Introduction
Members of the highly-conserved FXYD family are
differentially expressed in a wide variety of mammalian tissues and
cancer types (1,2). To date, the family comprises 12
water-insoluble, transmembrane proteins that serve as ion channels
and/or ion channel regulators (3–6). All
FXYD genes are expressed in early embryonic cells, and the
expression of certain FXYD proteins is tissue-specific in mammals.
FXYD1 is expressed in skeletal muscle and the myocardium, FXYD2 is
primarily expressed in kidney epithelial basement membranes, the
bile duct and in cholangiocarcinoma cells, FXYD3 is primarily
expressed in the liver, pancreas, stomach, colon, prostate, lung,
kidney, skeletal muscle and epidermal cells, FXYD4 is primarily
expressed in the kidney and distal colon, and FXYD5 is expressed in
the brain (7).
Certain FXYD proteins display altered expression in
cancer cells. For instance, FXYD2 is differentially expressed in
cholangiocarcinoma cells, as is FXYD5 in epithelioid sarcoma, head
and neck squamous cell carcinoma, small cell carcinoma, pancreatic
cancer cells and breast cancer cells. FXYD3 expression is
upregulated in breast cancer tissues and cancer cell lines,
intrahepatic cholangiocarcinoma, thyroid cancer, colon cancer,
certain prostate cancer cells and in urothelial cancers (8,9). It
has also been reported that FXYD3 expression is downregulated in
specific prostate cancer cells (10). FXYD proteins have garnered a high
level of research focus in recent years, as they appear to play
significant physiological and pathophysiological roles in human
biology.
As such, FXYD3 is being scrutinized as a potential
novel biomarker for cancer (11).
The human FXYD3 gene is located on chromosome 19q13.11-q13.12. This
gene is 8,428 base pairs long, and is comprised of 9 exons and 8
introns. FXYD3 belongs to the FXYD protein family. It interacts
with, and regulates the Na+/K+-ATPase enzyme,
but also acts independently as a chloride ion channel or chloride
channel regulator (12).
To the best of our knowledge, FXYD3 expression has
not been investigated in association with endometrial cancer.
Endometrial cancer is the most common gynecological malignancy.
Each year, 142,000 females are diagnosed, and 42,000 females die
from this disease worldwide. In the present study,
immunohistochemistry was used to detect the differential FXYD3
expression and corresponding pathological changes in endometrial
tissue samples obtained from patients diagnosed with endometrial
cancer. The correlation between endometrial cancer risk factors,
clinicopathological features and FXYD3 expression is analyzed and
discussed.
Materials and methods
Patients
For immunohistochemistry, formalin-fixed
paraffin-embedded tissue blocks were obtained from 50 patients with
endometrial cancer and integral clinical data at the First Hospital
of Hebei Medical University (Shijiazhuang, Hebei, China) between
2005 and 2007. The patients were diagnosed according to the
International Federation of Gynecology Obstetrics (FIGO) Surgical
Staging System for Endometrial Cancer (2000) (13). The study also included 18 atypical
endometrial hyperplasia and 21 normal endometrium samples. The
median age of the patients was 36, 40.5 and 57 years old (range, 22
to 60, 26 to 77, and 33 to 75 years old) for the normal
endometrium, atypical endometrial hyperplasia and endometrial
cancer groups, respectively. The study was approved by the Ethical
Committee at the First Hospital of Hebei Medical University.
Patients provided written informed consent.
Immunohistochemistry
The preparation, specificity and reliability of the
rabbit polyclonal FXYD3 antibody used in the study have been
described previously (14).
