 4.25 | 12.71 | 346 | - |
| August 30, 2011 | Chemoradiation | 1,581 | 3.44 | 8.90 | 0.5 | 4.08 | 11.25 | 182 | - |
| September 16,
2011 | Following concurrent
chemoradiation | 318.7 | 3.05 | 7.19 | 0.6 | 2.44 | 10.58 | 145 | CT of the soft tissue
mass showed a maximum cross sectional area of 5.3×4.6 cm and the
size of hilar lymph nodes were reduced to ~0.5×0.9 cm |
| September 29,
2011 | | 118.5 | 3.78 | 10.26 | 0.7 | 2.06 | 15.33 | 202 | - |
| October 12, 2011 | | - | 2.96 | - | - | - | - | 191 | Brain MRI showed no
abnormal enhanced nodules |
| November 22,
2011 | Following 4 cycles of
paclitaxel + cisplatin | 23.11 | 3.10 | 13.69 | 1.0 | 2.97 | 14.71 | 159 | The left lung apex to
the middle and posterior mediastinum, the area of the largest
cross-section was 3.3×4.2 cm |
| December 6, 2011 | Chemotherapy | | | | | | | |
| January 17, 2012 | | 70.33 | 3.69 | 9.83 | 0.7 | 2.02 | 10.06 | 186 | - |
| February 6, 2012 | Following 4 cycles of
docetaxel + nedaplatin chemotherapy | 193.6 | 4.24 | 8.02 | 0.5 | 1.66 | 9.84 | 129 | - |
| March 7, 2012 | Following 5 cycles of
docetaxel + nedaplatin chemotherapy | 386.8 | 3.18 | 7.54 | 0.5 | 1.35 | 9.03 | 141 | Pulmonary primary
lesions of the anterior and lateral to the adjacent irregular
nodules, the area of the largest cross section was 0.7×2.1 cm |
| March 19, 2012 | | 588.2 | 3.37 | 7.94 | 0.8 | 1.48 | 9.49 | 119 | - |
| May 14, 2012 | Follow-up | 2,070 | - | - | - | - | - | - | Pulmonary primary
lesions of the anterior and lateral to the adjacent irregular
nodules, the area of the largest cross section was 1.6×3.1 cm |
| August 1, 2012 | Following 3 cycles of
cytoxan + epirubicin + cisplatin chemotherapy | 5,303 | - | - | - | | - | - | The left lung lump
with the largest cross section was 4.9×4.8 cm, the large node of
metastatic carcinoma was ~0.4 cm |
| September 14,
2012 | Following 1 cycle
of gemcitabine + carboplatin | 5,682 | 4.01 | 11.24 | 0.6 | 1.92 | 11.43 | 269 | The nodules of
metastasis increased in number by five and enlarged, the largest
was ~1.2 cm |
| October 25,
2012 | Follow up | 6,438 | - | - | - | - | - | - | The left lung lump
with the largest cross section was 5.0×4.0 cm, the largest nodule
was ~2.1 cm |
| December 12,
2012 | Icaritin | 24,989 | - | - | - | - | - | - | - |
| January 10,
2013 | | 57,800 | - | - | - | - | - | - | - |
Discussion
Stomach HAC is the most common AFP-producing
carcinoma, accounting for ~2.5–15% of gastric cancers (3–5).
However, AFP-producing lung cancer is rarely reported, and its
pathological cell features and clinical symptoms remain unclear.
Thus far, no standard treatment strategy is available for
lung-originated HAC. The patient profile in the current study is of
a middle-aged male and heavy smoker with the primary clinical
symptom of back pain. In addition, the serum AFP level of the
patient was significantly elevated. The diagnosis of HAL was
primarily based on morphological features and immunohistochemical
markers facilitated the diagnosis. Mediastinal metastasis of this
disease has not yet been reported. According to the literature, the
combined treatment of surgical resection and adjuvant
tegafur-uracil chemotherapy is a common selection for HAL treatment
(6), and surgery was not considered
in the present study. To prevent the continuing enlargement of the
tumor from compressing the respiratory tracts and esophagus,
radiotherapy to the mediastinum and concurrent chemotherapy were
applied to control the disease. Radiotherapy was considered to be
an appropriate treatment, as the patient was in a condition of
physical tolerance, with a KPS score of 90 and was able to tolerate
radiotherapy. Additionally adenocarcinoma is relatively sensitive
to irradiation. However, radiotherapy has several disadvantages; it
is a type of local treatment approach and metastasis may occur
during the treatment. The relapse in this patient was detected via
observation of an increase in serum AFP level, and this was
identified significantly earlier than any indication from imaging
findings.
