Introduction
Leukemia is one of the leading causes of death among
patients suffering from malignant hematological disorders (1). Despite the fact that great advances
have been made in the treatment of leukemia, the overall survival
rate has not greatly elevated (1,2).
Hence, the understanding of the mechanism by which neoplastic cells
proliferate in leukemia is of great importance.
It is known that alternation between the processes
of dormancy and proliferation is very common in the human body,
such as during wound healing, liver tissue regeneration and
inflammatory proliferation (3–5).
Moreover, mucosal epithelium in the respiratory tract, the
digestive tract and the genital ducts is in a dynamic division
process (6). Initiation of the
abovementioned processes is based on the proliferation of original
cells that possess stem cell properties. However, all of the
aforementioned processes must occur under precise control,
otherwise neoplasms may occur.
Abnormal alternation of hematopoietic stem cells
(HSCs) is involved in the pathogenesis of leukemia. HSCs are the
precursors of all blood cells. They maintain the dynamic balance
between self-renewal and multipotential differentiation following
the formation of the hematopoietic stem cell pool. Hematopoiesis is
a complicated process that involves the interaction among
hematopoietic stem/progenitor cells, the hematopoietic
microenvironment and hematopoietic growth factors. It satisfies the
requirement for the renewal and replacement of 1,012 blood cells
from at least eight lines each day. Therefore, metabolism of
numerous blood cells may be sustained by a minor subset of HSCs
(7).
In nature, seed germination is also a process that
alternates between dormancy and proliferation (8). The common characteristic of seeds is
that oil is found in the cotyledon. In addition to providing
nutrients, the oil likely maintains the dormant state of the seeds.
The proliferation process begins when seeds incur water. Hence, we
hypothesize that the rehydration process may be the key point in
the initiation of cell proliferation. Therefore, in the human body,
there may be a process that is analogous to the oil/water exchange
that occurs in plants.
Aquaglyceroporin 9 (AQP9) was first discovered in
adipose tissue, then in leukocytes (9), the liver (10), testicle (10), spleen (10)and brain (10). It is the only known aquaglyceroporin
that expressed in the hematopoietic system (11). AQP9 is permeable to a number of
small molecules, such as water and glycerol (13). High viscosity of the concentrated
glycerol may slow down the biochemical processes in the cells and
its water-soluble properties facilitate the replacement of water
with glycerol. Therefore, we speculated that the difference in AQP9
expression between the quiescent normal HSCs and the proliferating
malignant HSCs, and the difference in intracellular glycerol
content between these two types of HSCs, may be involved in the
mechanisms of hematopoietic tumorigenesis. The aim of the present
study was to investigate the AQP9 expression in normal and leukemic
HSCs, and to identify the possible effects of AQP9 in leukemia
progression.
Materials and methods
Bone marrow and peripheral blood
specimens
For this study, bone marrow specimens were obtained
from six patients with leukemia and from thoracotomy patients
without hematology diseases and pathogen infection, respectively,
at the Hematology Department of the Affiliated Hospital of Xuzhou
Medical College (Xuzhou, China). The peripheral blood was obtained
from three healthy volunteers, who were students at Xuzhou Medical
College, and reverse transcription-polymerase chain reaction
(RT-PCR) and western blot analysis were performed to indicate the
white blood cell (WBC) groups.
Following isolation of the mononuclear cells from
each bone marrow sample using Ficoll-Hypaque screening (Shanghai
Biochemical Co., Ltd., Shanghai, China), the hematopoietic stem
cells were separated out by magnetic-activated cell sorting (MACS).
The study was approved by the ethics committee of the Affiliated
Hospital of Xuzhou Medical College, and all patients and healthy
volunteers provided informed consent.
MACS
StemSep® Human Primitive Hematopoietic
Progenitor Cell Enrichment kit (14057) and EasySep®
Magnetic Nanoparticles (19150.1) were purchased from Hangzhou
Baitong Biotech Co., Ltd. (Hangzhou, China). Cells were labeled
with primary monoclonal mouse anti-human CD34/38 antibody,
magnetically labeled with rabbit anti-mouse microbeads and
separated on MACS column (all Hangzhou Baitong Biotech Co., Ltd.).
All the procedures were carried out according to manufacturer’s
instructions. Cells were then positively enriched on micro
beads.