Continuous 5 μm sections from paraffin-embedded tissue were
deparaffinized, hydrated and rinsed in distilled H2O. In
order to expose masked epitopes, the sections were boiled in
citrate buffer (pH 9.0) in a high pressure cooker for 20 min, and
then kept at room temperature for 30 min, followed by a
phosphate-buffered saline (PBS; pH 7.4) wash. The activity of
endogenous peroxidase was blocked in 3% H2O2
in methanol for 10 min, and then the sections were washed 3 times
in PBS. Subsequent to being blocked with 1.5% horse serum in PBS
for 10 min, the sections were incubated with the primary mouse
anti-human monoclonal anti-FXYD3 antibody (kindly obtained from
Professor Hanswalter Zentgraf, Department of Applied Tumor
Virology, University of Heidelberg, Heidelberg, Germany) in 1:2
diluted in PBS (pH 7.4) at 4°C overnight. Next, a biotinlated
anti-rabbit Immunoglobulin G antibody (Fuzhou Maixin Biology
Technology, Fuzhou, China) was applied for 30 min, followed by
incubation of an avidin-biotin-peroxidase complex (Beijing
Zhongshan Biology Technology, Beijing, China) for 30 min. The
sections were rinsed in PBS between the incubations. The peroxidase
reactions were developed using diaminobenzidine (Beijing Zhongshan
Biology Technology) for 8 min. Following counterstaining with
hematoxylin, the sections were dehydrated and mounted. The breast
cancer sections known to be FXYD3-positive were included as
positive or negative controls. A negative control was designed for
every staining procedure, i.e., PBS instead of the primary
antibody.
Histological analyses
The stained sections were microscopically examined
and scored independently by two pathologists who were blinded to
the experimental conditions. Yellow-stained granules observed in
the cytoplasm and/or the membrane of glandular epithelial cells in
the normal endometrium, atypical endometrial hyperplasia and
endometrial cancer tumor cells were considered FXYD3-positive
cells. A total of 10 different high power fields (10×40) were
randomly selected for each sample, and the total number of cells
and FXYD3-positive cells were counted. The positive cell rate was
calculated as the following: Positive cell rate = ∑positive
cells/∑cells × 100. The staining intensity was graded on a scale of
0–3 based on the following criteria: 0 for negative cells or those
with no staining, 1 for yellow-stained cells, 2 for orange-stained
cells and 3 for brown-stained cells. The percentage of stained
cells was classified according to the following system: 0 for ≤5%
staining, 1 for 6–25%, 2 for 26–50% and 3 for >50%. The final
score was defined as the sum of the staining intensity and the
percentage of stained cells in each section, and sections scored
from 0 to 6 points. To avoid staining artifacts, the cells in areas
with necrosis, poor morphology and section margins were not
counted.
Statistical analyses
For statistical analyses, staining scores of 0 to 3
points were counted as negative and ≥4 points was counted as
positive. All data were analyzed using SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). The χ2 method and the Fisher’s
exact test were used to examine the correlation between FXYD3
expression in the normal endometrium, atypical endometrial
hyperplasia and endometrial carcinoma groups, and the correlation
between FXYD3 expression in cancer and clinicopathological
variables. All P-values were cited as two-sided, and P<0.05 was
considered to indicate a statistically significant difference.