Serum AFP was clearly increased in the patient of
the present study and was also detected in the cytoplasm by
immunohistochemical staining. However, AFP is not unique to HAL and
is more commonly found in hepatocellular carcinoma,
cholangiocarcinoma and teratomatous germ cell tumors (7). Therefore, other immunohistochemical
stains are necessary to confirm the diagnosis of HAL. Monoclonal
antibody hepatocyte expression appears to be confined to normal and
neoplastic liver cells and sensitivity for this is heightened
compared with AFP. Hepatocyte reactivity has been shown regularly
in hepatocellular carcinomas, hepatoblastomas and HAC. However
reactivity in cholangiocarcinomas and metastatic tumors to the
liver is rare. Immunostaining for CKs is helpful in defining HAL.
CK18, marker of simple parenchyma, was positive in HAL, while CK20
was negative in HAL; staining for CK7 can be positive (8) or negative (9).
Since HAL is an extremely heterogeneous type of
tumor, there is currently no standard treatment. HAL is generally
treated as an adenocarcinoma of the common type, derived from the
involved organ system. Patients with localized tumors undergo
surgery (10) and those with
mediastinal metastases are treated with concurrent chemoradiation
as opposed to surgery. The patient in the present study obtained an
initial partial response following four cycles of paclitaxel plus
cisplatin chemotherapy and concurrent chemoradiation. The patient
subsequently underwent five cycles of docetaxel plus nedaplatin
chemotherapy, until the disease progressed. Transbronchial needle
aspiration tissues were not enough to detect the molecular markers
of therapeutic significance, including epidermal growth factor
receptor and anaplastic lymphoma kinase mutations.
Another indication for the poor prognosis of HAL is
the production of AFP, which possesses immunosuppressive properties
(11). The poor prognosis was
considered to be associated with the extensive venous invasion and
locally advanced or metastatic presentation (12). The unfavorable outcome of the
patient in the present study may be due to a combination of various
factors, including late unresectable disease and a short
progression-free survival time following chemotherapy.
The diagnosis of HAL in the patient was confirmed
and the focus of the present study is the clinical significance of
the initial elevated serum AFP level. One possible reason for this
is that there were two primary tumors, lung cancer and liver
cancer. The timely diagnosis and treatment of the lung lesions
avoided the misdiagnosis of lung cancer. However, as this patient
had no previous history of liver disease and did not exhibit
abnormal liver function, it was unlikely to conclude that the AFP
abnormality was associated with liver disease. It was more likely
that the disease was associated with lung cancer and the multiple
lesions in the liver were pulmonary metastases. This type of lung
cancer with elevated serum AFP levels is a special variety. It is
noteworthy that certain lung cancers possess similar morphological
features to hepatocellular carcinoma or liver cell-like
differentiation. Immunohistochemical detection on a mediastinal
needle-biopsy specimen further confirmed the diagnosis, thereby
saving valuable time that could be used for treatment.
By identifying and exploring the aforementioned
circumstances, clinicians should be aware that in addition to liver
cancer, other malignancies, including lung cancer, may be
accompanied by elevated serum AFP levels. Comprehensive systemic
examinations and combined diagnostic examinations are, therefore,
required to prevent misdiagnosis.
In conclusion, the heterogeneity of HAC complicates
the diagnosis. Furthermore, the associated poor prognosis
emphasizes the requirement for an accurate and early diagnosis
using immunohistochemistry as an increase in serum AFP levels
occurs significantly earlier compared with any indications from
imaging examinations. However, the optimal management of HAL is
still not well defined and requires further investigation.
References
|
1
|
Adachi Y, Tsuchihashi J, Shiraishi N,
Yasuda K, Etoh T and Kitano S: AFP-producing gastric carcinoma:
multivariate analysis of prognostic factors in 270 patients.
