Introduction
Myeloid-derived suppressor cells (MDSCs) represent a
heterogeneous population of immature myeloid cells that are
characterized by their potential to suppress various T cell
functions (1). MDSCs are enriched
in tumor-bearing hosts, and since the accumulation of MDSCs has
been confirmed to be correlated with tumor progression (2), the control of MDSC accumulation is
crucial for directly targeting MDSCs against tumors. During tumor
development, tumor- and host-secreted factors, including vascular
endothelial growth factor, stem cell factor, granulocyte-macrophage
colony-stimulating factor, interleukin (IL)-1β, IL-6 and
prostaglandin E2, have been reported to promote the accumulation of
MDSCs. These factors can expand and differentiate MDSCs by
interacting with the receptors on their surface causing conversion
to a suppressive phenotype (1,3).
Certain molecules that are expressed on the surface of MDSCs
regulate the accumulation or suppressive activity of MDSCs,
including cluster of differentiation (CD) 80 (4), CD115 (5), IL-4 receptor α (IL-4Rα) (6), programmed cell death ligand-1
(7), CD40 (8) and CD95 (Fas) (9).
CD40, a member of the tumor necrosis factor (TNF)
receptor superfamily, is known to be expressed at various levels on
antigen-presenting cells, epithelial cells, hematopoietic
progenitor cells and activated T cells (10,11).
The interaction between the CD40-CD40 ligand has been demonstrated
to regulate immune responses, and CD40 has an important effect on
promoting tumor cell apoptosis or tumor growth. The dual role of
CD40 depends on the level of its expression (12) and different signaling activation
(24). Recent studies have shown
that the expression of CD40 on MDSCs regulates MDSC-mediated immune
suppression and the expansion of T regulatory cells (Tregs)
(8,13,14).
However, whether CD40 is a mediator of MDSC accumulation has not
yet been thoroughly investigated.
Therefore, the present study evaluated CD40
expression on the MDSCs of a gastric tumor model, with a focus on
the dynamics of tumor progression and CD40 expression on MDSCs. The
levels of CD40 expression were observed to significantly correlate
with MDSC accumulation and the relative contribution to MDSC
apoptosis. The present study aimed to analyze the regulation of
MDSC levels in order to determine a method for targeting MDSCs
against tumors.
Materials and methods
Cell line and tumor model
The mouse forestomach carcinoma (MFC) cell line was
obtained from the Shanghai Institute of Biochemistry and Cell
Biology, Shanghai Institute for Biological Sciences (Shanghai,
China) and 6–10 week old C57BL/6 (B6) mice were purchased from the
Shanghai Laboratory Animal Center, Chinese Academy of Sciences
(Shanghai, China). All mice were housed in pathogen-free conditions
at the Animal Center of the Medical College of Soochow University
(Suzhou, China) according to the institutional guidelines for
animal care and use. The cells were cultured in Roswell Park
Memorial Institute 1640 medium (Gibco-BRL, Carlsbad, CA, USA)
containing 10% fetal calf serum. A total of 1×106 MFC
cells were then subcutaneously injected into the B6 mice and the
tumor sizes were evaluated every two to three days. The study was
approved by the ethics committee of Soochow University (Suzhou,
China).
Antibodies and flow cytometry
Phycoerythrin-cyanine (PC)7-labeled anti-Gr-1
monoclonal antibody (mAb), PC5-labeled anti-CD11b mAb,
phycoerythrin (PE)-labeled anti-CD40 mAb and agonistic anti-CD40
antibody were provided by Biolegend (San Diego, CA, USA). The
spleens and primary tumors were harvested at varying tumor sizes,
placed in phosphate-buffered saline (PBS), gently pressed and then
filtered to obtain a single cell suspension. Following red blood
cell lysis and washing with PBS, the splenocytes and tumor tissue
cells were then stained with the aforementioned mouse-specific mAbs
for 30 min at 4°C. All samples were analyzed by flow cytometry
(Cytomics FC 500, Beckman Coulter, Miami, FL, USA).
MDSC apoptosis
Single cell suspensions (2×106 cells/ml)
were prepared from the spleen and tumor tissues and incubated with
PBS or agonistic anti-CD40 (5 μg/ml; Biolegend) for 24 h at 37°C in
a humidified atmosphere of 5% CO2. The cells were
subsequently collected and stained with anti-Gr-1 mAb, anti-CD11b
mAb and Annexin V (Invitrogen Life Technologies, Carlsbad, CA,
USA), as described previously (20). Finally, the proportion of the
Gr-1+/CD11b+/Annexin V+ cells was
determined by flow cytometric analysis.
