Introduction
The Toll-like receptors (TLRs) are key regulators of
the innate immune system and function as pattern recognition
proteins that detect microbe- and host-derived endogenous molecular
patterns (1,2). Thus far, 13 mammalian TLRs have been
identified and each TLR recognizes a specific ligand; TLR4 is the
receptor for bacterial lipopolysaccharide, TLR5 recognizes
flagellin and members of the TLR9 subfamily recognize RNA (TLR3, 7
and 8) and DNA (TLR9) structures (1,2).
Differences have also been identified in the subcellular
localization of the various TLRs; TLR1, 2 and 4 are typically cell
surface bound, whereas the nucleotide-sensing receptors, TLR3 and
TLR13, as well as the TLR9 subfamily, reside in the intracellular
compartments (3–6). Ligand binding to TLRs activates
transcription factors, most notably NF-κB and the eventual outcome
of TLR activation in cells of the immune system is an innate immune
reaction, which is characterized by increased cytokine and
interleukin production (2).
Eventually, this inflammatory reaction results in the activation of
the adaptive immune system and thereby, elimination of the invading
pathogens and infected cells (7).
It has been well established that in addition to the
cells of the immune system, TLR9 is frequently expressed in various
cancer cell lines, as well as in clinical cancer specimens
(5,8–16).
Furthermore, synthetic TLR9-ligands (CpG-sequence containing
oligonucleotides) have been demonstrated to stimulate the in
vitro invasion of TLR9-expressing cancer cells, including
astrocytoma and glioblastoma cells (14,16).
This increased invasion is mediated via the downregulation of the
tissue inhibitor of matrix metalloproteinases-3 (TIMP-3) and the
upregulation of matrix metalloproteinase-13 (MMP-13) activity
(15,16). Additionally, increased TLR9
expression has been associated with the poor differentiation of
cancer cells in breast, prostate and glioblastoma multiforme (GBM)
tumors (17–21). Furthermore, in GBM patients, high
expression of the TLR9 protein in tumors has been found to
correlate with a significantly shorter survival time (17). Despite the well-documented
expression of TLR9 in various cancers and invasive response to TLR9
ligands in vitro, the involvement of this protein in cancer
pathophysiology remains unclear. The regulation of TLR9 expression,
as well as the possible physiological ligands which may induce
invasion in TLR9-expressing cancer cells, also remain poorly
characterized. Synthetic TLR9 ligands have also been investigated
in preclinical models of brain cancer immunotherapy; CpG-sequence
containing oligonucleotides were shown to induce apoptosis in brain
cancer cells in vitro and in vivo and, therefore, may
provide long-term antitumor immunity against gliomas (22,23).
Hypoxia is a fundamental characteristic of solid
tumors and it induces various adaptive changes in cancer cells,
which eventually lead to increased tumor growth, invasion and
metastasis (24). Hypoxia is also
frequently detected in brain tumors and the detection of hypoxia in
these tumors by hypoxia markers is essential for the diagnosis of
GBM (25). Due to the previously
demonstrated associations between hypoxic tissue conditions and the
upregulation of TLR2 and 6 in various normal tissues, we
hypothesized that hypoxic tissue conditions may activate
TLR9-expression and the TLR9-mediated invasive pathway in brain
cancer cells (26).
Materials and methods
Cell culture
Human D54MG, U373MG and SNB191 glioma cell lines and
primary human GBM XD45 and JX10 xenolines (UAB Brain Tumor
Specialized Program Of Research Excellence) were cultured in
Dulbecco’s modified Eagle’s medium (Gibco-BRL, Paisley, UK)
supplemented with 10% heat-inactivated fetal bovine serum,
L-glutamine, penicillin/streptomycin and non-essential amino acids
(all Gibco-BRL) (27,28). The cells were cultured at 37°C, in a
humidified atmosphere of 5% CO2 and 95% air (~21%
pO2). For the hypoxia experiments, the cells were kept
for the indicated durations in a cell culture incubator (I-Glove;
BioSpherix, Inc., Lacona, NY, USA) with an oxygen level set to 1 or
5% pO2, as indicated. Chloroquine was purchased from
Sigma-Aldrich (St. Louis, MO, USA) and the wide-spectrum
MMP-inhibitor, GM6001, was obtained from Enzo Life Sciences Inc.,
(Farmingdale, NY, USA).
RNA isolation and quantitative polymerase
chain reaction (qPCR)
Total RNA was isolated from the cells using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and
purified using RNeasy mini kits (Qiagen, Valencia, CA, USA). All
reagents used for the qPCR experiments were purchased from Applied
Biosystems (Carlsbad, CA, USA). The cDNA was synthesized from 0.2
μg of total RNA, using MultiScribe reverse transcriptase and random
hexamers. TLR9 mRNA expression levels were then quantified using
the following primer and probe set, obtained from Applied
Biosystems: Forward, 5′-GGCCCTCCACGCATGAG-3′ and reverse,
5′-CTTGTCCTTTTCTGCCCTTGTAG-3′ for TLR9; and 5′-CCTGCAGAACTCTG-3′
for the probe. The primer and probe sets used for MMP-2, MMP-9,
MMP-13 and TIMP-3 were also purchased from Applied Biosystems. For
all qPCR assays, a standard amplification program was used as
follows: One cycle of 50°C for 2 min; one cycle of 95°C for 10 min;
and 40 cycles of 95°C for 15 sec and 60°C for 1 min. Following
normalization to the large ribosomal protein RPLPO expression
levels for each cDNA, a relative quantification of the target cDNA
was performed using 2−ΔΔCt values (29).
Western blot analysis
The cells were cultured in normal culture medium
until near confluency and then rinsed with sterile
phosphate-buffered saline (PBS; Fisher Scientific, Pittsburgh, PA,
USA), prior to culture in serum-free culture medium (Gibco-BRL).
After 24 h, the culture medium was discarded and the cells were
rapidly harvested in lysis buffer (Cell Signaling Technology, Inc.,
Danvers, MA, USA), prior to separation by centrifugation as
previously described (30). Next,
the supernatants were boiled in reducing SDS sample buffer and
equal amounts of protein (~100 μg) were loaded into each lane. The
samples were then electrophoresed into 10% or 4–20% gradient
polyacrylamide SDS gels (Bio-Rad, Hercules, CA, USA) and
transferred to nitrocellulose membranes (Bio-Rad). Following
blocking with 5% non-fat dry milk in Tris-buffered saline with
Tween-20 (TBST), the blots were incubated overnight at 4°C with the
following primary antibodies: TLR9 (IMG-431; 1:500, Imgenex
Corporation, San Diego, CA, USA), TIMP-3 (AB8106; 1:500, Millipore,
Billerica, MA, USA) and actin (A-2066; 1:1,000, Sigma-Aldrich, St.
Louis, MO, USA). All primary antibodies were diluted in 0.1% TBST
(v/v), with 5% non-fat dry milk. The secondary detection was
performed using the horseradish-peroxidase-linked secondary
antibodies (NA934-100UL, Anaspec, Fremont, CA, USA) and the protein
bands were visualized with Pierce Enhanced Chemiluminescence
Western Blotting substrate (Pierce Biotechnology, Inc., Rockford,
IL, USA) (30).
Zymograms
The cells were cultured on 12-well plates until
confluent, and then washed with sterile PBS and further cultured
for 24 h in serum-free media (500 μl per well). The supernatants
were then collected and concentrated using a centrifugal filter
device using a cut-off size of 3 kDa (UFC5-003-24; Millipore,
Billerica, MA, USA). An equal amount of protein (~20 μg) was added
to each lane of the zymogram gels (10% gelatin; Bio-Rad). The gels
were then run, renaturated and developed using BioRad zymogram
buffers, according to the manufacturer’s instructions (31).