Purity of the sorted cells measured by
flow cytometry
The final concentrations of the sorted stem cells
and non-stem cells were adjusted to 1×106 cells/ml, then
both mouse anti-human CD38 FITC monoclonal antibody (eBioscience,
Inc., San Diego, CA, USA) and mouse anti-human CD34 PE monoclonal
antibody (eBioscience, Inc.) were added at a concentration of 5
μl/ml. Following incubation on ice in the dark for 30 min, the
purity rate was analyzed on a FACScan flow cytometer (Becton
Dickinson, San Jose, CA, USA). The ratio of sorted stem cells
versus bone marrow cells as well as peripheral blood mononuclear
cells was determined using Trypan blue staining (Gibco-BRL,
Eggenstein, Germany).
Western blot analysis
Cells from the from L-HSC, L-nHSC, N-HSC, N-nHSC and
WBC groups were collected and lysed with 200 μl lysate (Beyotime
Institute of Biotechnology, Haimen, China), then centrifuged to
pellet the cell debris. Proteins were separated on SDS-PAGE gels
(Shanghai Shenggong Biotechnological Ltd., Shanghai, China) and
transferred to polyvinylidene difluoride (PVDF) membranes
(Millipore, Billerica, MA, USA). Following this, the PVDF membranes
were rinsed using washer buffer (phosphate-buffered saline with
Tween 20; Zhongshan Golden Bridge Biotech Co., Ltd., Beijing,
China), and then were blocked with 5% non-fat milk. Immunoblotting
was performed with primary mouse monoclonal antibodies specific for
AQP9 and β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), followed by secondary monoclonal rabbit anti-mouse alkaline
phosphatase-conjugated antibody (Zhongshan Golden Bridge Biotech
Co., Ltd.). The proteins were detected with NBT/BCIP
chemiluminescence reagent (Promega, Madison, WI, USA).
Densitometric intensity was measured with the Image-J microscopy
image analysis system (Shanghai Furi Science and Technology Co.,
Ltd., Shanghai, China) and normalized against a β-actin internal
control.
Detection of AQP-9 mRNA expression by
RT-PCR
Total RNA was isolated from the different groups of
cells using the RNAprep pure kit (Tiangen Biotech Co., Ltd.,
Tianjin, China), and the purity and concentration of RNA was
determined by the NanoDrop 1000 spectrophotometer (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The cDNA was then prepared
from 5 μg of total RNA by reverse transcription using TianScript
cDNA First Strand cDNA Synthesis kit (Tiangen Biotech Co., Ltd.),
and 2 μl of the cDNA was amplified for 40 cycles with specific
primers for AQP1, AQP9 and GAPDH (Tiangen Biotech Co., Ltd.)
(Table I). PCR reactions were
initiated with incubation at 94°C for 3 min, followed by 38 cycles
of 94°C for 30 sec, 58°C for 30 sec and 72°C for 1 min. Reactions
were completed with a 72°C, 5 min extension. Subsequently, the
targeted DNA was confirmed by agarose-gel electrophoresis, the
intensity of each band was determined by a gel digital image
analysis system (Furi FR-980; Shanghai Furi Science and Technology
Co., Ltd.) and normalized against a GAPDH internal control.
 | Table ISpecific primers for AQP9 and
GAPDH. |
Table I
Specific primers for AQP9 and
GAPDH.
| Primer | Sequence | Length |
|---|
| H-AQP9-P1 |
5-GAGCAGCTTAGCGAAAG-3 | 344 bp |
| H-AQP9-P2 | 5-
CACCAGCAAAGGACATA-3 | |
| H-GAPDH-P1 |
5-AGGTCGGAGTCAACGGATTTG-3 | 532 bp |
| H-GAPDH-P2 |
5-GTGATGGCATGGACTGTGGT-3 | |
Detection of the glycerol contents of
each group
The cell glycerol content was measured in all
experimental groups using the BG Glycerin ELISA kit (Shanghai Lanji
Biotech Co., Ltd., Shanghai, China). The cell concentration was
adjusted to 8×104 cells/ml; cells were lysed by
repeatedly freezing and thawing; and 50 μl of standard, sample or
distilled water was added to each well of a 96-well microtiter
plate, according to the manufacturer’s instructions. A standardized
preparation of the polyclonal mouse anti-human horseradish
peroxidase (HRP)-conjugated antibody (Shanghai Lanji Biotech Co.,
Ltd.) specific for glycerin was added to each well to bind the
glycerol immobilized on the plate, then the HRP-linked solution was
added. After incubating for 1 h at 37°C, the plate was washed
thoroughly to remove all unbound components. Then, substrate
solutions A and B were added to each well. The enzyme (HRP) and
substrate were allowed to react over a short period (5 min). The
enzyme-substrate reaction was terminated by the addition of a
sulfuric acid solution and the color change was measured
spectrophotometrically at a wavelength of 450 nm.