Statistical analysis
Statistical analysis of the data was performed using
FlowJo software (TreeStar Inc., Ashland, OR, USA), and all
statistical analyses were performed using GraphPad Prism software
(GraphPad Software, Inc., La Jolla, CA, USA). The statistical
differences were analyzed using a t-test and one-way or two-way
analysis of variance. The correlation between tumor progression and
the expression of CD40 on the MDSCs, as determined by
fluorescence-activated cell sorting, was assessed by Pearson’s
correlation analysis. Data corresponding to the expression of CD40
and MDSC apoptosis are presented as the mean ± standard error of
the mean (SEM). P<0.05 was considered to indicate a
statistically significant difference and all P-values were
two-sided.
Results
CD40 expression on MDSCs of gastric
tumor-bearing mice
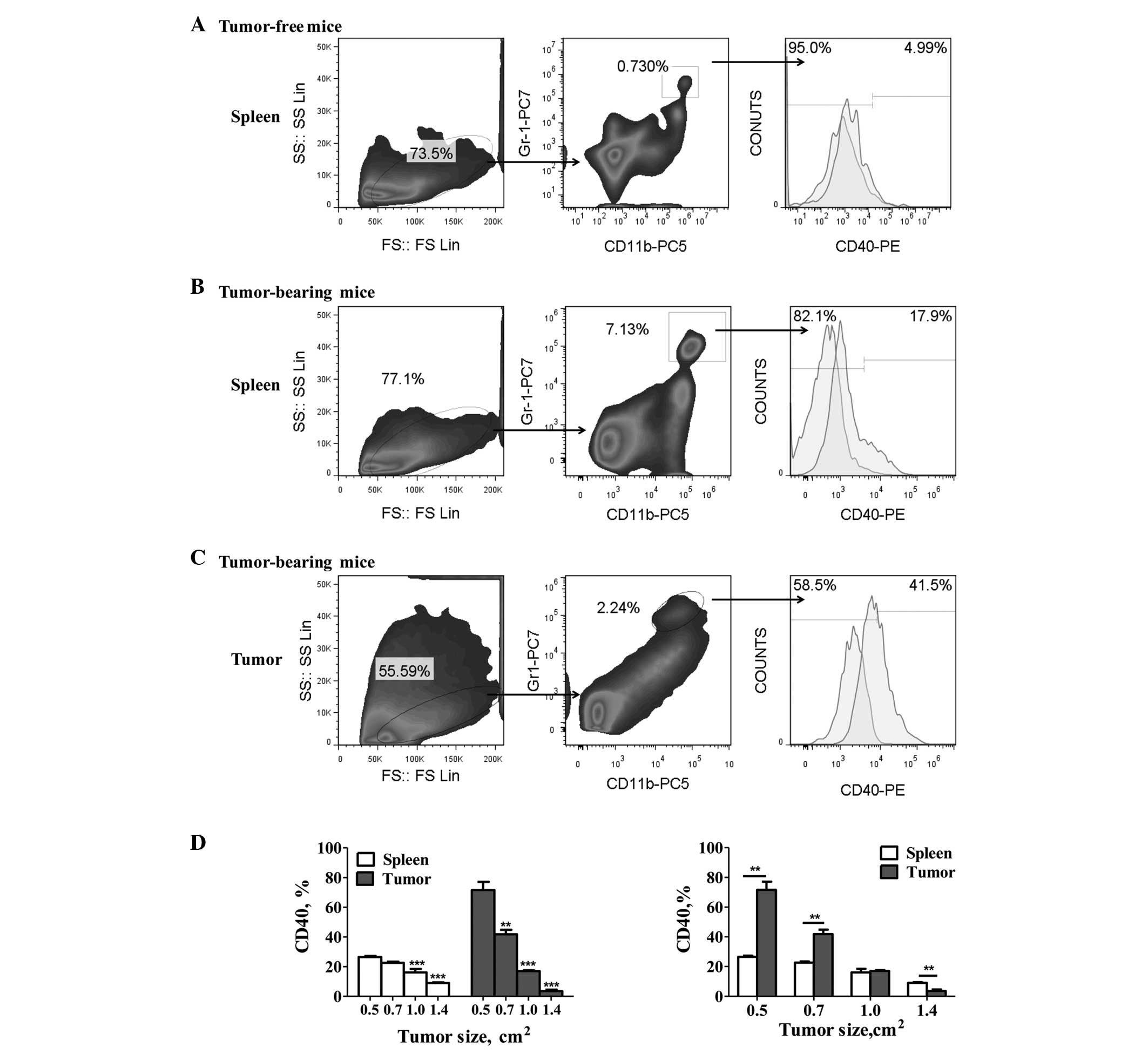
The levels of CD40 expression were detected on the
Gr-1+/CD11b+ MDSCs from gastric tumor-bearing
and tumor-free mice. The results showed a significant increase in
the percentage of CD40+ MDSCs in the tumor-bearing mice
compared with the tumor-free mice (Fig.