Results
FXYD3 expression in normal endometrium,
atypical endometrial hyperplasia and endometrial cancer
FXYD3 expression was examined in the normal
endometrium samples (n=21), the atypical endometrial hyperplasia
samples (n=18) and the endometrial cancer tissue samples from
surgically removed specimens (n=50). FXYD3 expression in the
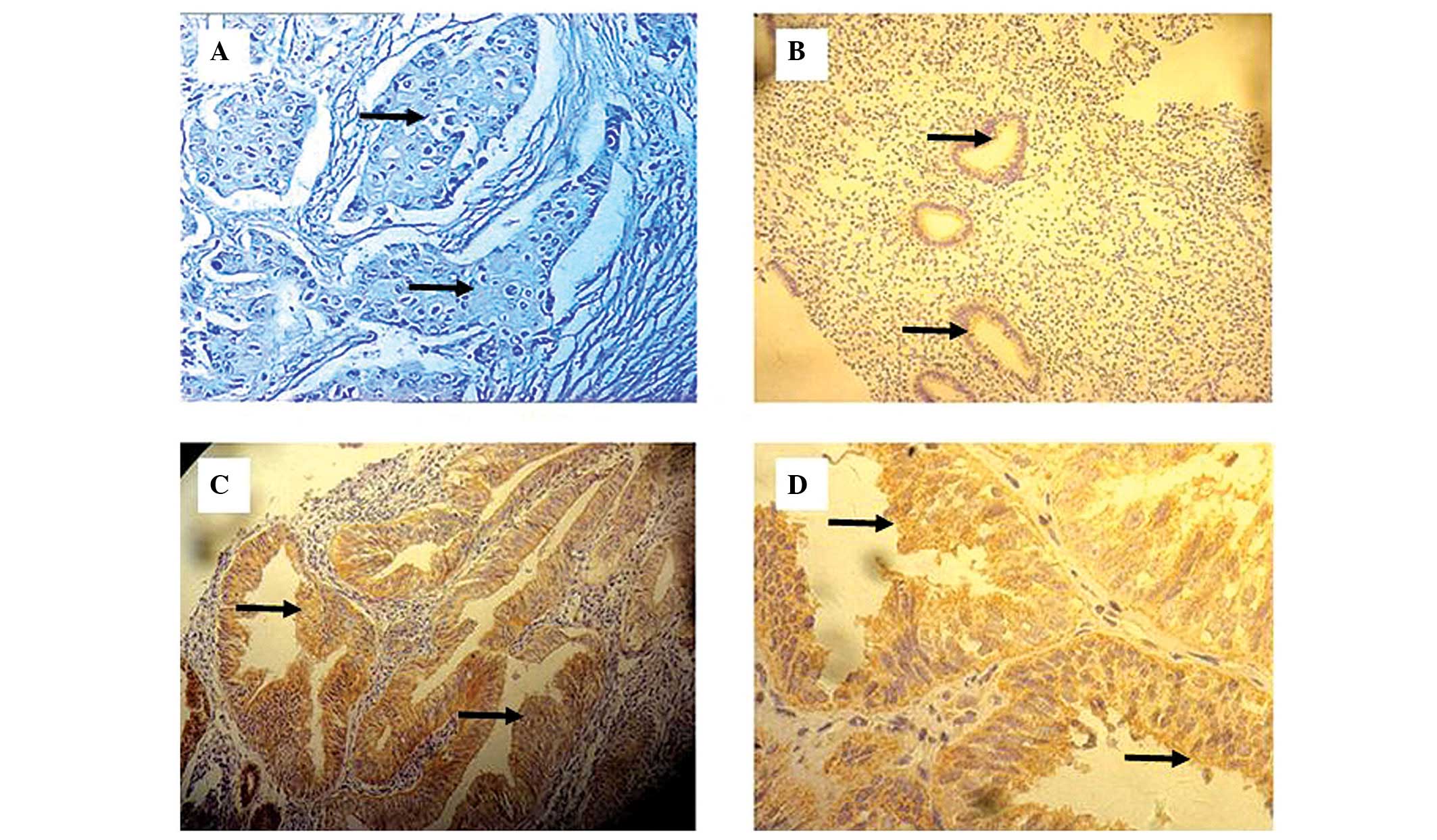
cytoplasm and/or normal epithelial membranes and tumor cells, and
the staining in the cytoplasm and/or the membrane was heterogeneous
and granulous. Among the 50 endometrial cancer tissue samples, 13
exhibited FXYD3-positive cells. However, FXYD3 expression in these
samples was heterogeneous, displaying great variation in the
numbers of FXYD3-positive cells and the staining intensity in
different regions of the same section (Fig. 1).
The percentage of FXYD3-positive cells in the normal
endometrium, atypical endometrial hyperplasia and endometrial
cancer tissue samples was 0, 22 and 26%, respectively (Table I). The percentage of FXYD3-positive
cells in the atypical hyperplasia and endometrial cancer tissues
were significantly increased when compared with samples in the
normal endometrium group (P=0.007 and P=0.037, respectively).