RNA interference
Downregulation of TLR9 with siRNA was performed
using a plasmid-based approach and a previously described TLR9 or
control (non-targeting siRNA) siRNA sequence, which were cloned
into the pSuper-EGFP vector (Oligoengine, Seattle, WA, USA)
(14,31). The plasmids were stably transfected
into the cells using standard techniques (14). The G418 (0.8 mg/ml) selection was
maintained and four cycles of green fluorescence-based cell sorting
was performed to obtain a pool of cells with a high percentage of
green fluorescent protein positivity. For the U373MG cells,
oligonucleotide siRNA molecules, the human TLR9 siRNA Smart pool or
control human non-targeting siRNA pool (Dharmacon, Inc., Lafayett,
CO, USA) were used. Briefly, 8 μl of siRNA stock (20 μM) was added
to 500 μl Optimem (Gibco-BRL, Grand Island, NY, USA) on six-well
plates, followed by the addition of 5 μl Lipofectamine RNAimax
(Invitrogen Life Technologies, Grand Island, NY, USA). The
components were mixed by pipetting and the plates were agitated on
a platform for 20 min at room temperature, followed by the addition
of 0.8 ml of cell suspension (45×104 cells/ml). After 2
h, 1.6 ml of regular growth medium was added to the cells and the
medium was changed on the following day. After 48 h, the cells were
trypsinized and subsequently used in invasion assays or cultured in
serum-free media for the final 24 h in normoxic or hypoxic
conditions, prior to western blot analysis.
Invasion assays
Invasion assays were performed as previously
described (16). Briefly, the cells
(20,000 per insert) were allowed to invade the extracellular
matrix-like Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) in
24-well plate inserts (Becton-Dickinson, Franklin Lakes, NJ, USA).
The membranes were stained using the Hema-3 set (Thermo Fisher
Scientific, Rockford, IL, USA) and the number of invading cells was
counted under a microscope (Nikon Labophot-2, Nikon Instech Co.,
Ltd., Tokyo, Japan) (16). In
certain experiments, a 22-mer DNA-oligonucleotide, which forms
G-quadruplex structures (sequence, aggg tta ggg tta ggc taa ggg in
a phosphodiester backbone; Midland Certified Reagent Company, Inc.,
Midland, TX, USA), was used as a positive control at a final
concentration of 10 μM and added to the upper invasion wells.
Through our ongoing studies we have determined that this DNA-ligand
induces TLR9-mediated invasion (32).
Cell viability assays
In total, 20,000 cells were plated on 96-well plates
(100 μl/well) in normal growth medium. The viability of the cells
was measured using the CellTiter 96 AQueous One Solution Cell
Proliferation assay (Promega, Madison, WI, USA), according to the
manufacturer’s instructions. In an additional set of assays, the