Smear of leukemia and normal bone marrow
cells and Wright-Giemsa staining
The two types of bone marrow were processed
routinely for smearing on slides coated with polylysine. After
being dried, the samples were stained with Wright-Giemsa dye
solution (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) in order to observe the cellular characteristics. An oil
microscope (Olympus BX51; Olympus, Tokyo, Japan) was used to
carefully observe the size and shape of the hematopoietic stem
cells, to determine whether the hematopoietic stem cells could be
distinguished from their morphology alone.
Statistical analysis
All data were analyzed with SPSS statistical
software (version 13.0; SPPS, Inc., Chicago, IL, USA). Results are
expressed as the mean ± standard deviation. Multiple comparisons
were assessed by one-way analysis of variance, and analysis of
differences between groups was carried out using
Student-Newman-Keuls analysis. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effective isolation of stem cells from
bone marrow
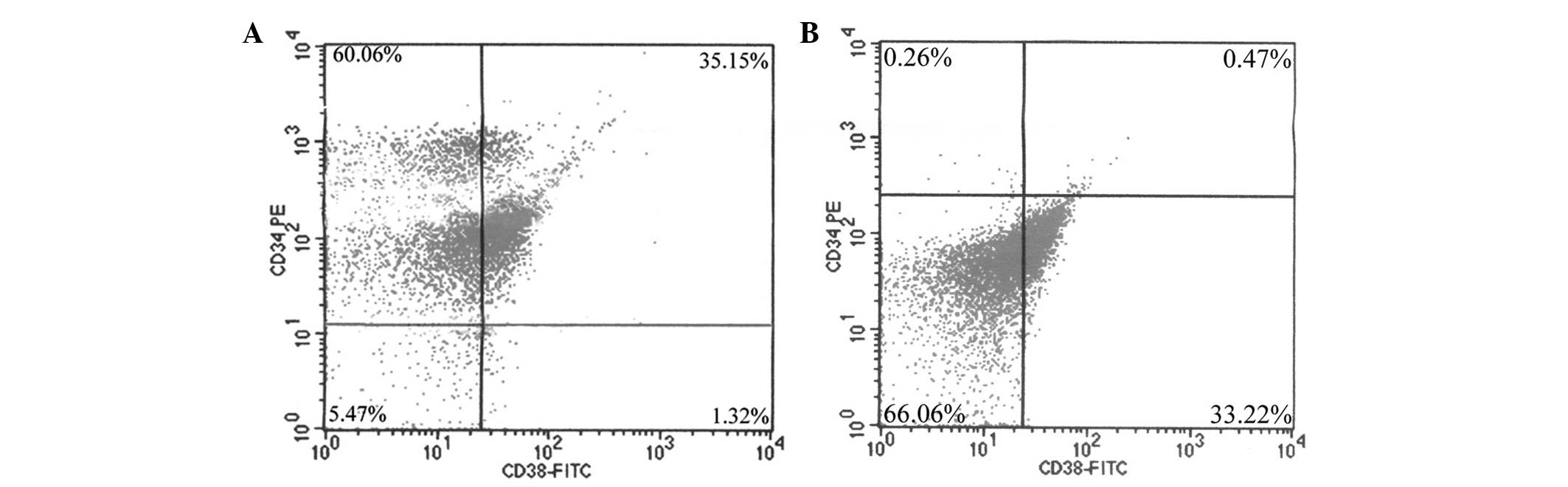
Stem cells and non-stem cells were isolated from the
bone marrow by MACS, labeled with mouse anti-human CD34 PE and CD38
FITC antibodies, and analyzed by FACS. With regard to stem cells,
the purity rate of the sorted CD34+/CD38−,
CD34+/CD38+,
CD34−/CD38− and
CD34−/CD38+ cells was 60.06, 35.15, 5.47 and
1.32%, respectively (Fig. 1A).
Additionally, with regard to non-stem cells, the purity rate of the
sorted CD34−/CD38−
CD34−/CD38+ CD34+/CD38−
and CD34+/CD38+ cells was 66.06, 33.22, 0.26
and 0.47%, respectively (Fig.
1B).