1A and B). Furthermore, tumor-infiltrating MDSCs exhibited
significantly higher CD40 expression levels than the spleen-derived
MDSCs (P<0.05; Fig. 1B and C).
However, significant downregulation of CD40 expression on the MDSCs
was observed with tumor progression (P<0.05; Fig. 1D). In addition, the levels of CD40
expression on the tumor-derived MDSCs decreased gradually from
71.67±5.42 to 3.56±0.99% (mean ± SEM), and that of the splenic
MDSCs decreased from 26.53±0.82 to 9.05±0.43% (mean ± SEM).
Correlation between CD40 downregulation
and MDSC accumulation with tumor progression
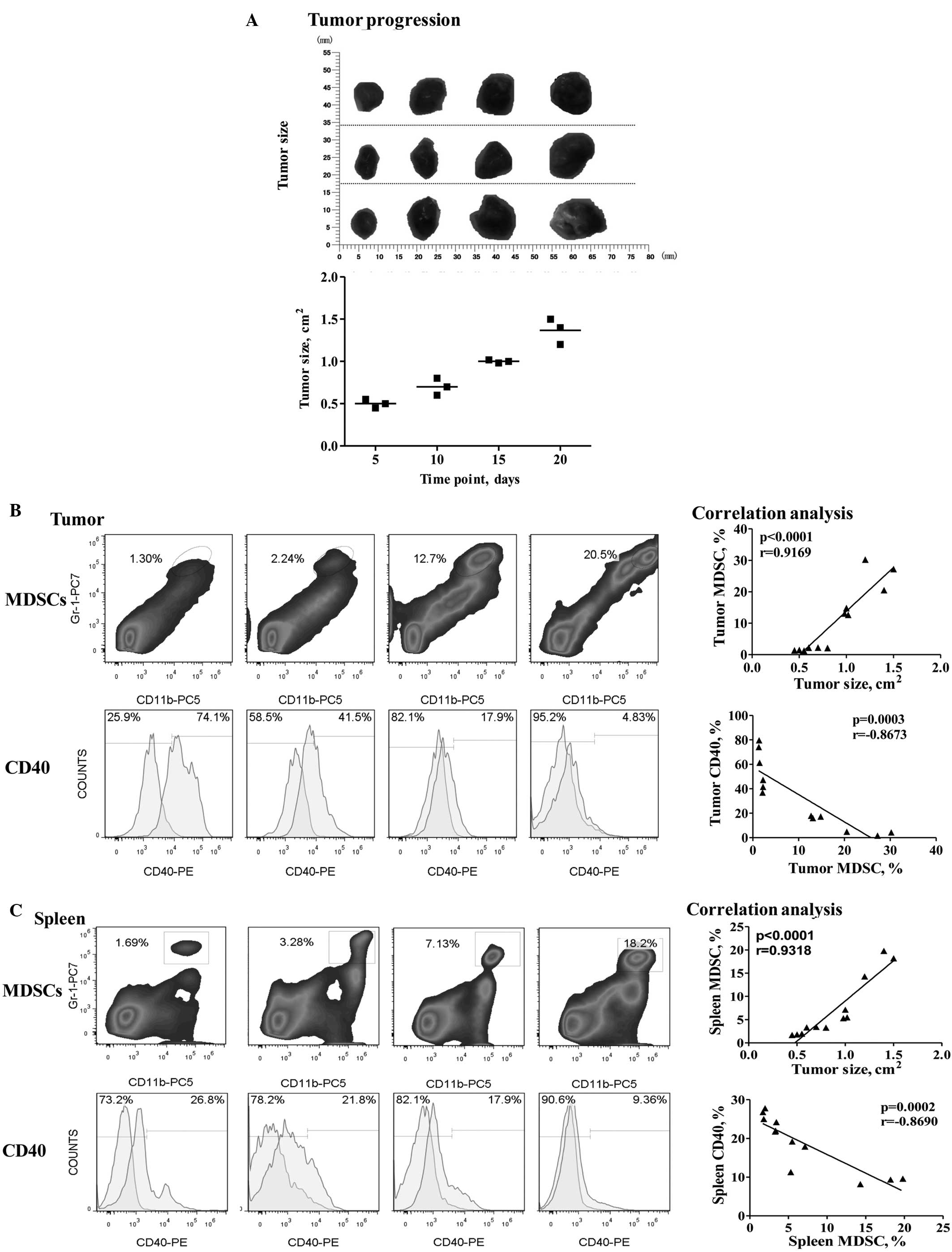
In gastric tumor-bearing mice, tumor burden was
found to induce MDSC accumulation. The proportion of MDSCs in
primary tumors increased from 1.38±0.05 to 25.97±2.87% (mean ± SEM)
and similarly, the proportion of MDSCs in the spleen increased from
1.82±0.08 to 17.43±1.63% (mean ± SEM). However, CD40 expression in
the MDSCs was significantly downregulated, as aforementioned. The
correlation analysis revealed a significant positive correlation
between the number of tumor-infiltrating MDSCs and tumor
progression (r=0.9169; P<0.0001; Fig. 2B), which was also observed between
the splenic MDSCs and tumor progression (r=0.9318; P<0.0001;
Fig. 2C). Furthermore, the CD40
expression levels were found to exhibit a significant inverse
correlation with MDSC accumulation in the tumors (r=−0.8673;
P=0.0003; Fig. 2B), which was also
observed in the spleen (r=−0.8690; P=0.002; Fig. 2C).
Regulation of MDSC accumulation through
CD40-induced apoptosis in vivo
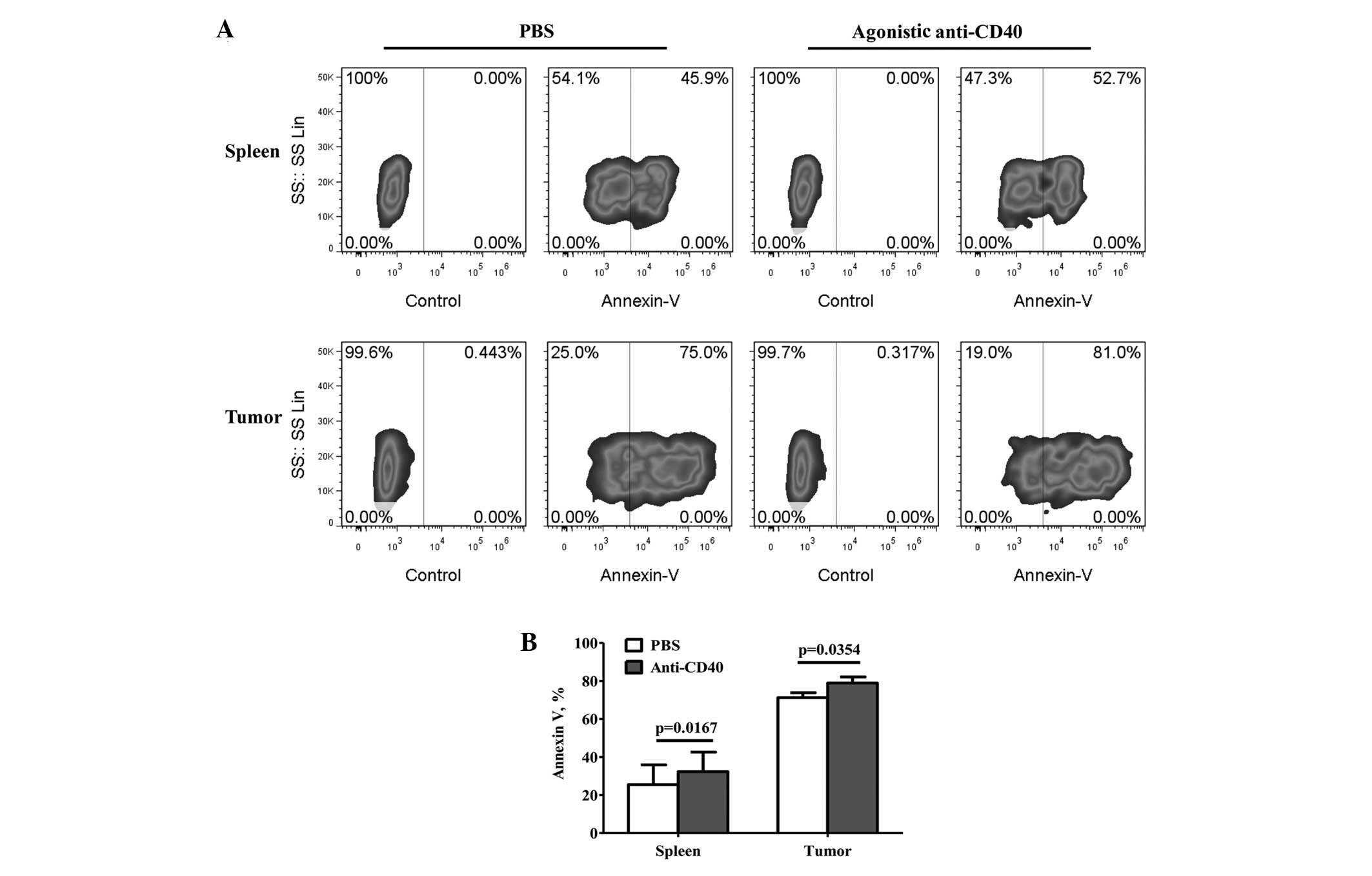
