Introduction
Dendritic cells (DCs) are antigen-presenting cells
with the unique ability to take up and process antigens in the
peripheral blood and tissues (1).
Subsequent migration to the draining lymph nodes then occurs, where
the antigens are presented to resting lymphocytes. Antigen
ingestion and processing is particularly efficient within immature
DCs, however, for an efficacious T-cell response, maturation to
fully activated DCs must occur; these cells express high levels of
cell-surface major histocompatibility complex (MHC)-antigen
complexes and costimulatory molecules. Tumor-associated dendritic
cells (TADCs) are DCs that present in the tumor microenvironment
with tolerogenic or suppressive functions, which exhibit lower
expression levels of costimulatory and MHC molecules (2). TADCs have also been associated with a
higher expression level of tolerogenic mediators, including
indoleamine-2,3-dioxygenase, arginase and transforming growth
factor (TGF)-β (3). In addition to
inducing immune surveillance, TADCs have been reported to secrete
factors that increase cancer progression (4–6).
Previous studies have shown that tolerogenic DCs
(tDCs) may be induced by the presence of interleukin (IL)-10 in
culture medium or in the tumor microenvironment (7–9). The
binding of IL-10 and the IL-10 receptor has been reported to
trigger several downstream pathways in mononuclear cells, including
the Janus kinase (JAK)-1/signal transducer and activator of
transcription-3 (STAT-3) (10,12),
p38 mitogen-activated protein kinase, extracellular
signal-regulated kinase and c-Jun N-terminal kinase signaling
pathways (12). Among the signaling
induced by IL-10, the JAK/STAT3 signaling pathway has been reported
to be regulated by a group of protein tyrosine phosphatases
(PTPases), termed suppressors of cytokine signaling (13,14).
Cluster of differentiation (CD)45 is a member of the
PTPase family that is distributed in the plasma membrane of
hematopoietic cells, with the exception of erythrocytes and
platelets, and exhibits a crucial function in T-cell
receptor-mediated signaling. Previous studies have shown that CD45
also regulates JAK (15–17) and Src (18,19)
families. In addition, the negative regulation of cytokine receptor
signaling by CD45 may explain the loss of CD45 activity observed in
several cancer types, including leukemia. CD45 has been found to
correlate with the proliferation of myeloma cells, and it may
therefore present a potential target for the treatment of multiple
myelomas (20). Despite its
abundant expression, the function of CD45 in cells of the myeloid
lineage is poorly understood. Fulcher et al (21) reported that CD45 may be a receptor
for galectin-induced cell activation and migration via Syk and
protein kinase C signaling in DCs. Previous studies regarding the
function of CD45 in cancer have focused on myelomas (22,23),
however, none of these studies have investigated the function of
CD45 in tumor immune surveillance. The present study demonstrated
that CD45 activation is important in tumor- and IL-10-induced
tDCs.
Materials and methods
Cell lines, cell cultures and condition
medium collection
Human breast adenocarcinoma MDA-MB-231 cells
(HTB-26) and human lung adenocarcinoma A549 cancer cells (CCL-185)
were obtained from the American Type Culture Collection (Manassas,
VA, USA). The human colorectal adenocarcinoma SW620 cells (BCRC
60343) and SW480 (BCRC 60249) cells were purchased from the
Bioresource Collection and Research Center (Hsinchu City, Taiwan).
The human lung adenocarcinoma A549 cancer cells were cultured in
minimum essential medium (Life Technologies, Inc., Grand Island,
NY, USA) with 10% fetal bovine serum (FBS), non-essential amino
acids and 0.1 mM sodium pyruvate (all Thermo Fisher Scientific,
Waltham, MA, USA). The human breast adenocarcinoma MDA-MB-231 cells
were cultured in F12K medium (Life Technologies, Inc.) with 10%
FBS, non-essential amino acids and 0.1 mM sodium pyruvate. The
SW620 and SW480 human colorectal adenocarcinoma cells were cultured
in Leibovitz’s L-15 medium (Life Technologies, Inc.) supplemented
with 10% FBS. The medium was changed once every 2–3 days and the
cells were channeled once a distinct cell density had been reached.