cells were plated on 24-well plates and, after the indicated time,
the cells were trypsinized and the viable cells were counted using
the TC10™ automated cell counter (Bio-Rad).
Statistical analysis
Data are presented as the mean ± standard deviation
or mean ± standard error of the mean, as stated. Student’s t-test
was used to determine whether the differences between the
corresponding cells under various circumstances were statistically
significant. P<0.05 was considered to indicate a statistically
significant difference.
Results
Hypoxia induces TLR9 expression in human
D54MG and U373MG cells
The D54MG and U373MG cells were selected for the
current study as they have been demonstrated to express TLR9 and
exhibit increased invasion in vitro in response to synthetic
TLR9-ligands (16). Furthermore,
brain cancers have been shown to be highly associated with the
tumor markers of hypoxia. In the current study, the cells were
cultured under normoxic and hypoxic conditions for 24 h and the
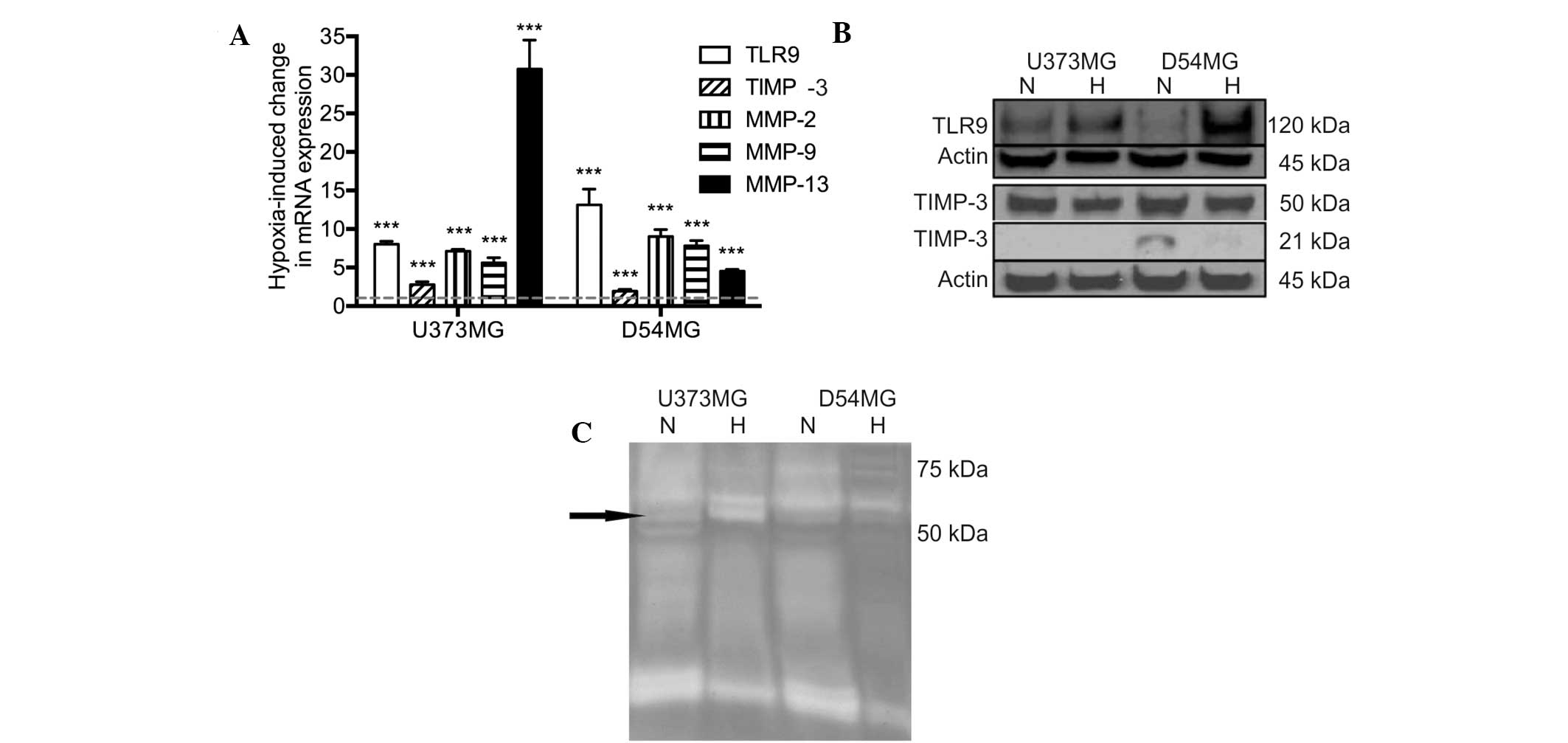
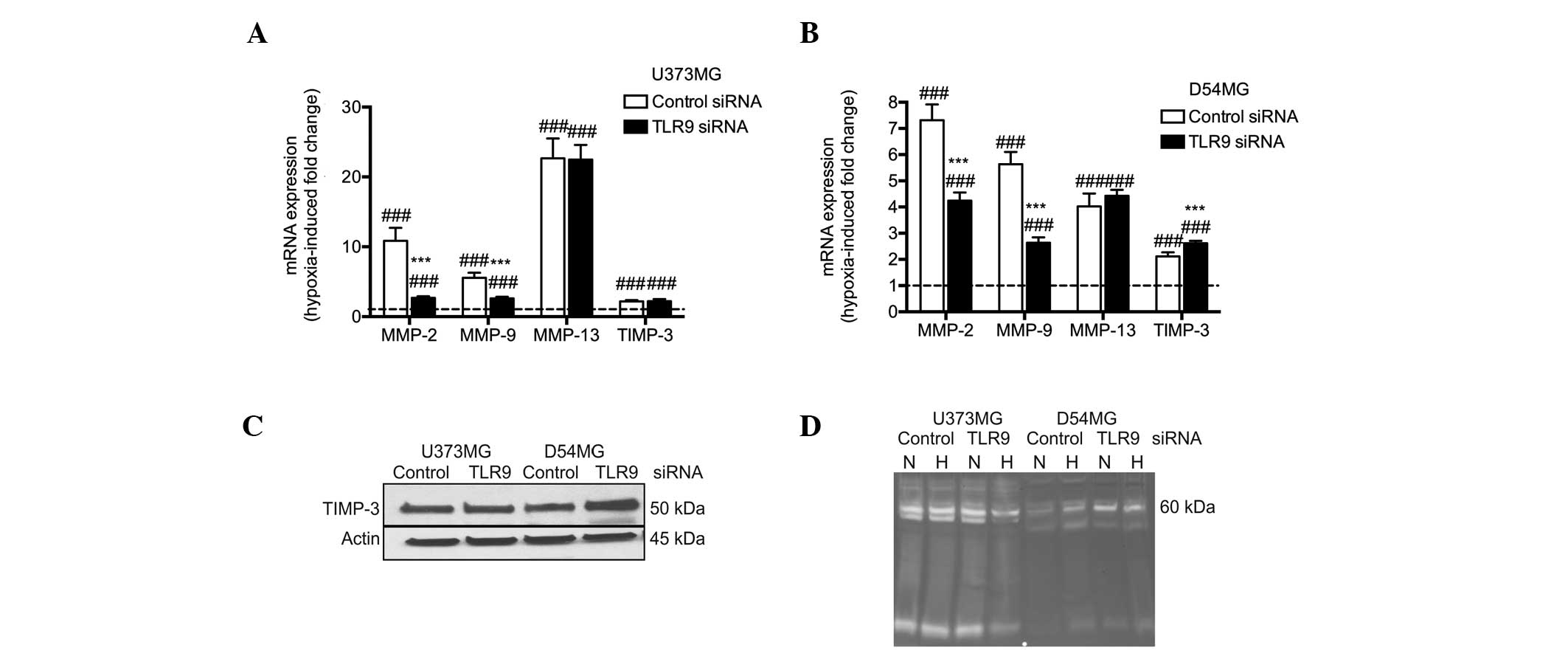
mRNA expression was determined using qPCR. Hypoxia was found to
induce a significant upregulation of TLR9 mRNA expression in the
two cell lines. In addition, the mRNA expression levels of TIMP-3
and MMP-2, -9 and -13 were also found to significantly increase
simultaneously (Fig. 1A). The
protein expression levels of TLR9 were found to correlate with the
mRNA levels in the two cell types. By contrast, the protein
expression levels of TIMP-3 were found to marginally decrease in
hypoxia in the D54MG and U373MG cells (Fig. 1B). The proteolytic activity of the
conditioned medium of the U373MG and D54MG cells was analyzed using
zymograms. A triple band of ~60 kDa was observed, which may present
latent MMP-2 and active MMP-2 and MMP-13. Additionally, an
increased level of MMP-2 activity in U373MG cells was detected in
hypoxia when compared with normoxia (Fig. 1C).
TLR9 regulates MMP and TIMP-3 expression
in normoxia
Hypoxia has been shown to induce invasion in various
types of cancer cells (31,33,34)
and, therefore, the involvement of TLR9 in the process of invasion
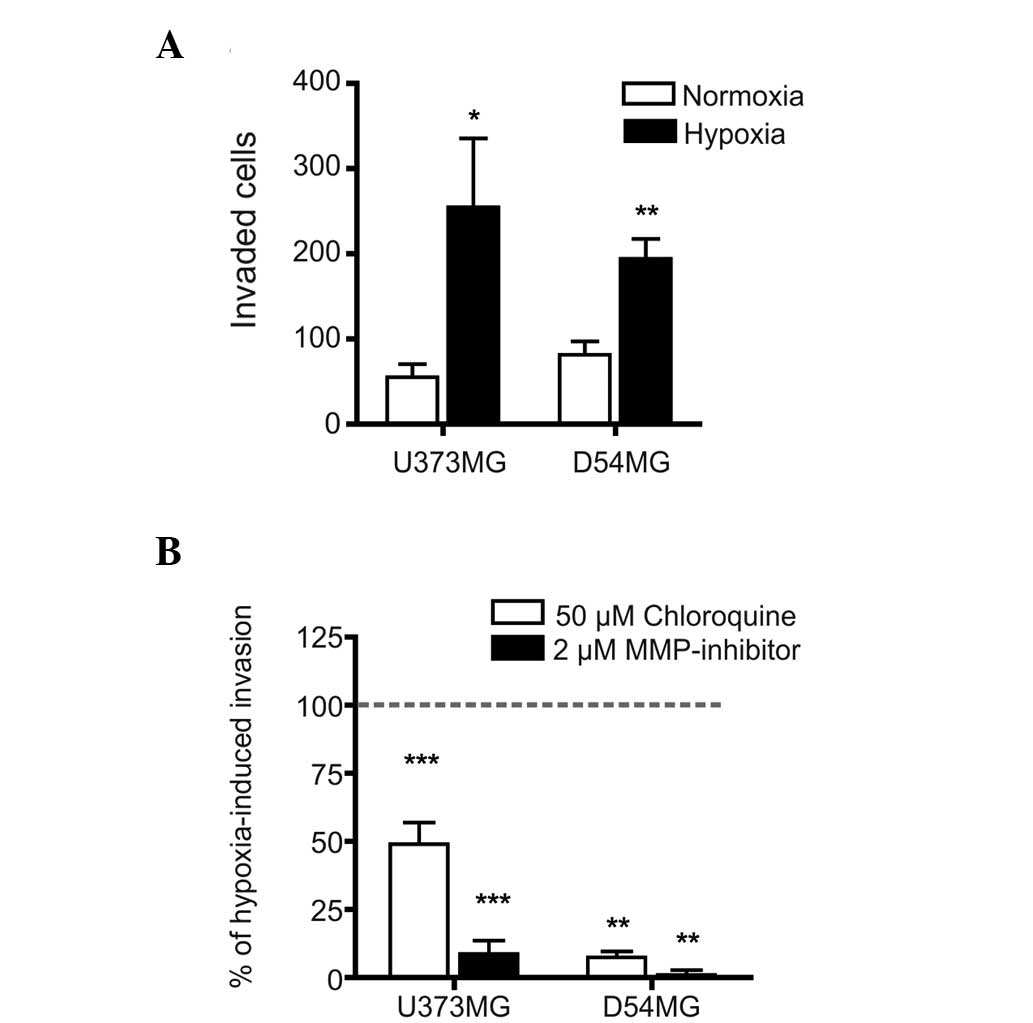
was investigated. The invasive effects of hypoxia were first
characterized in the parental U373MG and D54MG cells and, as
expected, hypoxia induced a significant increase in the invasion
rates of the two parental cell lines (Fig. 2A). Furthermore, the hypoxia-induced
invasion of these cells was significantly inhibited by chloroquine,
an important inhibitor of endosomal acidification (and, therefore,
possibly TLR9 signaling) and almost completely blocked by a
wide-spectrum of MMP-inhibitors (Fig.