To further investigate the correlation between the
CD40 expression levels and MDSC accumulation during tumor growth,
the apoptosis of the MDSCs was detected by treatment with agonistic
anti-CD40 or PBS. The apoptosis of the MDSCs was found to
significantly increase following treatment with agonistic anti-CD40
(Fig. 3B), and compared with the
PBS treatment, the percentage of apoptotic tumor-infiltrating MDSCs
increased from 71.37±2.68 to 79.09±3.29% (mean ± SEM; P=0.0352;
Fig. 3A and B). Furthermore, the
percentage of apoptotic splenic MDSCs increased from 25.43±10.45 to
32.30±10.27% (mean ± SEM; P=0.0167; Fig. 3A and B).
Discussion
MDSCs contribute to tumor progression via their
potent ability to suppress antitumor immunity and induce tumor
escape. Limiting MDSC accumulation using gene ablation, drugs or
antibodies enhances antitumor immunity and may result in tumor
regression (15–18). Thus, regulating MDSC accumulation is
a powerful tool to inhibit tumor progression. Previous studies have
reported the expression of CD40 on MDSCs to be important in
MDSC-mediated immune suppression and Treg expansion in certain
tumor models (8,13,14).
However, few studies have analyzed the direct effects of CD40
expression on MDSCs, including differentiation and expansion. Weiss
et al (19) revealed that
the combination of IL-2 with agonistic anti-CD40 reduces the number
of MDSCs in the tumor microenvironment by mediating MDSC
recruitment. Furthermore, Zhao et al (20) showed that TNF signaling mediates
MDSC accumulation by affecting their apoptosis rather than
proliferation. However, whether CD40 regulates MDSC accumulation