The ratio of leukemia bone marrow stem cells versus
bone marrow cells and mononuclear cells was ~3 and ~17.3%,
respectively. While the ratio of normal bone marrow stem cells
versus bone marrow cells and mononuclear cells was ~0.8 and ~8%,
respectively (Fig. 1).
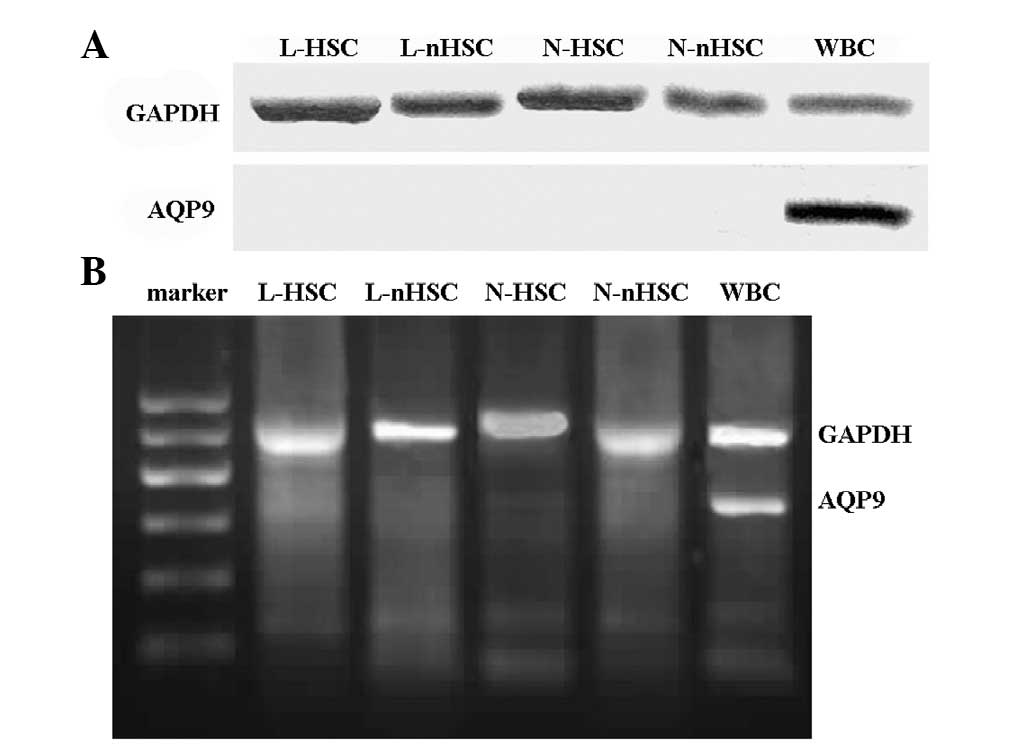
AQP9 protein expression is not observed
in bone marrow stem cells or non-stem cells
The expression of AQP9 protein was only detected in
the peripheral blood (in the WBCs) (Fig. 2A). The results confirmed that AQP9
protein was not expressed in the remainder of the experimental
groups.
AQP9 mRNA expression in peripheral blood
WBCs
The expression of AQP9 mRNA was only detected in
peripheral blood (in the WBCs) (Fig.
2B); AQP9 mRNA expression was not detected in the remainder of
the experimental groups.
Glycerol downregulation in non-leukemia
hematopoietic stem cells
The glycerol content in all experimental groups is
shown in Fig. 3. The glycerol
concentration was identified to be significantly higher in the
N-HSCs compared with the N-nHSCs, in the N-nHSC compared with the
L-nHSCs and in the L-HSCs compared with the L-nHSCs (P<0.05 for
all). The concentration of glycerol in the L-HSCs and N-HSCs was
significantly higher than that in the WBCs (P<0.05 for both),
and that in the L-nHSCs was significantly lower compared with that
in the WBCs (P<0.05).