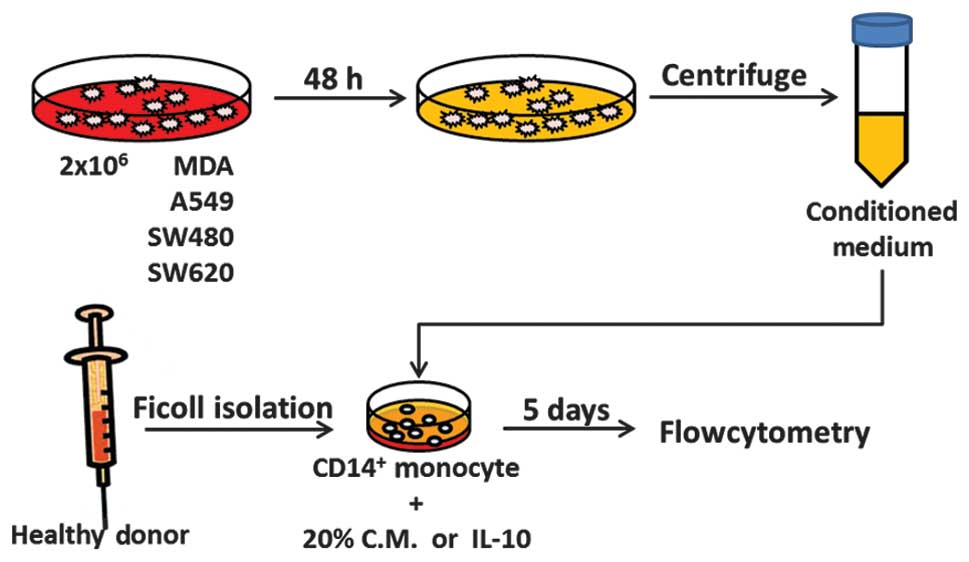
To obtain the conditioned medium, 2×106 cells/well were
seeded in a 100 mm dish and cultured for 24 h. The medium was then
replaced and the supernatants were harvested following 48 h of
incubation (Fig. 1).
Reagents and antibodies
Recombinant human granulocyte-macrophage
colony-stimulating factor (GM-CSF) and IL-4 were purchased from
Millipore (Bedford, MA, USA). Recombinant human IL-10 was purchased
from R&D Systems (Minneapolis, MN, USA). Phenylarsine oxide
(PAO), CD45 PTPase inhibitor and PTPase inhibitor XVIII were
purchased from Merck Millipore (Bedford, MA, USA). Fluorescein
isothiocyanate-conjugated anti-CD16, phycoerythrin-conjugated
anti-CD163, anti-CD11c, -CD80, -CD1a and -CD14, and APC-conjugated
anti-CD14 monoclonal antibodies (mAbs) were purchased from BD
Pharmingen (San Diego, CA, USA).
Monocyte isolation and
differentiation
Mononuclear cells were isolated from the blood of
healthy donors using the Ficoll-Hypaque gradient (GE Healthcare,
Little Chalfont, UK). Approval for this study was obtained from the
Institutional Review Board of Kaohsiung Medical University Hospital
(Kaohsiung, Taiwan), and informed consent was obtained from all
patients in accordance with the Declaration of Helsinki.
CD14+ monocytes were purified using CD14+
mAb-conjugated magnetic beads (MACS MicroBeads; Miltenyi Biotec,
Bisley, UK) according to the manufacturer’s instructions. A control
group of monocyte-derived dendritic cells was generated by
culturing CD14+ monocytes in RPMI 1640 medium containing
10% FBS (Invitrogen Life Technologies, Carlsbad, CA, USA), 20 ng/ml
GM-CSF and 20 ng/ml IL-4 (Millipore) for five days. In the IL-10,
A549, SW480, SW620 and MDA groups, an additional 10 ng/ml IL-10
(R&D Systems) or 20% cancer cell-conditioned medium was added.
The medium was replaced with fresh medium containing GM-CSF and
IL-4 on day three. Following five days of incubation, the presence
of CD14 and other surface markers was determined by
fluorescence-activated cell sorting array flow cytometry using
fluorochrome-conjugated mAbs (BD Pharmingen) (Fig. 1).
Assessment of CD45 PTPase activity
CD14+ monocytes were incubated in RPMI
containing GM-CSF and IL-4 with or without IL-10 for 30 min. The
activity of CD45 PTPase was determined by a Human Active CD45
activity assay (R&D Systems) according to the manufacturer’s
instructions. Briefly, 5×106 CD14+ monocytes
in 200 μl lysis buffer and 100 μl lysate were applied to the assay
plate. Absorbance at 620 nm was measured using the Biotek Powerwave
340 ELISA reader (BioTek Instruments, Inc., Winooski, VT, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were performed by analysis of variance and
two-sided t-tests using Excel 2010 (Microsoft, Tulsa, OK, USA).
P<0.05 was considered to indicate a statistically significant
difference between the means of the two test groups.