2B). These results suggested that TLR9 signaling and MMP
activity regulate hypoxia-induced invasion in these cells.
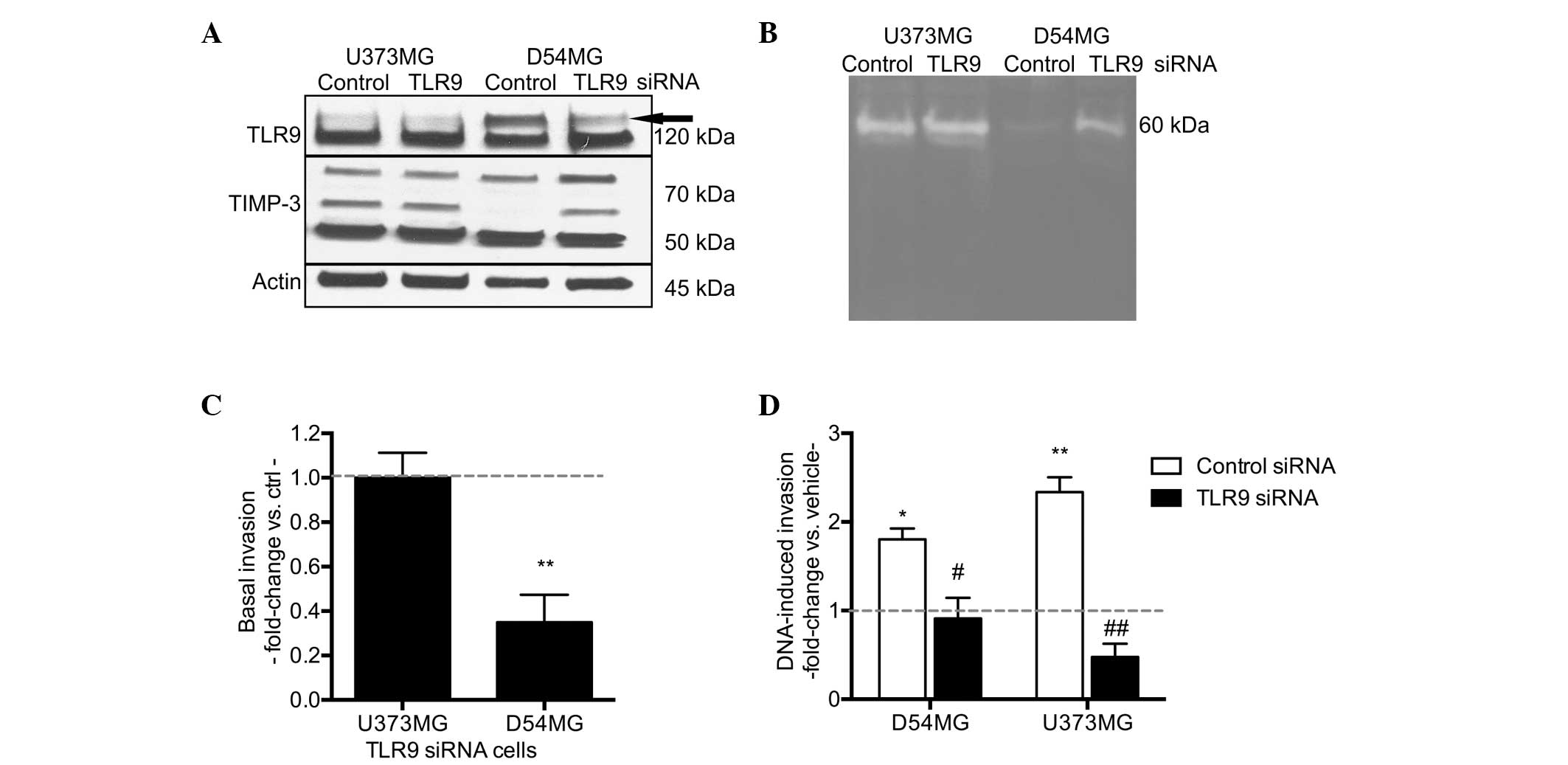
To study the involvement of TLR9 in this process
more specifically, stable TLR9 siRNA cells were established, in
which TLR9 expression was downregulated through a plasmid-based
siRNA approach (31). In a similar
manner, the control cells were transfected with a plasmid encoding
a non-targeting siRNA. Characterization of the control and TLR9
siRNA cells in normoxic, steady-state conditions is shown in
Table I. Briefly, relative to the
corresponding control siRNA cells, the TLR9 siRNA cells exhibited
downregulated TLR9 mRNA expression levels of ~37 and ~66% in the
U373MG and D54MG cells, respectively. Furthermore, the
downregulation of TLR9 induced cell-specific changes in cellular
MMP and TIMP-3 mRNA expression levels. The mRNA expression levels
for various MMPs (MMP-2, -9 and -13) remained unchanged or were
marginally upregulated in the two cell lines; however,
downregulation of TLR9 resulted in a significant upregulation of
TIMP-3 mRNA expression (Table
I.).
 | Table IBasal mRNA expression in the TLR9
siRNA cells relative to the corresponding control siRNA cells in
normoxia. |
Table I
Basal mRNA expression in the TLR9
siRNA cells relative to the corresponding control siRNA cells in
normoxia.
| mRNA | D54MG
TLR9 siRNA | U373MG
TLR9 siRNA |
|---|
| TLR9 | 0.34±0.03c | 0.63±0.08c |
| MMP-2 | 1.24±0.09b | 1.19±0.09a |
| MMP-9 | 0.99±0.12d | 1.27±0.14b |
| MMP-13 | 1.31±0.11b | 1.09±0.10d |
| TIMP-3 | 1.47±0.101c | 1.26±0.04c |
At the protein level, the changes in TLR9 expression
between the control and TLR9 siRNA cells in normoxia were subtle
and similar to that of the mRNA expression (Fig. 3A). Consistent with the changes at
the mRNA level, D54MG/TLR9 siRNA cells also exhibited increased
TIMP-3 protein expression; however, this upregulation was
particularly detected in TIMP-3 protein bands of high molecular
weight, which may subsequently present different glycosylation
forms of the protein (35,36). In the U373MG/TLR9 siRNA cells,
TIMP-3 protein expression in normoxia was similar to that of the
U373MG/control siRNA cells (Fig.
3A). A comparison of the proteolytic activity between the
control and TLR9 siRNA supernatants in normoxia demonstrated
differences between the control and TLR9 siRNA D54MG cells. More
specifically, the supernatants of the D54MG/TLR9 siRNA cells
exhibited enhancement in a 60-kDa proteolytic band, suggesting an
increase in latent MMP-2 (Fig. 3B).
No such differences were detected between the proteolytic
activities of the U373MG control siRNA and TLR9 siRNA supernatants
in normoxia. The basal invasive capacity of D54MG/TLR9 siRNA cells
in normoxia was significantly decreased, as compared with the
D54MG/control siRNA cells. However, no difference was detected
between the control and TLR9 siRNA U373MG cells (Fig. 3C).