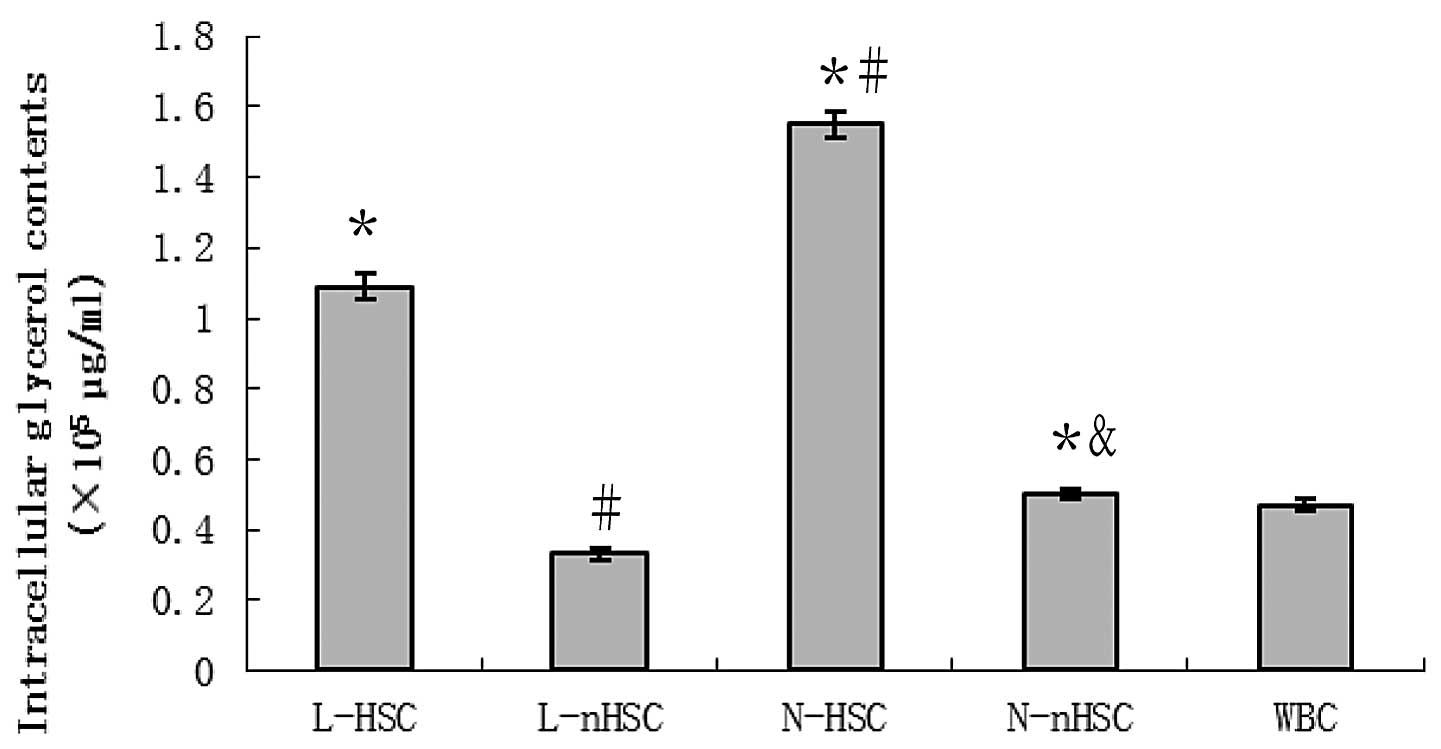 | Figure 3Intracellular glycerol contents in
L-HSCs, L-nHSCs, N-HSCs, N-nHSCs and WBCs. The N-HSCs exhibited the
greatest concentration of glycerol among the five groups, and the
glycerol content of the L-HSCs was less than that of the N-HSCs,
but greater than that of the remaining three groups.