Results
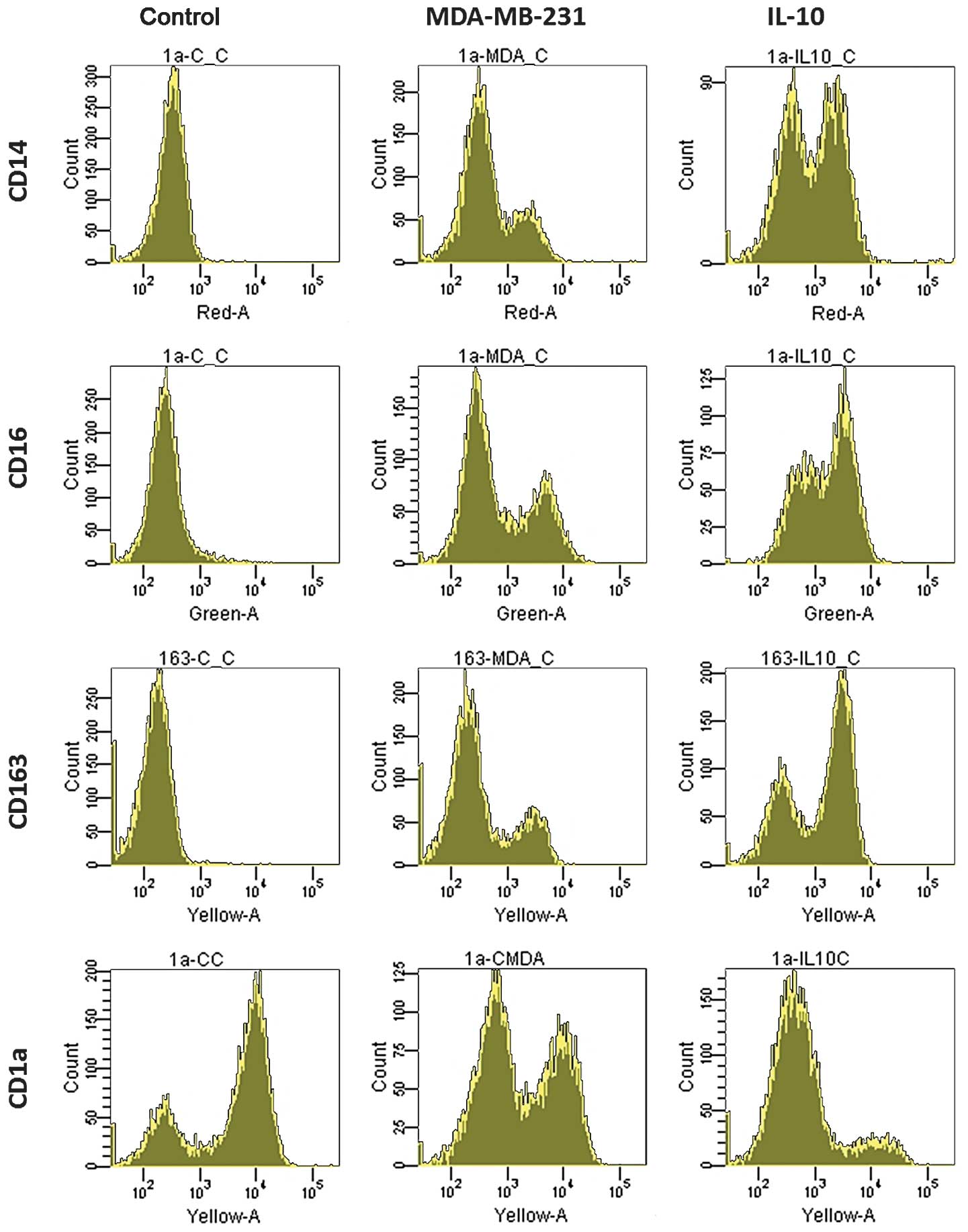
IL-10 may induce TADC-like DCs
The monocytes isolated from the healthy donors were
treated with 20 ng/ml GM-CSF and IL-4 and incubated for five days
for differentiation into resting DCs. In the MDA-MB-231 and IL-10
groups, an additional 20% of MDA-MB-231 breast cancer
cell-conditioned medium and 10 ng/ml IL-10 was added. Following
five days of incubation, the MCF and IL-10 groups exhibited a
different expression pattern of surface markers when compared with
the control group. In the control group, no CD14 expression was
detected, however, 25% of the cells expressed CD14 in the MDA
group. In addition, the CD14 expression was higher in the IL-10
group than in the MDA group. CD16 and CD163 expression showed the
same pattern as CD14 in the three groups, in contrast to the
expression of CD1a (Fig. 2).
However, no significant differences were identified in the
expression of CD80 and CD11c in the three groups (data not
shown).