However, there was no significant difference between the atypical
hyperplasia and endometrial cancer groups (P=1.000).
 | Table IFXYD3 expression in normal
endometrium, atypical endometrial hyperplasia and endometrial
cancer. |
Table I
FXYD3 expression in normal
endometrium, atypical endometrial hyperplasia and endometrial
cancer.
| | FXYD3 expression, n
(%) | |
|---|
| |
| |
|---|
| Groups | n | Positive | Negative | P-value |
|---|
| Normal
endometrium | 21 | 0 (0) | 21 (100) | 0.037a |
| Atypical
hyperplasia | 18 | 4 (22) | 14 (78) | 1.000b |
| Endometrial
cancer | 50 | 13 (26) | 37 (74) | 0.007c |
Correlation between FXYD3 expression in
endometrial cancer and clinicopathological features
The correlation between FXYD3 expression and
different clinicopathological features was examined. Table II shows the correlation between
FXYD3 expression and patient age, fertility frequency, blood
pressure, plasma sugar and lipid levels, family history of cancer,
age of menopause onset, FIGO stage, histopathological type,
histological grade, myometrial invasion, cervical involvement,
lymph nodal metastases and growth pattern. FXYD3 expression in the
endometrial carcinoma group was negatively correlated with
fertility frequency. A high fertility frequency corresponded with
lower FXYD3 expression (P=0.024).
 | Table IIFXYD3 expression in the endometrial
cancer tissue samples, and clinicopathological features. |
Table II
FXYD3 expression in the endometrial
cancer tissue samples, and clinicopathological features.
| | FXYD expression, n
(%) | |
|---|
| |
| |
|---|
| Variables | n | Negative | Positive | P-value |
|---|
| Age, years | | | | 0.990 |
| <55 | 23 | 17 (74) | 6 (26) | |
| ≥55 | 27 | 20 (74) | 7 (26) | |
| Births | | | | 0.024 |
| None | 5 | 4 (80) | 1 (20) | |
| 1 | 7 | 2 (29) | 5 (71) | |
| ≥2 | 37 | 30 (81) | 7 (19) | |
| Blood pressure,
mmHg | | | | 0.747 |
| <140/90 | 25 | 18 (72) | 7 (28) | |
| ≥140/90 | 25 | 19 (76) | 6 (24) | |
| Plasma glucose,
mmol/l | | | | 0.586 |
| <6.1 | 27 | 19 (70) | 8 (30) | |
| ≥6.1 | 22 | 17 (77) | 5 (23) | |
| Plasma lipids | | | | 0.405 |
| Normal | 13 | 8 (62) | 5 (38) | |
| High | 10 | 8 (80) | 2 (20) | |
| Family history of
cancer | | | | 1.000 |
| No | 43 | 31 (72) | 12 (28) | |
| Yes | 6 | 5 (83) | 1 (17) | |
| Menopause onset age,
years | | | | 0.794 |
| <49 | 21 | 17 (81) | 4 (19) | |
| 49–52 | 15 | 10 (67) | 5 (33) | |
| ≥52 | 14 | 10 (71) | 4 (29) | |
| FIGO stage | | | | 0.919 |
| I | 33 | 25 (76) | 8 (24) | |
| II | 10 | 8 (80) | 2 (20) | |
| III | 7 | 5 (71) | 2 (29) | |
| IV | 0 | 0 (0) | 0 (0) | |
| Histopathological
type | | | | 0.549 |
| Adenocarcinoma | 48 | 35 (73) | 13 (27) | |
| Undifferentiated
carcinoma | 1 | 1 (100) | 0 (0) | |
| Small cell
carcinoma | 1 | 1 (100) | 0 (0) | |
| Histological
grade | | | | 1.000 |
| I | 0 | 0 (0) | 0 (0) | |
| II | 25 | 19 (76) | 6 (24) | |
| III | 5 | 4 (80) | 1 (20) | |
| Myometrial
invasion | | | | 0.372 |
| No | 3 | 3 (100) | 0 (0) | |
| Superficial
myometrial invasion | 33 | 24 (73) | 9 (27) | |
| Deep myometrial
invasion | 13 | 9 (69) | 4 (31) | |
| Cervical
involvement | | | | 0.727 |
| No | 34 | 24 (71) | 10 (29) | |
| Yes | 15 | 12 (80) | 3 (20) | |
| Lymph nodal
metastases | | | | 0.556 |
| No | 36 | 26 (72) | 10 (28) | |
| Yes | 3 | 3 (100) | 0 (0) | |
