Introduction
Colorectal carcinoma (CRC) is the second leading
cause of cancer-related mortality worldwide (1). Although, surgical intervention is no
longer the only treatment option and chemotherapy presents an
important strategy for the treatment of the majority of CRC
patients (2). However, de
novo and acquired resistance to a variety of drugs is common
and, therefore, the drug-resistant phenotype of CRCs presents one
of the major obstacles in its eradication (3).
Gene silencing or inhibition of associated
downstream proteins is commonly used to understand gene function
(4). If multidrug resistance in CRC
cells is found to correlate with LIM domain-containing protein 1
(LIMD1) expression, it may be possible to reverse drug resistance
by interfering with the expression of this protein, thus providing
a potential treatment for CRC. This is an attractive option since
drugs that selectively inhibit LIMD1 in CRC are in the early phase
of development and little, if any, evidence exists regarding the
effects of blocking LIMD1 in CRC. RNA interference is a
post-transcriptional gene silencing mechanism in which mRNA is
degraded in a sequence-specific manner (5). The aim of this study was to
demonstrate that the specific silencing of LIMD1 by RNA
interference may effectively reverse drug resistance in
multidrug-resistant (MDR) CRC cells by enhancing cell apoptosis,
which may highlight novel investigational targets that will provide
therapeutic options for CRC.
Materials and methods
Experimental approval
The study was conducted in accordance with the
Declaration of Helsinki. All experimental protocols were approved
by the Review Committee for the Use of Human or Animal Subjects of
Xiamen University (Xiamen, China).
Cell culture and induction of MDR
The human CRC Colo205 and HCT-8 cell lines were
purchased from the Cell Bank of Shanghai Institute of Biochemistry
and Cell Biology, Chinese Academy of Sciences (Shanghai, China).
The cell lines were cultured in Dulbecco’s modified Eagle’s medium
(Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum
(Hyclone) at 37°C in a humidified atmosphere of 5% CO2.
The 5-fluorouracil (5-FU)-resistant human CRC cell sublines
(Colo205/5-FU and HCT-8/5-FU) were established by adding 5-FU
[Shanghai Pharmaceutical (Group) Co., Ltd., Shanghai, China] to
GIBCO® RPMI-1640 medium (Invitrogen Life Technologies,
Carlsbad, CA, USA) at concentrations ranging between 0.01 and 2
μg/ml.
siRNA transfection
siRNA was purchased from Thermo Fisher Scientific
(Waltham, MA, USA). The Colo205/5-FU and HCT-8 cells were seeded at
a density of 5×104 cells/well in six-well plates for 24
h prior to transfection. The cells were transfected with 25, 50 or
75 nM concentrations of siRNA-LIMD1 using Lipofectamine 2000
(Invitrogen Life Technologies) following the manufacturer’s
instructions.
Western blot analysis
The silencing effects of siRNA targeting the LIMD1
gene were assessed by western blot analysis. The cells were
cultured in a six-well plate at a density of 2.0×104
cells/well for 48 h, harvested, washed twice with ice-cold
phosphate-buffered saline and then lysed in
radioimmunoprecipitation assay buffer (Abcam, Cambridge, UK). The
cell lysates were briefly sonicated (Sonicator Q700; Qsonica, LLC,
Newton, CT, USA) and kept in ice-water for 30 min. The protein
concentrations were determined using a BCA Protein Assay kit
(Bio-Rad, Hercules, CA, USA). The protein bands were visualized by
chemiluminescence using enhanced chemiluminescence plus western
blotting reagent (GE Healthcare, Little Chalfont, UK), followed by
exposure to Fujifilm LAS-1000 equipment (Fujifilm, Tokyo, Japan).
The parallel membranes were incubated with 1:10,000 rabbit
anti-human monoclonal antibodies against β-actin (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and horseradish
peroxidase-coupled rabbit anti-mouse monoclonal secondary antibody
(Innova Biosciences, Ltds., New York, NY, USA).
Cell growth and chemosensitivity
The chemosensitivity resulting from the LIMD1
knockdown was assessed using an MTT-based cell growth determination
kit (Sigma-Aldrich, St. Louis, MO, USA). The sensitivity of
transfected CRC cells to the commonly used anticancer drugs, 5-FU
and l-oxaliplatin (Changsha Huir Biological-tech Co., Ltd.,
Changsha, China), was detected in accordance with a previous study
(6). The total cells were then
harvested at 12, 24, 36, 48 and 60 h following drug exposure. The
absorbance was measured at 490 nm using a microplate reader (Getein
Biotechnology Co., Ltd., Nanjing, China) and the value of 50%
inhibitory concentration (IC50), defined as the drug
concentration required to reduce cell survival to 50% as determined
by the relative absorbance of BrdU, was assessed by probit
regression analysis. The resistance index (RI) was calculated using
the following formula: RI (%) = (IC50 of treated
cells/IC50 of untreated or parental cells) × 100.