Finally, to further confirm the differences in
TLR9-mediated invasion between the control and TLR9 siRNA cell
pairs, the invasive capacity of these cells was also studied in
response to treatment with G-quadruplex-DNA TLR9-ligands in
normoxia. As compared with the vehicle, the G-quadruplex-DNA
induced an approximately two-fold increase in invasion in the
D54MG/control siRNA cells, but not in the corresponding TLR9 siRNA
cells. Similar results were observed with the stable U373MG cells,
confirming differences in the TLR9-mediated invasive responses of
the TLR9 siRNA cells (Fig. 3D).
Overall, these results suggest that the
downregulation of TLR9 in brain cancer cells results in decreased
basal and DNA ligand-induced invasion in normoxia, in a
cell-specific manner. The anti-invasive phenotype, particularly of
the D54MG/TLR9 siRNA cells, suggests that despite the observed
increases in MMP mRNA expression, the increased expression levels
of the endogenous MMP-inhibitors, such as TIMP-3, are possibly a
more significant determinant of their invasive behavior in
normoxia.
Hypoxia-induced invasion is inhibited in
TLR9 siRNA brain cancer cells
Next, the involvement of TLR9 in hypoxia-induced
invasion was studied more specifically, using the D54MG and U373MG
control and TLR9 siRNA cells. As with the parental cells, hypoxia
induced TLR9 mRNA expression in the control siRNA cell lines, as
well as in the TLR9 siRNA cells. The hypoxia-induced increases in
TLR9 mRNA expression were, however, significantly reduced in the
TLR9 siRNA cells (Fig. 4A). As
predicted, TLR9 protein expression levels were also found to
decrease under hypoxic conditions in the two TLR9 siRNA cells, when
compared with those of the control siRNA cells (Fig. 4B). Invasion assays were also used to
study these cells; hypoxia induced an increased level of invasion
in the control siRNA cells, however, invasion was evidently reduced
in the TLR9 siRNA cell lines (Fig.
4C). In an attempt to achieve an improved level of TLR9 protein
downregulation, TLR9 expression was also downregulated in the
U373MG cells using an oligo siRNA approach. With this approach, the
U373MG cells transfected with the TLR9 siRNA molecules demonstrated
a complete loss of hypoxia-inducible TLR9 protein expression
(Fig. 4D). Similar to the results
obtained with the plasmid-based siRNA, the oligo TLR9 siRNA U373MG
cells also demonstrated a significantly inhibited response to
G-quadruplex-DNA and hypoxia-induced invasion, as compared with the
corresponding control oligo siRNA cells (Fig. 4E). In conclusion, these results
suggest that the downregulation of TLR9 expression inhibits the
hypoxia-induced invasion of brain cancer cells in vitro.
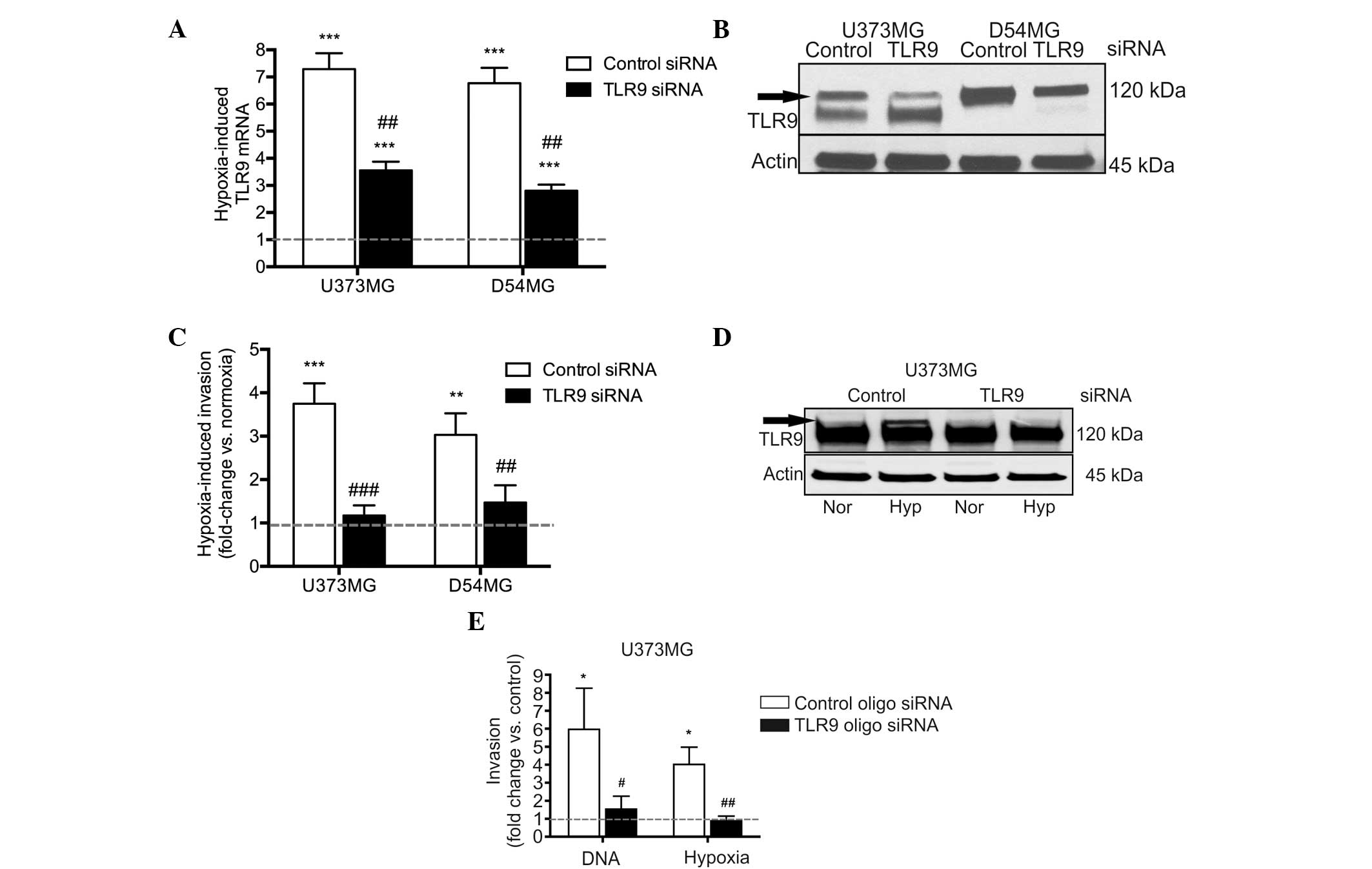 | Figure 4Hypoxia-induced TLR9 (A) mRNA and (B)
protein expression as indicated by the arrow in the U373MG and
D54MG cells. Actin bands were used to indicate equal loading. (C)
Hypoxia-induced invasion in the indicated cells. Data are presented
as a fold-increase of invasion in hypoxia, as compared with
invasion in normoxia (indicated by the dotted line; mean ±SEM;
n=7–12) **P<0.01 and ***P< 0.001, vs.
normoxia; and ##P<0.01 and ###P<0.001,
vs. the corresponding control siRNA cells. (D) Expression of TLR9
protein (indicated by the arrow) in normoxia and hypoxia in U373MG
cells transiently transfected with control or TLR9 oligo siRNA
molecules. The blot was stripped and reblotted with anti-actin
antibodies to demonstrate equal loading. (E) G-quadruplex-DNA (DNA)
and hypoxia-induced invasion of the U373MG cells demonstrated
differences in the invasive responses between control and TLR9
siRNA cells. Data are presented as a fold-change in invasion as
compared with the corresponding control (as indicated by the dotted
line, corresponding to vehicle treatment for
G-quadruplex-DNA-induced invasion, and invasion in normoxia for the
hypoxia-induced invasion) (mean ± SEM; n=7–9).