*P<0.05 vs. WBC; #P<0.05 vs. L-HSC;
&P<0.05 vs. N-HSC. L-HSCs, hematopoietic stem
cells (HSCs) isolated from leukemia patients; L-nHSCs, non-HSCs
(nHSCs) isolated from leukemia patients; N-HSCs, HSCs isolated from
thoracotomy patients; N-nHSCs, nHSCs isolated from thoracotomy
patients; WBCs, white blood cells. |
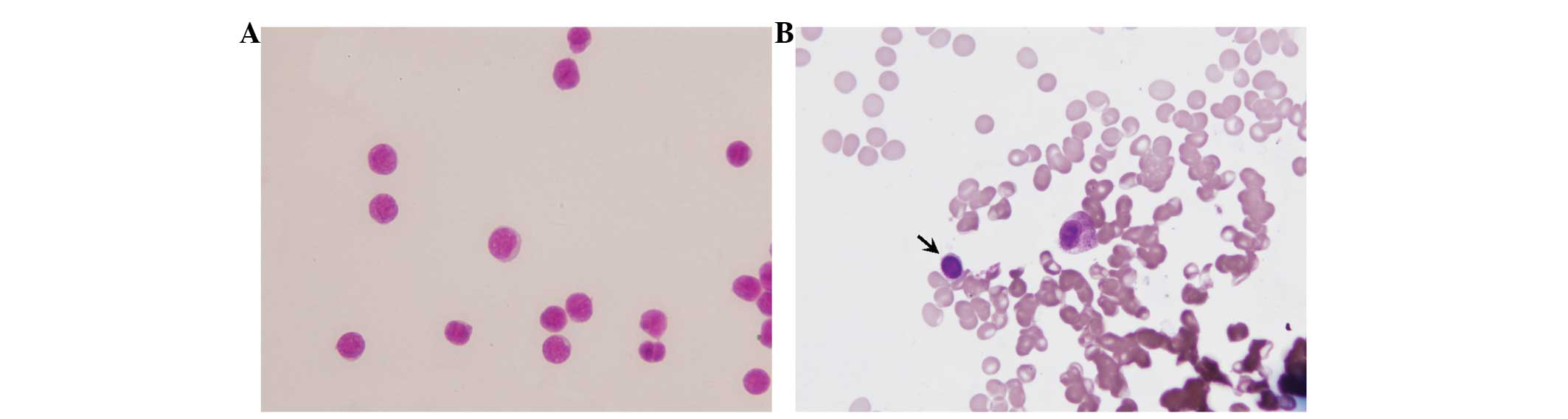
Bone marrow and stem cell smear
The morphology of stem cells of normal bone marrow
is typical, while that of the leukemia stem cell is atypical. Under
the oil microscope, primitive cells of the same shape and size were
observed in both the bone marrow and stem cell smears. These
primitive cells were round or oval, and the nuclei were round,
slightly oval or irregular in shape. In addition, the nuclei
occupied almost the entire cell, were located in the middle or were
biased toward one side, and were stained light purple or red. The
chromatin was tender, uniformly distributed and deeply stained,
without any phenomenon of aggregation. The nuclear membrane was
neat and thin, and the nucleus had three to six nucleoli. The
cytoplasm was seldom and without visible particles, and was stained
light blue (Fig. 4).
Discussion
AQP9 is widely expressed in a number of tissues
which are permeable to water, glycerol and urea. AQP9 is also
involved in the glycerol uptake by hepatocyte for gluconeogenesis
(14). Certain studies have found
that AQP9 is abundant in peripheral blood leukocytes, which is
consistent with the results of the current study (9). When the leukemia K562 cell line was
transfected with human AQP9 cDNA, trisenox uptake was found to
increase (11), which may lead to
leukemia cell death (15).
Therefore, the downregulation of AQP9 may present as a target for
cancer therapy (16). Qu et
al (17) verified via in
situ hybridization that AQP9 is expressed in cytotrophoblasts
and syncytiotrophoblasts of the placental epithelial cells of the
amnion in healthy pregnant women. It is speculated that AQP9 is
associated with maternal-fetal water and solution exchange. Damiano
et al (18) verified by
histochemistry and western blotting that the expression of AQP9 was
elevated in the preeclampsia placenta; however, the functional
ability of AQP9 for water permeability was decreased, suggesting
that AQP9 plays an important role in pre-eclampsia. Badaut et
al (19) suggested that AQP9
can regulate the metabolic balance between brain parenchyma and
cerebrospinal fluid. Yamamoto et al (20) showed that cerebral edema, which is
due to cerebral ischemia and hypoxia, is closely associated with
AQP9; AQP9 functions in maintaining the internal environment
homeostasis and in lactate buffering. As a member of the
aquaglyceroporin family, AQP9 is abundantly expressed on the
sinusoid surface of hepatocytes, indicating that AQP9 is involved
in the liver-blood glycerol exchange (21). Additionally, certain studies have
suggested that AQP9 is involved in hepatocyte lipid metabolism and
steatosis (22–24). Apart from its contribution to
neoplasm pathogenesis, AQP9 has also been implicated in the process
of inflammation; a previous study indicated that a strong increase
in AQP9 transcripts was observed in synovial tissues from patients
with osteoarthritis and rheumatoid arthritis (25).