Apoptosis detection assay
The apoptosis detection assay was performed to
determine the effects of LIMD1 knockdown on apoptosis in CRC in
response to chemotherapy agents. The Colo205/5-FU and HCT-8/5-FU
cells were transfected as previously described. The transfected
cells and controls were then exposed to 0.2 μg/ml of 5-FU for 48 h,
harvested by trypsinization and centrifuged at 2,000 × g for 5 min
at 4°C. Next, 0.5 μg/ml of Annexin V-fluorescein isothiocyanate
(Becton-Dickinson, Franklin Lakes, NJ, USA) and 0.6 μg/ml of
propidium iodide (Shanghai QF Biosciences Co., Ltd., Shanghai,
China) were added to the cell suspension. After 15 min, the stained
cells were immediately analyzed by BD FACSCalibur™ (BD Biosciences,
San Jose, CA, USA).
Statistical Analysis
Differences between the groups were analyzed by
one-way analysis of variance using Dunnett’s post hoc test for
continuous variables and the χ2 or Fisher’s exact tests
for categorical variables, as appropriate. P<0.05 was considered
to indicate a statistically significant difference. Data were
analyzed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) and
presented as the mean ± standard deviation.
Results
Establishment of MDR CRC cell lines
The Colo205/5-FU cells were 29.21 times more
resistant to 5-FU compared with the Colo205 cells, and the
HCT-8/5-FU cells were 16.04 times more resistant when compared with
the HCT-8 cells. The two cell lines also exhibited cross-resistance
to another common chemotherapeutic agent, 1-oxaliplatin (Table I).
 | Table IEstablishment of two 5-FU-resistant
colorectal carcinoma sublines. |
Table I
Establishment of two 5-FU-resistant
colorectal carcinoma sublines.
| IC50
(mg/l) | | IC50
(mg/l) | |
|---|
|
| |
| |
|---|
| Agents | Colo205 | Colo205/5-FU | RI | HCT-8 | HCT-8/5-FU | RI |
|---|
| 5-FU | 0.0113±0.011 | 0.2719±0.002 | 29.21 | 0.0056±0.018 | 0.1931±0.001 | 16.04 |
| l-OHP | 0.0061±0.004 | 0.1129±0.005 | 17.13 | 0.0097±0.004 | 0.1101±0.062 | 10.21 |
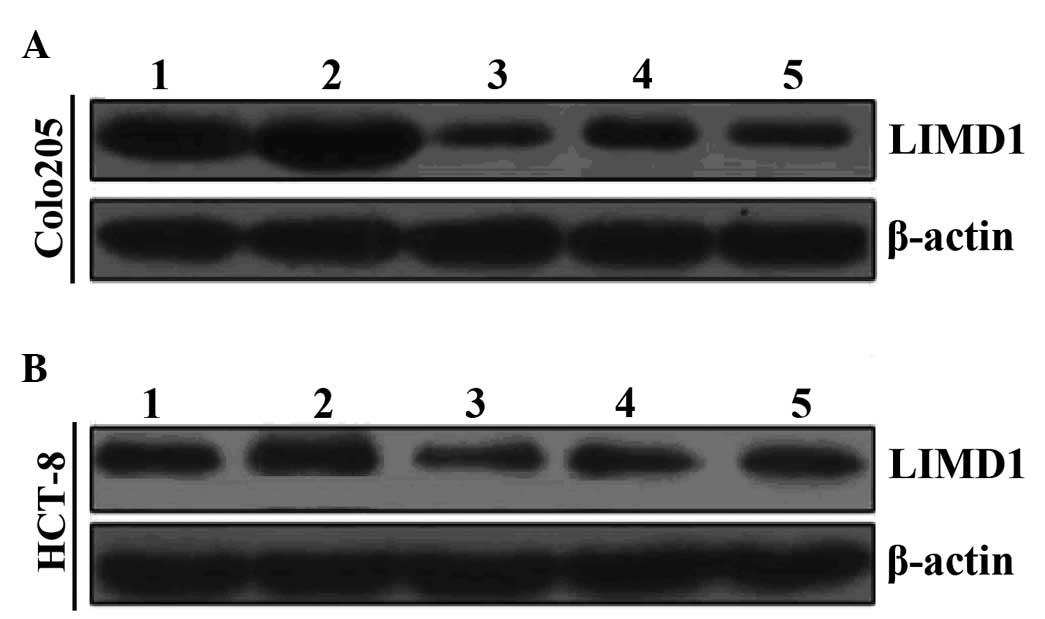
siRNA transfection decreases LIMD1
protein expression in MDR cells
Prior to inducing drug resistance in the Colo205 and
HCT-8 cells, the LIMD1 protein levels were detectable; although,
LIMD1 expression in the Colo205 and HCT-8 cells was relatively low
(Fig. 1). As the CRC cell
population developed with increasing MDR, LIMD1 protein expression
was also found to significantly increase (P=0.006 and P=0.002 for
LIMD1 in Colo205/5-FU and HCT-8/5-FU, respectively). Following
siRNA transfection, the expression of the LIMD1 protein was reduced
in MDR cells. In addition, LIMD1 protein expression was lower in
the siRNA-transfected MDR cells than that in the parental
controls.