*P<0.01, vs. the corresponding control condition; and
#P<0.05 and ##P<0.01, vs. the control
siRNA cells. TLR9, toll-like recptor-9; Nor, normoxia; Hyp,
hypoxia. |
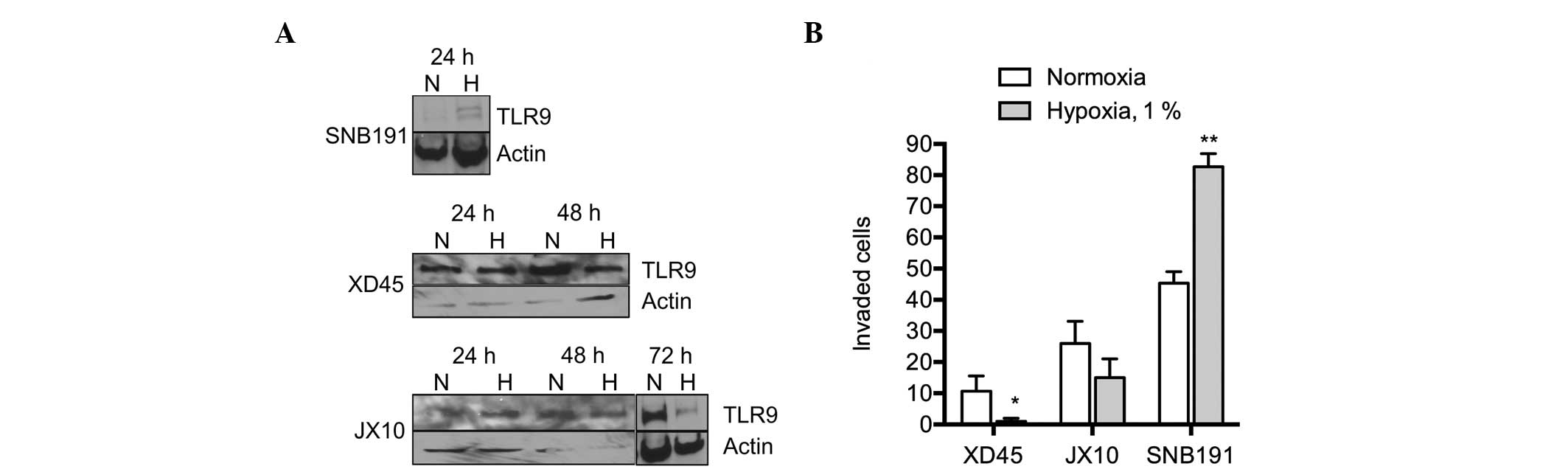
Finally, the effect of hypoxia on TLR9 expression
and invasion was investigated in other cell lines. Consistent with
the previously described observations, hypoxia increased invasion
in the SNB191 glioma cells, whereby the TLR9 protein was also
observed to be upregulated (Fig. 5A and
B). By contrast, invasion was not upregulated by hypoxia in the
primary JX10 and XD45 glioma cell lines, where the protein
expression of TLR9 was not found to increase, or to be
downregulated by hypoxia (Fig. 5A and
B). Therefore, these results suggest that TLR9 mediates
hypoxia-induced invasion in brain cancer cells in vitro.
TLR9 regulates MMP and TIMP-3 expression
in hypoxia
Next, the mechanisms that may explain the
TLR9-mediated invasion in hypoxia were investigated using control
and TLR9 siRNA D54MG and U373MG cells. Firstly, the hypoxia-induced
fold changes of selected mRNAs versus normoxia between the
corresponding control and TLR9 siRNA cells were compared. Relative
to normoxia and similar to the parental cells, hypoxia induced
significant increases in MMP-2, -9 and -13 expression in the two
control siRNA cells. With the exception of MMP-13 mRNA, the
hypoxia-induced changes were TLR9-dependent and significantly
reduced in the two TLR9 siRNA cell lines. Furthermore, similar to
the parental cells, hypoxia also induced TIMP-3 mRNA expression in
the two control cell lines, and this effect was significantly
enhanced in the D54MG/TLR9 siRNA cells. In the U373MG/TLR9 siRNA
cells, hypoxia-induced effects on TIMP-3 mRNA expression were
similar to those detected in the control siRNA cells (Fig. 6A). These changes in TIMP-3 mRNA
expression were also found to correlate with the TIMP-3 protein
expression levels; TIMP-3 protein expression was found to be
similar in U373MG/control siRNA and TLR9 siRNA cells in hypoxia.
However, in the D54MG/TLR9 siRNA cells, TIMP-3 protein expression
levels were increased compared with the levels observed in the
corresponding control siRNA cells in hypoxia. In contrast to the
normoxic conditions, the change in the TIMP-3 expression level was
detected as a ~50 kDa band (Fig.
6C). Zymograms of the supernatants of the corresponding cells
further indicated a decreased induction of proteolytic activity by
hypoxia, particularly in the U373MG/TLR9 siRNA cells. Among the
D54MG cells, hypoxia-induced proteolytic activity was observed in
the control siRNA cells, but not in the corresponding TLR9 siRNA
cells (Fig. 6D). Therefore, these
results suggest that hypoxia-induced proteolytic and invasive
activity is mediated by TLR9 in D54MG and U373MG cells, possibly
via the regulation of MMPs and their endogenous inhibitors.
Discussion
TLR9 is an innate immunity receptor, which
recognizes self- and microbe-derived DNA (37,38).
Although widely expressed in various tumors, including brain
cancer, the contribution of TLR9 to cancer pathophysiology remains
unclear (16,39) and the regulation of TLR9 expression
in cancer is also poorly understood.
The present study demonstrated that the surrounding
oxygen level has an important effect on TLR9 expression and
function in human brain cancer cells in vitro. In addition,
our studies further suggest that TLR9 expression is significantly
associated with the invasive machinery in brain cancer cells and
may mediate hypoxia-induced invasion in brain cancer. This novel
observation may have therapeutic implications in brain cancers,
particularly for those tumors that exhibit high hypoxia-associated
TLR9 expression at diagnosis. The marginal changes in TLR9
expression observed in the TLR9 siRNA cells in normoxia, which
appear to regulate MMP and TIMP-3 expression, suggest that TLR9, as
a DNA-binding protein, may exhibit transcriptional activity.
However, this hypothesis requires further study. Although hypoxia
also induces TLR9 expression in breast cancer cells, we previously
demonstrated, particularly in triple-negative breast cancers, that
decreased TLR9 expression does not inhibit hypoxia-induced invasion
but rather augments it. This effect was associated with a complete
lack of TIMP-3 expression and was not detected in breast cancer
cells that express the estrogen receptor (31). Overall, these observations suggest
that, although hypoxia appears to regulate TLR9 expression in
various cancer cells, the effects of TLR9 inhibition on
hypoxia-induced invasion are specific to cancer type. This is to be
expected, as it was recently demonstrated that TLR9 may utilize as
many as 190 different cofactors to facilitate various cellular
responses (40).