The present study indicated that glycerol was
present in the cells of all five groups, and that the glycerol
content in the L-HSCs was significantly lower than that in the
N-HSCs (P<0.05). Similarly, the concentration of glycerol in the
L-nHSCs was significantly lower compared with that in the N-nHSCs
(P<0.05). Moreover, the glycerol content in the L-HSCs was
significantly higher than that in the L-nHSCs (P<0.05), and the
same trend was observed between the N-HSCs and the N-nHSCs
(P<0.05). Since the proliferation rate of L-HSCs is markedly
higher than that of N-HSCs, and that of L-nHSCs is markedly higher
than that of N-nHSCs, these results indicated that the cell
proliferation state maybe associated with the intracellular
glycerol content; the higher the intracellular glycerol content,
the lower the proliferation rate. This is a novel insight into the
mechanism underlying leukemia cell proliferation, which may be
useful for the growth intervention of leukemia cells, as altering
the intracellular glycerol content may be beneficial for decreasing
the tumor cell proliferation rate.
However, our results also showed that no AQP9
expression was detected in the L-HSCs, L-nHSCs, N-HSCs or N-nHSCs;
while AQP9 was identified to be expressed in the WBCs of the normal
peripheral blood. Although AQP9 is the only known aquaglyceroporin
that is expressed in the hematopoietic system, there may be other
pathway for the glycerol to enter these normal or abnormal bone
marrow hematopoietic cells. The possible mechanism for the cell
growth inhibition induced by an increase in the intracellular
glycerol concentration may lead to an increase in the viscosity of
the cytoplasmic fluid, which may subsequently lead to the
decelerated movement of signal molecules. As a result, the cell
growth is inhibited. The concrete mechanisms are required to be
further investigated.
Glycerol is the carbon backbone of triacylglycerol
and represents an important metabolite for the control of fat
accumulation and glucose homeostasis; glycerol serves as the major
substrate for hepatic gluconeogenesis during periods of fasting
(26). It has been demonstrated
that glucose metabolism is altered in neoplastic cells; the
glycolytic rate is much higher than that in normal cells. Aerobic
glycolysis is a hallmark of cancer, and cancer cells become highly
glycolytic (27). Hence, we
speculate that the influx of glycerol into leukemia stem cells or
normal bone marrow stem cells is markedly greater than that of
non-stem leukemia cells or non-stem normal bone marrow cells;
however, the increased glycerol is immediately metabolized for
producing glucose, and thus the cells enter into aerobic glycolysis
to produce sufficient energy. Previously, Sun et al
(27) indicated that pyruvate
kinase isoenzyme type M2 is involved in this process. It is
therefore possible that the glycerol content is merely a reflection
of the active glucose metabolism of leukemia stem cells.
In conclusion, the present study identified that the
glycerol content of leukemia stem cells was significantly lower
than that of normal bone marrow stem cells, while both cell types
were deficient of AQP9. The glycerol content is reduced in leukemia
stem cells compared with that in normal bone marrow stem cells. The
excessive aerobic glycolysis is not only attributable to glycerol,
but also to other substances, such as amino acids. It is possible
that amino acids dominate glycerol in this aerobic glycolysis for
leukemia stem cells. This study provides a novel insight into
understanding leukemia tumorigenesis, which may be utilized for
leukemia prevention.
Acknowledgements
The authors would like to thank Mrs. Hong Liu for
the technical assistance and valuable discussions.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013.
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012.
|
|
3
|
Iredale JP: Models of liver fibrosis:
exploring the dynamic nature of inflammation and repair in a solid
organ. J Clin Invest. 117:539–548. 2007.
|
|
4
|
Martin P: Wound healing - aiming for
perfect skin regeneration. Science. 276:75–81. 1997.
|
|
5
|
Michalopoulos GK and DeFrances MC: Liver
regeneration. Science. 276:60–66. 1997.
|
|
6
|
Arnold I and Watt FM: c-Myc activation in
transgenic mouse epidermis results in mobilization of stem cells
and differentiation of their progeny. Curr Biol. 11:558–568.
2001.
|
|
7
|
Ogawa M: Differentiation and proliferation
of hematopoietic stem cells. Blood. 81:2844–2853. 1993.
|
|
8
|
Joel DM, Bar H, Mayer AM, et al: Seed
ultrastructure and water absorption pathway of the root-parasitic
plant Phelipanche aegyptiaca (Orobanchaceae). Ann Bot.
109:181–195. 2011.
|
|
9
|
Ishibashi K, Kuwahara M, Gu Y, et al:
Cloning and functional expression of a new aquaporin (AQP9)
abundantly expressed in the peripheral leukocytes permeable to
water and urea, but not to glycerol. Biochem Biophys Res Comm.