Silencing LIMD1 reverses the MDR cell
phenotype
The silencing of LIMD1 was found to enhance the
chemosensitivity of the two MDR CRC sublines to 5-FU (P<0.001;
Table II).
 | Table IIRestoration of drug sensitivity
following the knockdown of LIMD1 by RNA interference resensitized
5-FU-resistant colorectal carcinoma sublines to the applied agents
(n=five per group). |
Table II
Restoration of drug sensitivity
following the knockdown of LIMD1 by RNA interference resensitized
5-FU-resistant colorectal carcinoma sublines to the applied agents
(n=five per group).
| IC50
(mg/l) | | IC50
(mg/l) | |
|---|
|
| |
| |
|---|
| Agents | Colo205/5-FU |
Colo205/5-FU/siLIMD1 | RI | HCT-8/5-FU |
HCT-8/5-FU/siLIMD1 | RI |
|---|
| 5-FU | 0.2657±0.003 | 0.0608±0.033 | 1.23 | 0.1181±0.002 | 0.0108±0.006 | 1.09 |
| l-OHP | 0.0062±0.004 | 0.0112±0.003 | 2.32 | 0.0098±0.004 | 0.0149±0.003 | 1.78 |
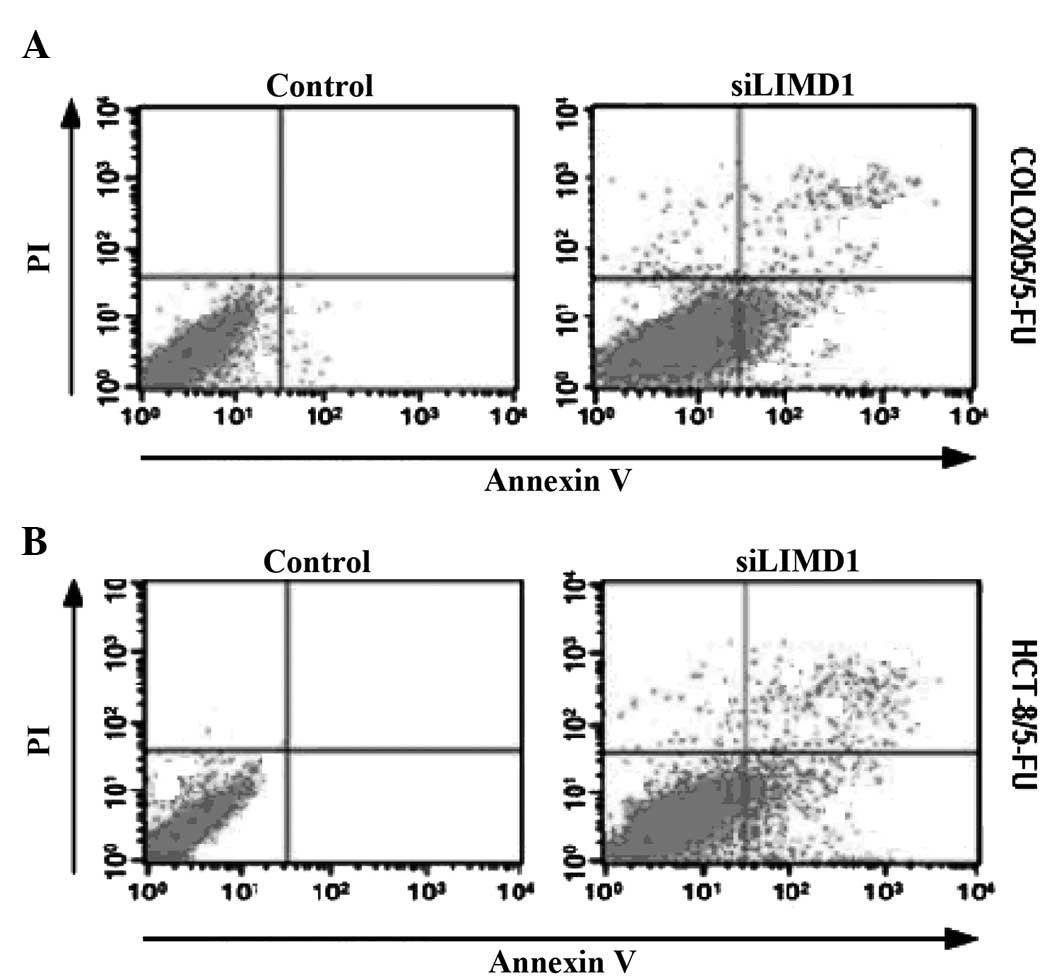
Silencing LIMD1 induces apoptosis in
5-FU-resistant CRC cells
The average apoptotic rate in LIMD1
siRNA-transfected Colo205/5-FU and HCT-8/5-FU cells was 8.50 and
8.11%, respectively (Fig. 2), which
was significantly increased compared with that in the MDR sublines
[0.16% (P=0.001) and 0.19% (P<0.05), respectively].
Discussion
CRC responds poorly to chemotherapy owing to
multidrug resistance, which contributes to poor treatment outcomes
of CRC with chemotherapeutic drugs (7). However, a recent study demonstrated a
close correlation between LIMD1 expression in CRCs (8). In the present study, the CRC Colo205
and HCT-8 cell lines were shown to express LIMD1, albeit at low
levels, prior to 5-FU-induced multidrug resistance. As the CRC
cells acquired increasing tolerance to 5-FU, LIMD1 expression was
found to significantly increase in the Colo205 and HCT-8 MDR
phenotypes. However, when the LIMD1 gene was silenced by siRNA,
LIMD1 protein expression returned to levels comparable to those of
the parental cell populations. The results of the current study
indicated that the modulation of LIMD1 expression using RNA
interference may reverse drug resistance in the CRC MDR