| Growth pattern | | | | 0.682 |
| Limitations | 24 | 17 (71) | 7 (29) | |
| Diffusibility | 25 | 19 (76) | 6 (24) | |
Discussion
The present study investigated the correlation
between FXYD3 expression and endometrial cancer using
immunohistochemical analyses of normal endometrium, atypical
hyperplasia and endometrial cancer tissue samples. The correlation
between differential FXYD3 expression and several different
clinicopathological features was also analyzed. FXYD3 expression in
different human tissues has been extensively studied using various
methods. FXYD3 is expressed in normal human tissues, including the
liver, colon, prostate, lung, pancreas and brain and epithelium. In
addition, a growing body of evidence indicates that FXYD3
expression is upregulated in numerous different tumor tissues and
tumor cell lines. Moreover, certain studies indicate that tumor
malignancy is positively correlated with FXYD3 expression (15–18).
For example, Morrison et al used quantitative
(q)PCR and northern blotting to demonstrate that FXYD3 was
expressed at a significantly higher level in the primary breast
cancer tissues obtained from 16 patients, and in eight different
human breast cancer cell lines (15). Notably, studies investigating FXYD3
expression in prostate tissues have yielded conflicting results.
Grzmil et al found that FXYD3 was highly expressed in
prostate cancer tissue samples when using cDNA chip technology and
qPCR (10). In the same study, the
suppression of FXYD3 expression caused a significant decrease in
the cellular proliferation of prostate cancer cell lines.
Studies on pancreatic cancer show that FXYD3
expression in cancerous tissues and pancreatic cancer cell lines is
significantly higher than in normal pancreatic tissues (16) and in chronic pancreatitis (16–18).
In non-small cell lung cancer, FXYD3 expression in tumors for
patients with poor prognoses is higher than in those with better
prognoses. This indicates that FXYD3 could be an important
prognostic secondary indicator (19).
To the best of our knowledge, the present study is
the first to examine FXYD3 expression in endometrial cancer
tissues. FXYD3 expression was analyzed and compared in tissue
sample sections by immunohistochemistry using grading scales that
quantified the number of FXYD3-positive cells and the staining
intensity of these cells. The percentage of FXYD3-positive cells in
the normal endometrium, endometrial hyperplasia and endometrial
cancer tissue samples was 0, 22, and 26%, respectively. These
results indicate that FXYD3 is expressed in the early stages of
endometrial carcinoma formation, suggesting that the upregulation
of FXYD3 may be an early event in the progression of endometrial
cancer. From these study results, we propose that FXYD3 may be a
promising biomarker for endometrial cancer.
The female reproductive system is the target organ
for the sex hormones, estrogen and progesterone. Each hormone
mediates multiple effects via their specific receptors. Estrogen
promotes endometrial cell hyperplasia and vascular proliferation,
and induces estrogen receptor and progesterone receptor expression.
Progesterone stimulates endometrial cell differentiation and
promotes apoptosis in atypical hyperplasia endometrial cells, thus
inhibiting excessive growth or transformation (20).