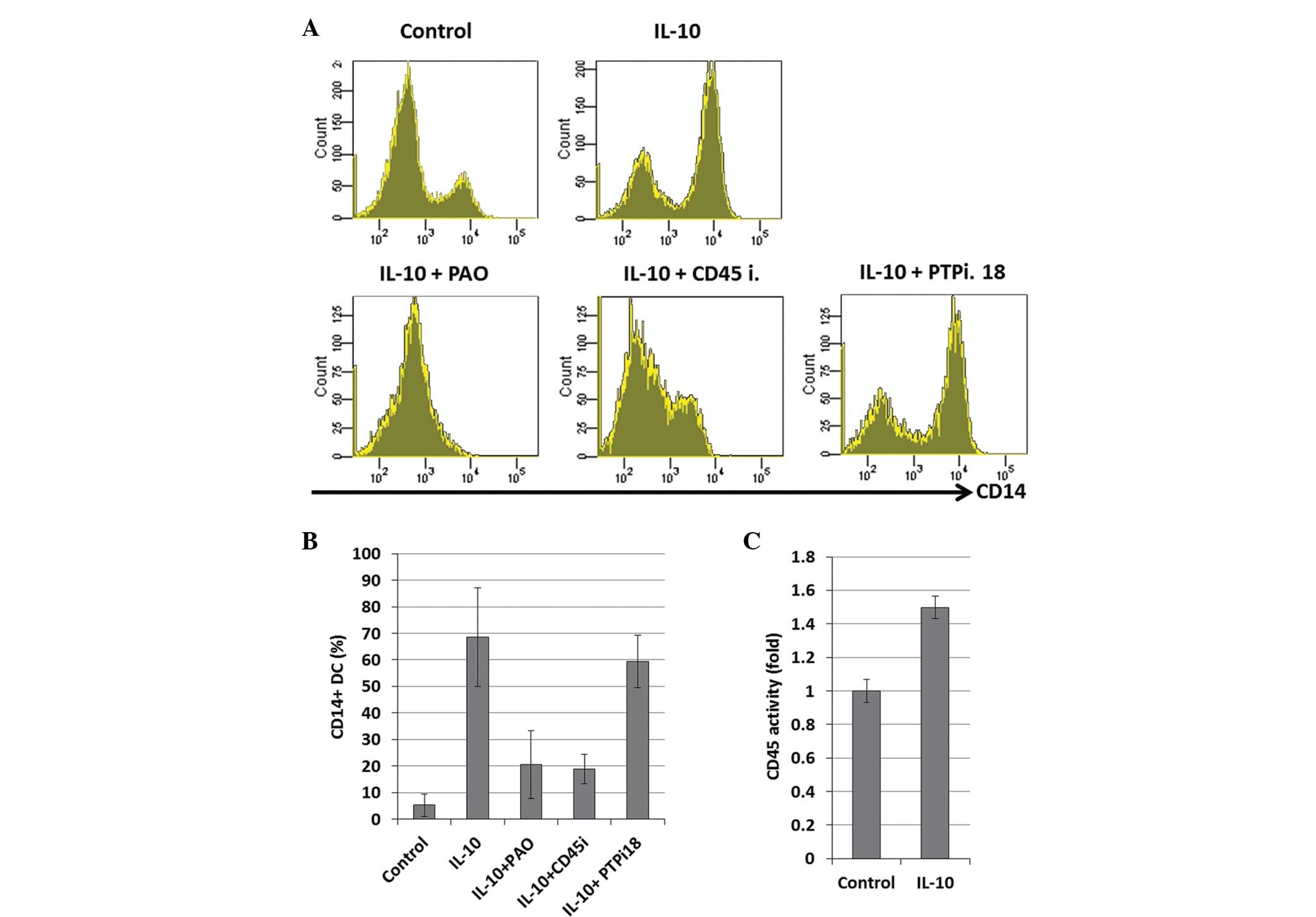
CD45 PTPase is involved in IL-10-induced
impaired DC differentiation
To investigate whether the PTPases are involved in
IL-10 signaling, three types of PTPase inhibitors were used in the
present study. PAO is a general A membrane-permeable protein
tyrosine phosphatase inhibitor, and PTP inhibitor XVIII inhibits
the enzymatic activity of PTP1B, tyrosine-protein phosphatase
non-receptor type 6, pathogenic Yersinia PTPase (YOP),
T-cell protein tyrosine phosphatase, and yeast PTP1. PTP CD45
inhibitor (CD45i) is a selective and reversible inhibitor of CD45.
Prior to the addition of IL-10 and GM-CSF/IL-4 to the culture
medium, the CD14+ monocytes isolated from the healthy
donors were incubated with the different inhibitors for 1 h.
Following five days of incubation, the higher CD14 expression level
mediated by IL-10 was reversed by the PAO and CD45 inhibitors,
however, the CD14 expression of the DCs was not found to be
downregulated by PTP inhibitor XVIII (Fig. 3A and B). The activation of CD45 was
also confirmed by a CD45 tyrosine phosphatase assay kit (Enzo Life
Sciences, Inc., Farmingdale, NY, USA), which showed that IL-10
activated CD45 phosphatase activity (Fig. 3C).