phenotype.
The observation that the resistance of tumor cells
to chemotherapy correlates with the overexpression of transport
proteins, including LIMD1, has prompted efforts to develop agents
with the ability to inhibit LIMD1-mediated drug transport (9). At present, no evidence exists to
suggest that the inhibition of LIMD1 may reduce multidrug
resistance in CRC. However, it has been reported that the
suppression of the LIMD1 gene by RNA interference reverses the drug
resistance in CRC cells (Colo205/5-FU and HCT-8/5-FU) (10). The results of the current study,
derived from a similar CRC subline, showed that siRNA targeting
LIMD1 effectively reverses MDR. This was demonstrated using
chemosensitivity assays, and these results were as efficacious as
blocking LIMD1. 5-FU and its analogs are widely used as first-line
chemotherapeutic agents for patients with advanced CRC and these
chemotherapeutics are known to function through various mechanisms
(11,12). It has been suggested that
upregulated LIMD1 gene expression may be the major mechanism
underlying acquired 5-FU resistance in CRC (13). In the context of the agents applied
in the present study, the modulation of MRP1 expression was found
to clearly sensitize the MDR CRC phenotype to chemotherapy.
However, these observations raise the question of
what mechanisms are involved in reversing the acquired drug
resistance of CRC when LIMD1 is silenced. The results of the
current study support this theory, as increased LIMD1 suppression
was found to result in increased apoptosis.
In conclusion, the current study demonstrated that
the suppression of LIMD1 expression may reverse drug resistance in
the CRC MDR cells and enhance apoptosis. The results highlight the
possibility that RNA interference targeting LIMD1 may be a useful
approach in the treatment of CRC.
Acknowledgements
The current study was supported by the Foundations
of Health Bureau (Xiamen, China; grant nos. 3502Z20124029 and
3502Z20134025) and the Medical Innovation Fund of Nanjing Military
Command of Chinese PLA (grant no. MS090).