Endometrial cancer progression is correlated with
endometrial hyperplasia, elevated estrogen levels and decreased
progesterone levels (21). Studies
have shown that large doses of estrogen replacement therapy
increase the risk of endometrial cancer 2–10-fold (22). Obesity, hypertension and diabetes
are three other factors associated with endometrial cancer. The
risk of endometrial cancer in diabetic patients or patients with
impaired glucose tolerance is 2.8 times greater than that of
healthy individuals (23). The
present data indicated that FXYD3 expression in endometrial cancer
tissues was not significantly correlated with the patient age,
blood pressure, menopause onset, plasma glucose and lipid levels,
family history of cancer, myometrial invasion, cervical invasion,
lymphatic metastasis, clinical cancer stage, growth pattern and
histological type of the endometrial cancer tumor (P>0.05).
However, a correlation was detected between FXYD3 expression and
fertility. This data indicates that lifelong infertility is a risk
factor for endometrial cancer. We hypothesize that the effects of
estrogen on endometrial tissues are uncontrolled in individuals
lacking sufficient amounts of progesterone. During pregnancy,
progesterone inhibits menstruation (24). The cell damage, repair, and injury
responses in endometrial epithelial cells shut down, and the risk
of developing endometrial cancer during pregnancy is reduced. The
present study found that females who have never been pregnant are
twice as likely to develop endometrial cancer than those who have
given birth once. This is particularly true for females who are
unable to become pregnant due to failed ovulation and insufficient
progesterone levels. This results in endometrial hyperplasia that
could progress to endometrial cancer. Our results show that FXYD3
expression in endometrial cancer tissues is correlated with
fertility frequency (P=0.024). The risk of developing endometrial
carcinoma appears to be higher in females who have never become
pregnant when compared with those who have given birth. With each
birth, the risk of developing endometrial carcinoma decreases.
Whether this correlation is due to progesterone-regulated levels of
FXD3 levels or vice versa is unclear. To address this question,
future studies examining the correlation between estrogen,
progesterone and FXYD3 expression in normal and endometrial cancer
cells are required.
Acknowledgements
The authors would like to thank Professor Hanswalter
Zentgraf (University of Heidelberg, Heidelberg, Germany) for
providing the FXYD3 antibody.
References
|
1
|
Tipsmark CK: Identification of FXYD
protein genes in a teleost: tissue-specific expression and response
to salinity change. Am J Physiol Regul Integr Comp Physiol.
294:R1367–R1378. 2008.
|
|
2
|
Sweadner KJ and Rael E: The FXYD gene
family of small ion transport regulators or channels: cDNA
sequence, protein signature sequence, and expression. Genomics.
68:41–56. 2000.
|
|
3
|
Wang PJ, Lin CH, Hwang HH and Lee TH:
Branchial FXYD protein expression in response to salinity change
and its interaction with Na+/K+-ATPase of the
euryhaline teleost Tetraodon nigroviridis. J Exp Biol.
211:3750–3758. 2008.
|
|
4
|
Cornelius F and Mahmmoud YA: Modulation of
FXYD interaction with Na,K-ATPase by anionic phospholipids and
protein kinase phosphorylation. Biochemistry. 46:2371–2379.
2007.
|
|
5
|
Kadowaki K, Sugimoto K, Yamaguchi F, et
al: Phosphohippolin expression in the rat central nervous system.
Brain Res Mol Brain Res. 125:105–112. 2004.
|
|
6
|
Lubarski I, Karlish SJ and Garty H:
Structural and functional interactions between FXYD5 and the
Na+-K+-ATPase. Am J Physiol Renal Physiol.
293:F1818–F1826. 2007.
|
|
7
|
Franzin CM, Gong XM, Teriete P and Marassi
FM: Structures of the FXYD regulatory proteins in lipid micelles
and membranes. J Bioenerg Biomembr. 39:379–383. 2007.
|
|
8
|
Morrison BW and Leder P: neu and ras
initiate murine mammary tumors that share genetic markers generally
absent in c-myc and int-2-initiated tumors. Oncogene. 9:3417–3426.
1994.
|
|
9
|
Subrungruanga I, Thawornkunob C,
Chawalitchewinkoon-Petmitrc P, Pairojkul C, Wongkham S and Petmitrb
S: Gene expression profiling of intrahepatic cholangiocarcinoma.