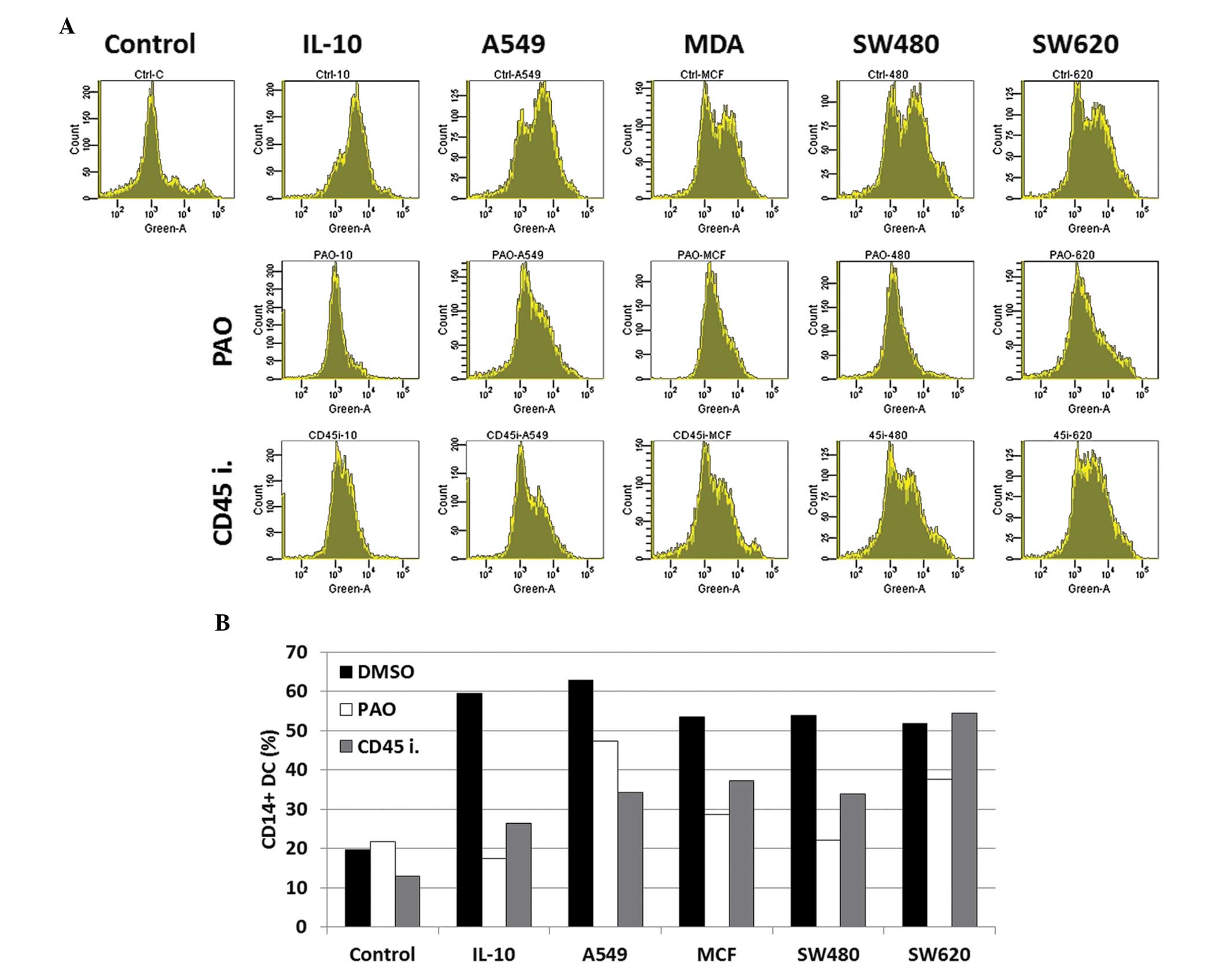
PAO and CD45i reduces the number of
tumor-mediated TADCs
To investigate whether PAO and CD45i were able to
block the differentiation of the TADCs, the conditioned medium of
the varying cancer types, including that of the lung (A549), breast
(MDA-MB-231) and colorectal cancer (SW480 and SW620) cells, was
collected. The CD14+ monocytes isolated from the healthy
donors were treated with PAO or CD45i prior to the addition of 20%
cancer cell-conditioned medium. Following five days of incubation,
the surface CD14 expression was determined, and it was found that
PAO and CD45i reversed cancer cell-mediated TADC differentiation in
the IL-10, A549, MDA and SW480 groups (Fig. 4A). However, only PAO, but not CD45i,
reversed the induction of TADCs by SW620 (Fig. 4B).
Discussion
DCs are antigen-presenting cells of hemopoietic
origin with potent effects on primary T-cell differentiation and
activation, and are thus of central relevance to antitumor immune
responses and vaccine development (24,25).
However, normal DC function is usually impaired in cancer patients
(26,27). Previous studies have shown that
immature or tDCs are induced by vascular endothelial growth factor
and IL-10 (28–30), which is consistent with the results
of the present study (Fig. 2). In
the current study, cancer cell-conditioned medium and IL-10 induced
higher expression levels of CD14, CD16 and CD163 with normal DC
markers, including CD11c and CD209. This indicates that the TADCs
or IL-10-induced DCs were similar to the circulating tDC-10
(31).