Hypoxic tumor regions are common in brain cancer,
including GBM, and thus hypoxia is used as diagnostic criteria
(41). Increased tumor hypoxia is
also associated with poor prognosis in GBM, as hypoxia is important
in GBM invasion (42,43). The less invasive phenotype of TLR9
siRNA cells in normoxia and hypoxia suggests that TLR9 may mediate
the high levels of brain cancer cell invasion into the healthy
brain tissue in clinical tumors, through regulation MMP expression
and activity. Notably, hypoxia has also been shown to activate
MMP-2, -9 and -13 in brain tissues; however, the current study is
the first to demonstrate that these proteases may be TLR9-regulated
in brain cancer cells (42,44). The results from a previous study
suggesting that the absence of TLR9 expression protects against
ischemia-reperfusion injury in the liver also imply that
hypoxia-induced changes may be TLR9-mediated in other tissues
(45). Furthermore, since the
procedures of the current study were performed without the addition
of exogenous ligands, the results additionally suggest that TLR9
expression is sufficient to control cancer cell invasion. However,
the presence of TLR9 ligands can not be ignored, since apoptotic
DNA and other endogenous ligands have been demonstrated to
stimulate TLR9 expression in mammalian cells and, therefore, may
have been present under the experimental circumstances (38,46).
These observations add TLR9 to the list of molecules that have been
previously suggested to mediate hypoxia-induced invasion, including
MMP-2, Jagged-2, vascular endothelial growth factor, fibroblast
growth factors and semaphorin 3F (47,48).
Hypoxia-inducible factor (HIF)-1 is a major
regulator of hypoxic responses in cells, that comprises of two
subunits (HIF-1α and HIF-1β), which binds to hypoxia responsive
elements upstream of the hypoxia-regulated target genes (49). HIF-1α is particularly responsible
for the cellular changes in acute hypoxia and, furthermore, HIF-1
expression has been detected in clinical cohorts of glioma;
inhibition of HIF-1α expression reduces hypoxia-induced invasion in
glioma cells in vitro and in vivo. An association has
been established between HIF-1α and TLRs, as stimulation with TLR
ligands has been shown to result in the accumulation of the HIF-1α
protein, or to result in cellular responses that are mediated by
HIF-1α (50,51). However, hypoxia has also been shown
to upregulate TLR2, 4, 6 and 9 via HIF-1α (26,31,52).
The reciprocal interaction between TLRs and HIF-1α suggests that
hypoxia-induced TLR9 expression may also be HIF-1α-regulated in
brain cancer cells. A recent study by Sinha et al further
suggested that such regulation may also occur in states other than
hypoxia (53); however, further
study is required to confirm these theories.
In conclusion, hypoxia regulates TLR9 expression in
brain cancer cells in vitro and TLR9 also mediates the
hypoxia-induced invasion of these cells. Overall, these
observations suggest that TLR9 expression may contribute to the
increased invasion of brain cancer cells under hypoxic tissue
conditions. Although this phenomenon requires further study in
vivo, the results of the current study suggest that the
suppression of TLR9 expression and activity may present a novel
molecular target in brain cancer.
Acknowledgements
The authors would like to thank Ms Christine Pressey
for skillful assistance with the qPCR assays. The present study was
funded by grants from the Oulu University Scholarship Foundation
and Cancer Foundation of Northern Finland (J.H.K.), the Lapland
Cultural Foundation (K.S.S.) and the Department of Defense (K.S.S.
and D.G., grant no. BC095831).
References
|
1
|
Akira S and Hemmi H: Recognition of
pathogen-associated molecular patterns by TLR family. Immunol Lett.
85:85–95. 2003.
|
|
2
|
Wagner H: The immunobiology of the TLR9
subfamily. Trends Immunol. 25:381–386. 2004.
|
|
3
|
Matsumoto M, Funami K, Tanabe M, et al:
Subcellular localization of Toll-like receptor 3 in human dendritic
cells. J Immunol. 171:3154–3162. 2003.
|
|
4
|
Nishiya T and DeFranco AL:
Ligand-regulated chimeric receptor approach reveals distinctive
subcellular localization and signaling properties of the Toll-like
receptors. J Biol Chem. 279:19008–19017. 2004.
|
|
5
|
Schmausser B, Andrulis M, Endrich S, et
al: Expression and subcellular distribution of toll-like receptors
TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter
pylori infection. Clin Exp Immunol. 136:521–526. 2004.
|
|
6
|
Leifer CA, Kennedy MN, Mazzoni A, Lee C,
Kruhlak MJ and Segal DM: TLR9 is localized in the endoplasmic
reticulum prior to stimulation. J Immunol. 173:1179–1183. 2004.
|
|
7
|
Palm NW and Medzhitov R: Pattern
recognition receptors and control of adaptive immunity. Immunol
Rev. 227:221–233. 2009.
|
|
8
|
Schmausser B, Andrulis M, Endrich S,
Müller-Hermelink HK and Eck M: Toll-like receptors TLR4, TLR5 and
TLR9 on gastric carcinoma cells: an implication for interaction
with Helicobacter pylori. Int J Med Microbiol. 295:179–185.
2005.
|
|
9
|
Schaefer TM, Desouza K, Fahey JV, Beagley
KW and Wira CR: Toll-like receptor (TLR) expression and
TLR-mediated cytokine/chemokine production by human uterine
epithelial cells. Immunology. 112:428–436. 2004.
|
|
10
|
Bowman CC, Rasley A, Tranguch SL and
Marriott I: Cultured astrocytes express toll-like receptors for
bacterial products. Glia. 43:281–291. 2003.
|
|
11
|
Platz J, Beisswenger C, Dalpke A, et al:
Microbial DNA induces a host defense reaction of human respiratory
epithelial cells. J Immunol. 173:1219–1223. 2004.
|
|
12
|
Mempel M, Voelcker V, Köllisch G, et al:
Toll-like receptor expression in human keratinocytes: nuclear
factor kappaB controlled gene activation by Staphylococcus
aureus is toll-like receptor 2 but not toll-like receptor 4 or
platelet activating factor receptor dependent. J Invest Dermatol.
121:1389–1396. 2003.
|
|
13
|
Zaks-Zilberman M, Zaks TZ and Vogel SN:
Induction of proinflammatory and chemokine genes by
lipopolysaccharide and paclitaxel (Taxol) in murine and human
breast cancer cell lines. Cytokine. 15:156–165. 2001.
|
|
14
|
Ilvesaro JM, Merrell MA, Li L, et al:
Toll-like receptor 9 mediates CpG oligonucleotide-induced cellular
invasion. Mol Cancer Res. 6:1534–1543. 2008.
|
|
15
|
Ilvesaro JM, Merrell MA, Swain TM, et al:
Toll like receptor-9 agonists stimulate prostate cancer invasion in
vitro. Prostate. 67:774–781. 2007.
|
|
16
|
Merrell MA, Ilvesaro JM, Lehtonen N, et
al: Toll-like receptor 9 agonists promote cellular invasion by
increasing matrix metalloproteinase activity. Mol Cancer Res.
4:437–447. 2006.
|
|
17
|
Wang C, Cao S, Yan Y, et al: TLR9
expression in glioma tissues correlated to glioma progression and
the prognosis of GBM patients. BMC Cancer. 10:4152010.
|
|
18
|
Jukkola-Vuorinen A, Rahko E, Vuopala KS,
et al: Toll-like receptor-9 expression is inversely correlated with
estrogen receptor status in breast cancer. J Innate Immun. 1:59–68.
2009.
|
|
19
|
Väisänen MR, Jukkola-Vuorinen A, Vuopala
KS, Selander KS and Vaarala MH: Expression of Toll-like receptor-9
is associated with poor progression-free survival in prostate
cancer. Oncol Lett. 5:1659–1663. 2013.
|
|
20
|
Väisänen MR, Väisänen T, Jukkola-Vuorinen
A, et al: Expression of toll-like receptor-9 is increased in poorly
differentiated prostate tumors. Prostate. 70:817–824. 2010.
|
|
21
|
Berger R, Fiegl H, Goebel G, et al:
Toll-like receptor 9 expression in breast and ovarian cancer is
associated with poorly differentiated tumors. Cancer Sci.