References
|
1
|
Levin TR and Corley DA: Colorectal-cancer
screening - coming of age. N Engl J Med. 369:1164–1166. 2013.
|
|
2
|
Mastalier B, Tihon C, Ghiţă B, Botezatu C,
Deaconescu V, Mandisodza P, Drăghici C and Simion S: Surgical
treatment of colon cancer: Colentina surgical clinic experience. J
Med Life. 5:348–353. 2012.
|
|
3
|
Ito Y, Yamada Y, Asada K, Ushijima T,
Iwasa S, Kato K, Hamaguchi T and Shimada Y: EGFR L2 domain mutation
is not correlated with resistance to cetuximab in metastatic
colorectal cancer patients. J Cancer Res Clin Oncol. 139:1391–1396.
2013.
|
|
4
|
Zhu XS, Dai YC, Chen ZX, Xie JP, Zeng W,
Lin YY and Tan QH: Knockdown of ECHS1 protein expression inhibits
hepatocellular carcinoma cell proliferation via suppression of Akt
activity. Crit Rev Eukaryot Gene Expr. 23:275–282. 2013.
|
|
5
|
Lin YL and Pasero P: Interference between
DNA replication and transcription as a cause of genomic
instability. Curr Genomics. 13:65–73. 2012.
|
|
6
|
Magge D, Zureikat AH, Bartlett DL,
Holtzman MP, Choudry HA, Beumer JH, Pingpank JF, Holleran JL,
Strychor S, Cunningham DE, et al: A phase I trial of isolated
hepatic perfusion (IHP) using 5-FU and oxaliplatin in patients with
unresectable isolated liver metastases from colorectal cancer. Ann
Surg Oncol. 20:2180–2187. 2013.
|
|
7
|
Mirakhorli M, Rahman SA, Abdullah S,
Vakili M, Rozafzon R and Khoshzaban A: Multidrug resistance protein
2 genetic polymorphism and colorectal cancer recurrence in patients
receiving adjuvant FOLFOX-4 chemotherapy. Mol Med Rep. 7:613–617.
2013.
|
|
8
|
Sharp TV, Munoz F, Bourboulia D, Presneau
N, Darai E, Wang HW, Cannon M, Butcher DN, Nicholson AG, Klein G,
et al: LIM domains-containing protein 1 (LIMD1), a tumor suppressor
encoded at chromosome 3p21.3, binds pRB and represses E2F-driven
transcription. Proc Natl Acad Sci USA. 101:16531–16536. 2004.
|
|
9
|
Xu K, Liang X, Shen K, Sun L, Cui D, Zhao
Y, Tian J, Ni L and Liu J: MiR-222 modulates multidrug resistance
in human colorectal carcinoma by down-regulating ADAM-17. Exp Cell
Res. 318:2168–2177. 2012.
|
|
10
|
Maddalena F, Laudiero G, Piscazzi A,
Secondo A, Scorziello A, Lombardi V, Matassa DS, Fersini A, Neri V,
Esposito F and Landriscina M: Sorcin induces a drug-resistant
phenotype in human colorectal cancer by modulating Ca(2+)
homeostasis. Cancer Res. 71:7659–7669. 2011.
|
|
11
|
Andersen V, Ostergaard M, Christensen J,
Overvad K, Tjønneland A and Vogel U: Polymorphisms in the
xenobiotic transporter Multidrug Resistance 1 (MDR1) and
interaction with meat intake in relation to risk of colorectal
cancer in a Danish prospective case-cohort study. BMC Cancer.
9:4072009.
|
|
12
|
Andersen V, Agerstjerne L, Jensen D,
Østergaard M, Saebø M, Hamfjord J, Kure E and Vogel U: The
multidrug resistance 1 (MDR1) gene polymorphism G-rs3789243-A is
not associated with disease susceptibility in Norwegian patients
with colorectal adenoma and colorectal cancer; a case control
study. BMC Med Genet. 10:182009.
|
|
13
|
Vilaça N, Amorim R, Machado AF, Parpot P,
Pereira MF, Sardo M, Rocha J, Fonseca AM, Neves IC and Baltazar F:
Potentiation of 5-fluorouracil encapsulated in zeolites as drug
delivery systems for in vitro models of colorectal carcinoma.
Colloids Surf B Biointerfaces. 112:237–244. 2013.
|