via apoptosis has not yet been investigated.
The results of the current study showed that CD40 is
highly expressed on the MDSCs of a gastric tumor model. The tumors
were found to induce MDSC accumulation, and the MDSCs exhibited
different levels of CD40 expression with tumor progression. CD40
was preferentially expressed on tumor-infiltrating MDSCs at the
early phase of progression, but was gradually downregulated with
tumor progression. These differences may be attributed to the
diverse tumor types or the various sites (including the spleen,
tumor and blood) and progression stages.
Since tumor progression affects the differentiation
of MDSCs towards a suppressive phenotype, the downregulation of
CD40 may correlate with the functional state of the MDSCs (21). Notably, a marked correlation was
identified among MDSC accumulation, CD40 expression and tumor
progression. The downregulation of CD40 was found to significantly
correlate with MDSC accumulation, which indicated that CD40 may be
involved in MDSC accumulation. Considering that CD40 has a dual
role in the regulation of cell apoptosis, which relies on CD40
expression levels, whereby high levels of CD40 expression induce
apoptosis and low levels prevent apoptosis, we hypothesized that
MDSC accumulation may be regulated by CD40 via apoptosis. The
results of the present study revealed that treatment with agonistic
anti-CD40 significantly increases MDSC apoptosis, which indicates
that CD40 activation induces MDSC apoptosis. Therefore, the
downregulation of CD40 expression may facilitate MDSC resistance to
apoptosis and thereby promote the accumulation of the MDSCs.