101:1059–1066. 2010.
|
|
22
|
Grauer OM, Molling JW, Bennink E, et al:
TLR ligands in the local treatment of established intracerebral
murine gliomas. J Immunol. 181:6720–6729. 2008.
|
|
23
|
Zhao D, Alizadeh D, Zhang L, et al: Carbon
nanotubes enhance CpG uptake and potentiate antiglioma immunity.
Clin Cancer Res. 17:771–782. 2011.
|
|
24
|
Ruan K, Song G and Ouyang G: Role of
hypoxia in the hallmarks of human cancer. J Cell Biochem.
107:1053–1062. 2009.
|
|
25
|
Bar EE: Glioblastoma, cancer stem cells
and hypoxia. Brain Pathol. 21:119–129. 2011.
|
|
26
|
Kuhlicke J, Frick JS, Morote-Garcia JC,
Rosenberger P and Eltzschig HK: Hypoxia inducible factor (HIF)-1
coordinates induction of Toll-like receptors TLR2 and TLR6 during
hypoxia. PLoS One. 2:e13642007.
|
|
27
|
Filippova N, Yang X, King P and Nabors LB:
Phosphoregulation of the RNA-binding protein Hu antigen R (HuR) by
Cdk5 affects centrosome function. J Biol Chem. 287:32277–32287.
2012.
|
|
28
|
Filippova N, Yang X, Wang Y, et al: The
RNA-binding protein HuR promotes glioma growth and treatment
resistance. Mol Cancer Res. 9:648–659. 2011.
|
|
29
|
Sandholm J, Kauppila JH, Pressey C, et al:
Estrogen receptor-α and sex steroid hormones regulate Toll-like
receptor-9 expression and invasive function in human breast cancer
cells. Breast Cancer Res Treat. 132:411–419. 2012.
|
|
30
|
Merrell M, Suarez-Cuervo C, Harris KW,
Väänänen HK and Selander KS: Bisphosphonate induced growth
inhibition of breast cancer cells is augmented by p38 inhibition.
Breast Cancer Res Treat. 81:231–241. 2003.
|
|
31
|
Tuomela J, Sandholm J, Karihtala P, et al:
Low TLR9 expression defines an aggressive subtype of
triple-negative breast cancer. Breast Cancer Res Treat.
135:481–493. 2012.
|
|
32
|
Kauppila JH, Karttunen TJ, Saarnio J, et
al: Short DNA sequences and bacterial DNA induce esophageal,
gastric, and colorectal cancer cell invasion. APMIS. 121:511–522.
2013.
|
|
33
|
Muñoz-Nájar UM, Neurath KM, Vumbaca F and
Claffey KP: Hypoxia stimulates breast carcinoma cell invasion
through MT1-MMP and MMP-2 activation. Oncogene. 25:2379–2392.
2006.
|
|
34
|
Arvelo F and Cotte C: Hypoxia in cancer
malignity. Review Invest Clin. 50:529–546. 2009.(In Spanish).
|
|
35
|
Langton KP, Barker MD and McKie N:
Localization of the functional domains of human tissue inhibitor of
metalloproteinases-3 and the effects of a Sorsby’s fundus dystrophy
mutation. J Biol Chem. 273:16778–16781. 1998.
|
|
36
|
Stricklin GP: Human fibroblast tissue
inhibitor of metalloproteinases: glycosylation and function. Coll
Relat Res. 6:219–228. 1986.
|
|
37
|
Hemmi H, Takeuchi O, Kawai T, et al: A
Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745.
2000.
|
|
38
|
Lande R, Gregorio J, Facchinetti V, et al:
Plasmacytoid dendritic cells sense self-DNA coupled with
antimicrobial peptide. Nature. 449:564–569. 2007.
|
|
39
|
Meng Y, Kujas M, Marie Y, et al:
Expression of TLR9 within human glioblastoma. J Neurooncol.
88:19–25. 2008.
|
|
40
|
Chiang CY, Engel A, Opaluch AM, et al:
Cofactors required for TLR7- and TLR9-dependent innate immune
responses. Cell Host Microbe. 11:306–318. 2012.
|
|
41
|
Yang L, Lin C, Wang L, Guo H and Wang X:
Hypoxia and hypoxia-inducible factors in glioblastoma multiforme
progression and therapeutic implications. Exp Cell Res.
318:2417–2426. 2012.
|
|
42
|
Chen W, Hartman R, Ayer R, et al: Matrix
metalloproteinases inhibition provides neuroprotection against
hypoxia-ischemia in the developing brain. J Neurochem. 111:726–736.
2009.
|
|
43
|
Méndez O, Zavadil J, Esencay M, et al:
Knock down of HIF-1alpha in glioma cells reduces migration in vitro
and invasion in vivo and impairs their ability to form tumor
spheres. Mol Cancer. 9:1332010.
|
|
44
|
Lu DY, Yu WH, Yeh WL, et al:
Hypoxia-induced matrix metalloproteinase-13 expression in
astrocytes enhances permeability of brain endothelial cells. J Cell
Physiol. 220:163–173. 2009.
|
|
45
|
Bamboat ZM, Balachandran VP, Ocuin LM,
Obaid H, Plitas G and DeMatteo RP: Toll-like receptor 9 inhibition
confers protection from liver ischemia-reperfusion injury.
Hepatology. 51:621–632. 2010.
|
|
46
|
Rifkin IR, Leadbetter EA, Busconi L,
Viglianti G and Marshak-Rothstein A: Toll-like receptors,
endogenous ligands, and systemic autoimmune disease. Immunol Rev.
204:27–42. 2005.
|
|
47
|
Tuomela J, Grönroos TJ, Valta MP, et al:
Fast growth associated with aberrant vasculature and hypoxia in
fibroblast growth factor 8b (FGF8b) over-expressing PC-3 prostate
tumour xenografts. BMC Cancer. 10:5962010.
|
|
48
|
Barcellos-Hoff MH, Newcomb EW, Zagzag D
and Narayana A: Therapeutic targets in malignant glioblastoma
microenvironment. Semin Radiat Oncol. 19:163–170. 2009.
|
|
49
|
Kimbro KS and Simons JW: Hypoxia-inducible
factor-1 in human breast and prostate cancer. Endocr Relat Cancer.
13:739–749. 2006.
|
|
50
|
Nicholas SA, Oniku AE and Sumbayev VV:
Myeloid cell death associated with Toll-like receptor 7/8-mediated
inflammatory response. Implication of ASK1, HIF-1 alpha, IL-1 beta
and TNF-alpha. Mol Immunol. 48:240–247. 2010.
|
|
51
|
Paone A, Galli R, Gabellini C, et al:
Toll-like receptor 3 regulates angiogenesis and apoptosis in
prostate cancer cell lines through hypoxia-inducible factor 1
alpha. Neoplasia. 12:539–549. 2010.
|
|
52
|
Kim SY, Choi YJ, Joung SM, Lee BH, Jung YS
and Lee JY: Hypoxic stress up-regulates the expression of Toll-like
receptor 4 in macrophages via hypoxia-inducible factor. Immunology.
129:516–524
|
|
53
|
Sinha S, Koul N, Dixit D, Sharma V and Sen
E: IGF-1 induced HIF-1α-TLR9 cross talk regulates inflammatory
responses in glioma. Cell Signal. 23:1869–1875. 2011.
|