The IL-10 receptor (IL-10R) is a receptor tyrosine
kinase, and the binding of IL-10 and IL-10R activates the JAK1
signaling pathway and its downstream factors, including STAT3,
phosphoinositide 3-kinase (PI3K) and p38 (13,32,33).
Notably, CD45 has been reported to not only regulate the JAK/STAT3
pathway (17), but also to activate
p38 in B cells (34) and PI3K in
monocytes (35). This indicates
that CD45 may regulate the IL-10-mediated signaling pathway. The
results of the present study also support the hypothesis that CD45
is crucial in the IL-10 signaling pathway. The general PTPase
inhibitor, PAO, and CD45i reversed the IL-10-induced TADCs,
however, PTP inhibitor XVIII was not found to block CD45 activity
or the IL-10-mediated differentiation of the TADCs. These results
also indicate that additional PTPases may not be involved in the
IL-10 induction of TADCs. In addition, PAO and the CD45i were not
found to impair normal DC differentiation (data not shown). PAO
appeared to exhibit an increased efficacy in blocking the
differentiation of TADCs, which may be due to the multiple targets
of PAO, including internalization of ligand-receptor complexes
(36), protein kinase C activity,
phosphotyrosine phosphatase (37)
and formyl peptide-stimulated and phorbol ester-stimulated
phospholipase D (38). Therefore
the present study indicates that PAO or a novel signaling pathway
of IL-10 may represent a novel target.
Notably, PAO was found to completely block the
differentiation of the TADCs induced by cancer cell-conditioned
medium, however, CD45i was not found to block the induction of
TADCs in SW620-conditioned medium. This suggests that SW620 may
secrete factors other than IL-10 to induce TADCs. The comparison
between SW620 and SW480 is a well-established model used to study
the metastasis of colorectal cancer, whereby SW620 cells are
isolated from the metastatic lymph nodes of the patients from whom
SW480 cells are isolated. Furthermore, secretome studies have
revealed that SW620 cells may secrete trefoil factor 3,
growth/differentiation factor 15 (39), chemokine (C-X-C motif) ligand 8
(40), galectin-1 (41), TGFs and platelet-derived growth
factor (42–44). Further studies to investigate the
abilities of these factors in TADC induction are required, and the
pathways also require clarification to prevent immune surveillance
in distal metastatic organs. In conclusion, the present study is
the first to demonstrate that CD45 PTPase activity is required for
IL-10-mediated TADC differentiation, and that PAO and CD45
inhibitors block this process, which indicates that there is
potential for the use of small molecules to modulate the immune
system in the tumor microenvironment.
Acknowledgements
This study was supported by Biosignature in
Colorectal Cancers, Academia Sinica, Taiwan, the National Science
Council of Taiwan (grant nos. NSC99-2320-B-037-014-MY3,
101-2628-B-037-001-MY3, 102-2628-B-037-002-MY3,
101-2320-B-037-043-MY3, 102-2632-B-037-001-MY3 and
102-2314-B-037-035-MY3), the Excellence for Cancer Research Center
Grant, the Ministry of Health and Welfare, Executive Yuan, Taipei,
Taiwan (grant no. MOHW103-TD-B-111-05) and the Kaohsiung Medical
University Hospital (grant nos. KMUH101-1M09 and KMUH101-1M24). The
authors would like to thank the Center for Resources, Research and
Development of Kaohsiung Medical University for their support with
instrumentation.
References
|
1
|
Steinman RM: The dendritic cell system and
its role in immunogenicity. Annu Rev Immunol. 9:271–296. 1991.
|
|
2
|
Nestle FO, Burg G, Fäh J, Wrone-Smith T
and Nickoloff BJ: Human sunlight-induced
basal-cell-carcinoma-associated dendritic cells are deficient in T
cell co-stimulatory molecules and are impaired as
antigen-presenting cells. Am J Pathol. 150:641–651. 1997.
|
|
3
|
Watkins SK, Zhu Z, Riboldi E, et al: FOXO3
programs tumor-associated DCs to become tolerogenic in human and
murine prostate cancer. J Clin Invest. 121:1361–1372. 2011.
|
|
4
|
Kuo CH, Chen KF, Chou SH, et al: Lung
tumor-associated dendritic cell-derived resistin promoted cancer
progression by increasing Wolf-Hirschhorn syndrome candidate
1/Twist pathway. Carcinogenesis. 34:2600–2609. 2013.
|
|
5
|
Kuo PL, Huang MS, Cheng DE, Hung JY, Yang
CJ and Chou SH: Lung cancer-derived galectin-1 enhances tumorigenic
potentiation of tumor-associated dendritic cells by expressing
heparin-binding EGF-like growth factor. J Biol Chem. 287:9753–9764.