Furthermore, the present study showed that treatment with agonistic
anti-CD40 was effective in promoting the apoptosis of MDSCs and
inhibiting MDSC accumulation.
The mechanisms underlying CD40-mediated MDSC
apoptosis remain unclear. To date, studies have reported that Fas
signaling mediates MDSC apoptosis (9,22). In
addition, inhibition of IL-4Rα/signal transducer and activator of
transcription (STAT)-6 (15) or
knockout of TNF signaling enhances MDSC apoptosis (20) and subsequently results in tumor
regression. CD40, as a member of the TNF superfamily, may use
similar signaling pathways as TNF to regulate MDSC apoptosis. In
addition, CD40 has been demonstrated to interact with Fas to
mediate cell apoptosis (23).
Furthermore, CD40 downstream signaling molecules, including
activator protein 1 (c-Jun and c-Fos) and STAT-3, are also
associated with cell apoptosis (24,25).
Therefore, the possible mechanisms of CD40-mediated MDSC apoptosis
remain to be elucidated. Further studies are required to
investigate whether these factors function separately, in a
synergistic manner or via an interaction with CD40 to regulate MDSC
accumulation.
In conclusion, immunotherapeutic interference with
MDSC accumulation and its functions present a potential strategy
for tumor treatment. In addition, CD40 is the most promising
remedial molecular target against tumors. The current study
demonstrated that CD40 mediates the accumulation of MDSCs via the
induction of MDSC apoptosis, therefore, CD40 may present a novel
target for decreasing MDSC levels in the tumor environment.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant. no 81272737) and the
Ministry of Education of Jiangsu Province (grant no. CXLX11-0084).
The authors would like to thank Dr Xinliang Mao for the language
support, Gehua Yu, Yu Shen and Yumin Hu for their technical
assistance and Dr Jindong Ji for the invaluable discussions.
Abbreviations:
|
MDSCs
|
myeloid-derived suppressor cells
|
|
Tregs
|
T regulatory cells
|
|
MFC
|
mouse forestomach carcinoma
|
|
mAb
|
monoclonal antibody
|
References
|
1
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009.
|
|
2
|
Yang L, DeBusk LM, Fukuda K, Fingleton B,
Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP and Lin PC:
Expansion of myeloid immune suppressor Gr+CD11b+ cells in
tumor-bearing host directly promotes tumor angiogenesis. Cancer
Cell. 6:409–421. 2004.
|
|
3
|
Ostrand-Rosenberg S and Sinha P:
Myeloid-derived suppressor cells: linking inflammation and cancer.
J Immunol. 182:4499–4506. 2009.
|
|
4
|
Yang R, Cai Z, Zhang Y, Yutzy WH IV, Roby
KF and Roden RB: CD80 in immune suppression by mouse ovarian
carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res.
66:6807–6815. 2006.
|
|
5
|
Huang B, Pan PY, Li Q, Sato AI, Levy DE,
Bromberg J, Divino CM and Chen SH: Gr-1+CD115+ immature myeloid
suppressor cells mediate the development of tumor-induced T
regulatory cells and T-cell anergy in tumor-bearing host. Cancer
Res. 66:1123–1131. 2006.
|
|
6
|
Serafini P, Mgebroff S, Noonan K and
Borrello I: Myeloid-derived suppressor cells promote
cross-tolerance in B-cell lymphoma by expanding regulatory T Cells.
Cancer Res. 68:5439–5449. 2008.
|
|
7
|
Greenwald RJ, Freeman GJ and Sharpe AH:
The B7 family revisited. Annu Rev Immunol. 23:515–548. 2005.
|
|
8
|
Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang
G, Yin B, Divino CM and Chen SH: Immune stimulatory receptor CD40
is required for T-cell suppression and T regulatory cell activation
mediated by myeloid-derived suppressor cells in cancer. Cancer Res.