Asian Pac J Cancer Prev. 14:557–563. 2013.
|
|
10
|
Grzmil M, Voigt S, Thelen P, Hemmerlein B,
Helmke K and Burfeind P: Up-regulated expression of the MAT-8 gene
in prostate cancer and its siRNA-mediated inhibition of expression
induces a decrease in proliferation of human prostate carcinoma
cells. Int J Oncol. 24:97–105. 2004.
|
|
11
|
Kiyamova R, Garifulin O, Gryshkova V, et
al: Preliminary study of thyroid and colon cancers-associated
antigens and their cognate autoantibodies as potential cancer
biomarkers. Biomarkers. 17:362–371. 2012.
|
|
12
|
Geering K, Béguin P, Garty H, et al: FXYD
proteins: new tissue- and isoform-specific regulators of
Na,K-ATPase. Ann NY Acad Sci. 986:388–394. 2003.
|
|
13
|
Kuroda Y, Murakami N, Morota M, et al:
Impact of concurrent chemotherapy on definitive radiotherapy for
women with FIGO IIIb cervical cancer. J Radiat Res. 53:588–593.
2012.
|
|
14
|
Campana WM, Myers RR and Rearden A:
Identification of PINCH in Schwann cells and DRG neurons: shuttling
and signaling after nerve injury. Glia. 41:213–223. 2003.
|
|
15
|
Morrison BW, Moorman JR, Kowdley GC,
Kobayashi YM, Jones LR and Leder P: Mat-8, a novel
phospholemman-like protein expressed in human breast tumors,
induces a chloride conductance in Xenopus oocytes. J Biol Chem.
270:2176–2182. 1995.
|
|
16
|
Logsdon CD, Simeone DM, Binkley C, et al:
Molecular profiling of pancreatic adenocarcinoma and chronic
pancreatitis identifies multiple genes differentially regulated in
pancreatic cancer. Cancer Res. 63:2649–2657. 2003.
|
|
17
|
Iacobuzio-Donahue CA, Maitra A, Olsen M,
et al: Exploration of global gene expression patterns in pancreatic
adenocarcinoma using cDNA microarrays. Am J Pathol. 162:1151–1162.
2003.
|
|
18
|
Friess H, Ding J, Kleeff J, et al:
Microarray-based identification of differentially expressed growth-
and metastasis-associated genes in pancreatic cancer. Cell Mol Life
Sci. 60:1180–1199. 2003.
|
|
19
|
Gordon GJ, Richards WG, Sugarbaker DJ,
Jaklitsch MT and Bueno R: A prognostic test for adenocarcinoma of
the lung from gene expression profiling data. Cancer Epidemiol
Biomarkers Prev. 12:905–910. 2003.
|
|
20
|
Saegusa M and Okayasu I: Changes in
expression of estrogen receptors alpha and beta in relation to
progesterone receptor and pS2 status in normal and malignant
endometrium. Jpn J Cancer Res. 5:510–518. 2000.
|
|
21
|
Gielen SC, Hanekamp EE, Hanifi-Moghaddam
P, et al: Growth regulation and transcriptional activities of
estrogen and progesterone in human endometrial cancer cells. Int J
Gynecol Cancer. 16:110–120. 2006.
|
|
22
|
Tang ZY, Ye SL, Liu YK, et al: A decade’s
studies on metastasis of hepatocellular carcinoma. J Cancer Res
Clin Oncol. 130:187–196. 2004.
|
|
23
|
Kaaks R, Lukanova A and Kurzer MS:
Obesity, endogenous hormones, and endometrial cancer risk: a
synthetic review. Cancer Epidemiol Biomarkers Prev. 11:1531–1543.
2002.
|
|
24
|
Maybin JA and Critchley HO: Steroid
regulation of menstrual bleeding and endometrial repair. Rev Endocr
Metab Disord. 13:253–263. 2012.
|