2012.
|
|
6
|
Hsu YL, Huang MS, Cheng DE, et al: Lung
tumor-associated dendritic cell-derived amphiregulin increased
cancer progression. J Immunol. 187:1733–1744. 2011.
|
|
7
|
Han Y, Chen Z, Yang Y, et al: Human
CD14+ CTLA-4+ regulatory dendritic cells
suppress T cell response via cytotoxic T-lymphocyte
antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase
production in hepatocellular carcinoma. Hepatology. 59:567–579.
2013.
|
|
8
|
Lindenberg JJ, Oosterhoff D, Sombroek CC,
et al: IL-10 conditioning of human skin affects the distribution of
migratory dendritic cell subsets and functional T cell
differentiation. PloS One. 8:e702372013.
|
|
9
|
Kuo PL, Hung JY, Huang SK, et al: Lung
cancer-derived galectin-1 mediates dendritic cell anergy through
inhibitor of DNA binding 3/IL-10 signaling pathway. J Immunol.
186:1521–1530. 2011.
|
|
10
|
Lindenberg JJ, van de Ven R, Lougheed SM,
et al: Functional characterization of a STAT3-dependent dendritic
cell-derived CD14 cell population arising upon IL-10-driven
maturation. Oncoimmunology. 2:e238372013.
|
|
11
|
Donnelly RP, Dickensheets H and Finbloom
DS: The interleukin-10 signal transduction pathway and regulation
of gene expression in mononuclear phagocytes. J Interferon Cytokine
Res. 19:563–573. 1999.
|
|
12
|
Sato K, Nagayama H, Tadokoro K, Juji T and
Takahashi TA: Extracellular signal-regulated kinase,
stress-activated protein kinase/c-Jun N-terminal kinase, and
p38mapk are involved in IL-10-mediated selective repression of
TNF-α-induced activation and maturation of human peripheral blood
monocyte-derived dendritic cells. J Immunol. 162:3865–3872.
1999.
|
|
13
|
Staples KJ, Smallie T, Williams LM, et al:
IL-10 induces IL-10 in primary human monocyte-derived macrophages
via the transcription factor Stat3. J Immunol. 178:4779–4785.
2007.
|
|
14
|
Niemand C, Nimmesgern A, Haan S, et al:
Activation of STAT3 by IL-6 and IL-10 in primary human macrophages
is differentially modulated by suppressor of cytokine signaling 3.
J Immunol. 170:3263–3272. 2003.
|
|
15
|
Porcu M, Kleppe M, Gianfelici V, et al:
Mutation of the receptor tyrosine phosphatase PTPRC (CD45) in
T-cell acute lymphoblastic leukemia. Blood. 119:4476–4479.
2012.
|
|
16
|
Xu D and Qu CK: Protein tyrosine
phosphatases in the JAK/STAT pathway. Front Biosci. 13:4925–4932.
2008.
|
|
17
|
Irie-Sasaki J, Sasaki T, Matsumoto W, et
al: CD45 is a JAK phosphatase and negatively regulates cytokine
receptor signalling. Nature. 409:349–354. 2001.
|
|
18
|
McFarland EC, Hurley TR, Pingel JT, Sefton
BM, Shaw A and Thomas ML: Correlation between Src family member
regulation by the protein-tyrosine-phosphatase CD45 and
transmembrane signaling through the T-cell receptor. Proc Natl Acad
Sci USA. 90:1402–1406. 1993.
|
|
19
|
Hurley TR, Hyman R and Sefton BM:
Differential effects of expression of the CD45 tyrosine protein
phosphatase on the tyrosine phosphorylation of the lck, fyn, and
c-src tyrosine protein kinases. Mol Cell Biol. 13:1651–1656.
1993.
|
|
20
|
Bataille R, Robillard N, Pellat-Deceunynck
C and Amiot M: A cellular model for myeloma cell growth and
maturation based on an intraclonal CD45 hierarchy. Immunol Rev.
194:105–111. 2003.
|
|
21
|
Fulcher JA, Chang MH, Wang S, et al:
Galectin-1 co-clusters CD43/CD45 on dendritic cells and induces
cell activation and migration through Syk and protein kinase C
signaling. J Biol Chem. 284:26860–26870. 2009.
|
|
22
|
Descamps G, Pellat-Deceunynck C, Szpak Y,
Bataille R, Robillard N and Amiot M: The magnitude of
Akt/phosphatidylinositol 3′-kinase proliferating signaling is
related to CD45 expression in human myeloma cells. J Immunol.
173:4953–4959. 2004.
|
|
23
|
Ishikawa H, Mahmoud MS, Fujii R, Abroun S
and Kawano MM: Proliferation of immature myeloma cells by
interleukin-6 is associated with CD45 expression in human multiple
myeloma. Leuk Lymphoma. 39:51–55. 2000.
|
|
24
|
Timmerman JM and Levy R: Dendritic cell
vaccines for cancer immunotherapy. Ann Rev Med. 50:507–529.