Oncology. 65:95–101. 2003.
|
|
2
|
Ishikura H, Kanda M, Ito M, Nosaka K and
Mizuno K: Hepatoid adenocarcinoma: a distinctive histological
subtype of alpha-fetoprotein-producing lung carcinoma. Virchows
Arch A Pathol Anat Histopathol. 417:73–80. 1990.
|
|
3
|
Kim TB and Yu WS: Prognostic value of
preoperative serum alpha-fetoprotein level in resectable gastric
cancer. J Korean Gastric Cancer Assoc. 3:33–37. 2003.
|
|
4
|
Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long
Z, et al: Clinicopathologic features and prognostic factors in
alpha-fetoprotein-producing gastric cancers: analysis of 104 cases.
J Surg Oncol. 102:249–255. 2010.
|
|
5
|
McIntire KR, Waldmann TA, Moertel CG and
Go VL: Serum alpha-fetoprotein in patients with neoplasms of the
gastrointestinal tract. Cancer Res. 35:991–996. 1975.
|
|
6
|
Kitada M, Ozawa K, Sato K, Matsuda Y,
Hayashi S, Tokusashi Y, et al: Alpha-fetoprotein-producing primary
lung carcinoma: a case report. World J Surg Oncol. 9:472011.
|
|
7
|
Lau SK, Prakash S, Geller SA and Alsabeh
R: Comparative immunohistochemical profile of hepatocellular
carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum
Pathol. 33:1175–1181. 2002.
|
|
8
|
Tsung JS and Yang PS: Hepatoid carcinoma
of the ovary: characteristics of its immunoreactivity. A case
report. Eur J Gynaecol Oncol. 25:745–748. 2004.
|
|
9
|
Karayiannakis AJ, Kakolyris S,
Giatromanolaki A, Courcoutsakis N, Bolanaki H, Chelis L, et al:
Hepatoid adenocarcinoma of the gallbladder: Case report and
literature review. J Gastrointest Cancer. Sep 22–2011.(Epub ahead
of print).
|
|
10
|
Slotta JE, Jüngling B, Kim YJ, Wagner M,
Igna D and Schilling MK: Hepatoid adenocarcinoma of the transverse
colon. Int J Colorectal Dis. 27:989–991. 2012.
|
|
11
|
Inagawa S, Shimazaki J, Hori M, Yoshimi F,
Adachi S, Kawamoto T, et al: Hepatoid adenocarcinoma of the
stomach. Gastric Cancer. 4:43–52. 2001.
|
|
12
|
Maitra A, Murakata LA and Albores-Saavedra
J: Immunoreactivity for hepatocyte paraffin 1 antibody in hepatoid
adenocarcinomas of the gastrointestinal tract. Am J Clin Pathol.
115:689–694. 2001.
|
|
13
|
Yoshino I, Hayashi I, Yano T, Takai E,
Mizutani K and Ichinose Y: Alpha-fetoprotein-producing
adenocarcinoma of the lung. Lung Cancer. 15:125–130. 1996.
|
|
14
|
Arnould L, Drouot F, Fargeot P, Bernard A,
Foucher P, Collin F and Petrella T: Hepatoid adenocarcinoma of the
lung: report of a case of an unusual alpha-fetoprotein-producing
lung tumor. Am J Surg Pathol. 21:1113–1118. 1997.
|
|
15
|
Nasu M, Soma T, Fukushima H, Kudo K and
Matsubara O: Hepatoid carcinoma of the lung with production of
alpha-fetoprotein and abnormal prothrombin: an autopsy case report.
Mod Pathol. 10:1054–1058. 1997.
|
|
16
|
Hayashi Y, Takanashi Y, Ohsawa H, Ishii H
and Nakatani Y: Hepatoid adenocarcinoma in the lung. Lung Cancer.
38:211–214. 2002.
|
|
17
|
Hiroshima K, Iyoda A, Toyozaki T, Haga Y,
Baba M, Fujisawa T, Ishikura H and Ohwada H:
Alpha-fetoprotein-producing lung carcinoma: report of three cases.
Pathol Int. 52:46–53. 2002.
|
|
18
|
Kitada M, Ozawa K, Sato K, Matsuda Y,
Hayashi S, Tokusashi Y, et al: Alpha-fetoprotein-producing primary
lung carcinoma: a case report. World J Surg Oncol. 9:472011.
|

