70:99–108. 2010.
|
|
9
|
Sinha P, Chornoguz O, Clements VK,
Artemenko KA, Zubarev RA and Ostrand-Rosenberg S: Myeloid-derived
suppressor cells express the death receptor Fas and apoptose in
response to T cell-expressed FasL. Blood. 117:5381–5390. 2011.
|
|
10
|
Rizvi M, Pathak D, Freedman JE and
Chakrabarti S: CD40-CD40 ligand interactions in oxidative stress,
inflammation and vascular disease. Trends Mol Med. 14:530–538.
2008.
|
|
11
|
Van Kooten C and Banchereau J: CD40-CD40
ligand. J Leukoc Biol. 67:2–17. 2000.
|
|
12
|
Murugaiyan G, Martin S and Saha B:
CD40-induced countercurrent conduits for tumor escape or
elimination? Trends Immunol. 28:467–473. 2007.
|
|
13
|
Schlom J, Jochems C, Gulley JL and Huang
J: The role of soluble CD40L in immunosuppression. Oncoimmunology.
2:e225462013.
|
|
14
|
Huang J, Jochems C, Talaie T, Anderson A,
Jales A, Tsang KY, Madan RA, Gulley JL and Schlom J: Elevated serum
soluble CD40 ligand in cancer patients may play an
immunosuppressive role. Blood. 120:3030–3038. 2012.
|
|
15
|
Roth F, De La Fuente AC, Vella JL, Zoso A,
Inverardi L and Serafini P: Aptamer-mediated blockade of IL4Rα
triggers apoptosis of MDSCs and limits tumor progression. Cancer
Res. 72:1373–1383. 2012.
|
|
16
|
Ribechini E, Leenen PJ and Lutz MB: Gr-1
antibody induces STAT signaling, macrophage marker expression and
abrogation of myeloid-derived suppressor cell activity in BM cells.
Eur J Immunol. 39:3538–3551. 2009.
|
|
17
|
Vincent J, Mignot G, Chalmin F, Ladoire S,
Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C and
Ghiringhelli F: 5-Fluorouracil selectively kills tumor-associated
myeloid-derived suppressor cells resulting in enhanced T
cell-dependent antitumor immunity. Cancer Res. 70:3052–3061.
2010.
|
|
18
|
Veltman JD, Lambers ME, van Nimwegen M,
Hendriks RW, Hoogsteden HC, Aerts JG and Hegmans JP: COX-2
inhibition improves immunotherapy and is associated with decreased
numbers of myeloid-derived suppressor cells in mesothelioma.
Celecoxib influences MDSC function. BMC Cancer. 10:4642010.
|
|
19
|
Weiss JM, Back TC, Scarzello AJ, Subleski
JJ, Hall VL, Stauffer JK, Chen X, Micic D, Alderson K, Murphy WJ
and Wiltrout RH: Successful immunotherapy with IL-2/anti-CD40
induces the chemokine-mediated mitigation of an immunosuppressive
tumor microenvironment. Proc Natl Acad Sci USA. 106:19455–19460.
2009.
|
|
20
|
Zhao X, Rong L, Zhao X, Li X, Liu X, Deng
J, Wu H, Xu X, Erben U, Wu P, et al: TNF signaling drives
myeloid-derived suppressor cell accumulation. J Clin Invest.
122:4094–4104. 2012.
|
|
21
|
Youn JI, Nagaraj S, Collazo M and
Gabrilovich DI: Subsets of myeloid-derived suppressor cells in
tumor-bearing mice. J Immunol. 181:5791–5802. 2008.
|
|
22
|
Ostrand-Rosenberg S, Sinha P, Chornoguz O
and Ecker C: Regulating the suppressors: apoptosis and inflammation
govern the survival of tumor-induced myeloid-derived suppressor
cells (MDSC). Cancer Immunol Immunother. 61:1319–1325. 2012.
|
|
23
|
Humphreys EH, Williams KT, Adams DH and
Afford SC: Primary and malignant cholangiocytes undergo CD40
mediated Fas dependent apoptosis, but are insensitive to direct
activation with exogenous Fas ligand. PLoS One. 5:e140372010.
|
|
24
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: a leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009.
|
|
25
|
Ahmed-Choudhury J, Williams KT, Young LS,
Adams DH and Afford SC: CD40 mediated human cholangiocyte apoptosis
requires JAK2 dependent activation of STAT3 in addition to
activation of JNK1/2 and ERK1/2. Cell Signal. 18:456–468. 2006.
|