1999.
|
|
25
|
Théry C and Amigorena S: The cell biology
of antigen presentation in dendritic cells. Curr Opin Immunol.
13:45–51. 2001.
|
|
26
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: from immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002.
|
|
27
|
Almand B, Resser JR, Lindman B, et al:
Clinical significance of defective dendritic cell differentiation
in cancer. Clin Cancer Res. 6:1755–1766. 2000.
|
|
28
|
Gabrilovich DI, Chen HL, Girgis KR, et al:
Production of vascular endothelial growth factor by human tumors
inhibits the functional maturation of dendritic cells. Nat Med.
2:1096–1103. 1996.
|
|
29
|
Steinbrink K, Jonuleit H, Müller G,
Schuler G, Knop J and Enk AH: Interleukin-10-treated human
dendritic cells induce a melanoma-antigen-specific anergy in CD8(+)
T cells resulting in a failure to lyse tumor cells. Blood.
93:1634–1642. 1999.
|
|
30
|
Qin Z, Noffz G, Mohaupt M and Blankenstein
T: Interleukin-10 prevents dendritic cell accumulation and
vaccination with granulocyte-macrophage colony-stimulating factor
gene-modified tumor cells. J Immunol. 159:770–776. 1997.
|
|
31
|
Gregori S, Tomasoni D, Pacciani V, et al:
Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic
DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood.
116:935–944. 2010.
|
|
32
|
Bhattacharyya S, Sen P, Wallet M, Long B,
Baldwin AS and Tisch R: Immunoregulation of dendritic cells by
IL-10 is mediated through suppression of the PI3K/Akt pathway and
of IkappaB kinase activity. Blood. 104:1100–1109. 2004.
|
|
33
|
Song GY, Chung CS, Schwacha MG, Jarrar D,
Chaudry IH and Ayala A: Splenic immune suppression in sepsis: A
role for IL-10-induced changes in P38 MAPK signaling. J Surg Res.
83:36–43. 1999.
|
|
34
|
Arimura Y, Ogimoto M, Mitomo K, et al:
CD45 is required for CD40-induced inhibition of DNA synthesis and
regulation of c-Jun NH2-terminal kinase and p38 in BAL-17 B cells.
J Biol Chem. 276:8550–8556. 2001.
|
|
35
|
Hayes AL, Smith C, Foxwell BM and Brennan
FM: CD45-induced tumor necrosis factor alpha production in
monocytes is phosphatidylinositol 3-kinase-dependent and nuclear
factor-kappaB-independent. J Biol Chem. 274:33455–33461. 1999.
|
|
36
|
Hoffman JF, Linderman JJ and Omann GM:
Receptor up-regulation, internalization, and interconverting
receptor states Critical components of a quantitative description
of N-formyl peptide-receptor dynamics in the neutrophil. J Biol
Chem. 271:18394–18404. 1996.
|
|
37
|
Kutsumi H, Kawai K, Johnston RB Jr and
Rokutan K: Evidence for participation of vicinal dithiols in the
activation sequence of the respiratory burst of human neutrophils.
Blood. 85:2559–2569. 1995.
|
|
38
|
Planat V, Tronchere H, Record M, Ribbes G
and Chap H: Involvement of vicinal dithiols in differential
regulation of fMLP and phorbol ester-activated phospholipase D in
stimulated human neutrophils. Biochem Biophys Res Commun.
218:847–853. 1996.
|
|
39
|
Dowling P and Clynes M: Conditioned media
from cell lines: a complementary model to clinical specimens for
the discovery of disease-specific biomarkers. Proteomics.
11:794–804. 2011.
|
|
40
|
Bailey C, Negus R, Morris A, et al:
Chemokine expression is associated with the accumulation of tumour
associated macrophages (TAMs) and progression in human colorectal
cancer. Clin Exp Metastasis. 24:121–130. 2007.
|
|
41
|
Ghosh D, Yu H, Tan XF, et al:
Identification of key players for colorectal cancer metastasis by
iTRAQ quantitative proteomics profiling of isogenic SW480 and SW620
cell lines. J Proteome Res. 10:4373–4387. 2011.
|
|
42
|
Ma C, Rong Y, Radiloff DR, et al:
Extracellular matrix protein betaig-h3/TGFBI promotes metastasis of
colon cancer by enhancing cell extravasation. Genes Dev.
22:308–321. 2008.
|
|
43
|
Coffey RJ Jr, Shipley GD and Moses HL:
Production of transforming growth factors by human colon cancer
lines. Cancer Res. 46:1164–1169. 1986.
|
|
44
|
Anzano MA, Rieman D, Prichett W,
Bowen-Pope DF and Greig R: Growth factor production by human colon
carcinoma cell lines. Cancer Res. 49:2898–2904. 1989.
|