244:268–274. 1998.
|
|
10
|
Elkjaer M, Vajda Z, Nejsum LN, et al:
Immunolocalization of AQP9 in liver, epididymis, testis, spleen,
and brain. Biochem Biophys Res Commun. 276:1118–1128. 2000.
|
|
11
|
Bhattacharjee H, Carbrey J, Rosen BP and
Mukhopadhyay R: Drug uptake and pharmacological modulation of drug
sensitivity in leukemia by AQP9. Biochem Biophys Res Commu.
322:836–841. 2004.
|
|
12
|
Hara-Chikuma M and Verkman A:
Physiological roles of glycerol-transporting aquaporins: the
aquaglyceroporins. Cell Mol Life Sci. 63:1386–1392. 2006.
|
|
13
|
Verkman AS: Physiological importance of
aquaporin water channels. Ann Med. 34:192–200. 2002.
|
|
14
|
Carbrey JM, Gorelick-Feldman DA, Kozono D,
et al: Aquaglyceroporin AQP9: solute permeation and metabolic
control of expression in liver. Proc Nat Acad Sci USA.
100:2945–2950. 2003.
|
|
15
|
Leung J, Pang A, Yuen WH, et al:
Relationship of expression of aquaglyceroporin 9 with arsenic
uptake and sensitivity in leukemia cells. Blood. 109:740–746.
2007.
|
|
16
|
Monzani E, Shtil AA and La Porta CA: The
water channels, new druggable targets to combat cancer cell
survival, invasiveness and metastasis. Curr Drug Targets.
8:1132–1137. 2007.
|
|
17
|
Qu F, Wang FF, Lu XE, et al: Altered
aquaporin expression in women with polycystic ovary syndrome:
hyperandrogenism in follicular fluid inhibits aquaporin-9 in
granulosa cells through the phosphatidylinositol 3-kinase pathway.
Human Reprod. 25:1441–1450. 2010.
|
|
18
|
Damiano AE, Zotta E and Ibarra C:
Functional and molecular expression of AQP9 channel and UT-A
transporter in normal and preeclamptic human placentas. Placenta.
27:1073–1081. 2006.
|
|
19
|
Badaut J, Brunet JF and Petit JM:
Induction of brain aquaporin-9 (AQP9) in catecholaminergic neurons
in diabetic rats. Brain Res. 1188:17–24. 2008.
|
|
20
|
Yamamoto N, Yoneda K, Asai K, et al:
Alterations in the expression of the AQP family in cultured rat
astrocytes during hypoxia and reoperation. Brain Res Mol Brain Res.
90:26–38. 2001.
|
|
21
|
Jelen S, Wacker S, Aponte-Santamaría C, et
al: Aquaporin-9 protein is the primary route of hepatocyte glycerol
uptake for glycerol gluconeogenesis in mice. J Bio Chem.
286:44319–44325. 2011.
|
|
22
|
Miranda M, Ceperuelo-Mallafré V, Lecube A,
et al: Gene expression of paired abdominal adipose AQP7 and liver
AQP9 in patients with morbid obesity: Relationship with glucose
abnormalities. Metabolism. 58:1762–1768. 2009.
|
|
23
|
Calamita G, Fanelli E, Svelto M, et al:
Biophysical assessment of AQP9 as real membrane pathway in
hepatocyte glycerol uptake. FASEB J. 24(Meeting Abstract
Supplement): 10002010.
|
|
24
|
Lebeck J: Metabolic impact of the glycerol
channels AQP7 and AQP9 in adipose tissue and liver. J Mol
Endocrinol. 52:R165–178. 2014.
|
|
25
|
Nagahara M, Waguri-Nagaya Y, Yamagami T,
et al: TNF-alpha-induced aquaporin 9 in synoviocytes from patients
with OA and RA. Rheumatology. 49:898–906. 2010.
|
|
26
|
Rodríguez A, Catalán V, Gómez-Ambrosi J
and Frühbeck G: Aquaglyceroporins serve as metabolic gateways in
adiposity and insulin resistance control. Cell Cycle. 10:1548–1556.
2011.
|
|
27
|
Sun Q, Chen X, Ma J, et al: Mammalian
target of rapamycin up-regulation of pyruvate kinase isoenzyme type
M2 is critical for aerobic glycolysis and tumor growth. PNAS.
108:4129–4234. 2011.
|