Introduction
Acute leukemias (ALs) are the most frequent type of
cancer occurring in children (1).
In Mexico City, ~85% of the cases were acute lymphoblastic leukemia
(ALL) and 14.5% were acute myeloblastic leukemia, with a low
percentage of acute biphenotypic or non-differentiated AL (1). From 1996 to 2000, a mortality rate of
63.7 per million children was recorded, which is one of the highest
rates reported worldwide (2). In
2005, leukemia was the second highest cause of mortality in
Guerrero in children <15 years old (3). An antineoplastic agent commonly used
for the treatment of ALL is methotrexate (MTX), which was
introduced to clinical oncology ~50 years ago. Folylpolyglutamate
synthase (FPGS) catalyzes the polyglutamation of MTX to produce
highly active metabolites (4).
Certain polymorphisms at specific sites in the FPGS gene may
decrease the affinity for its substrate, causing deficient
polyglutamation of MTX (4). The
A22G polymorphism (rs10760502), which replaces Ile with Val at
position 22 of the FPGS protein, was identified in
African-American, Caucasian-American, Chinese-American and
Mexican-American populations (4);
however, this polymorphism has not yet been studied as a factor for
ALL. The present study retrospectively evaluated whether the A22G
polymorphism in the FPGS gene is associated with an increased risk
and survival for ALL.
Materials and methods
Study population
Patients (n=70) with ALL at the Pediatric Oncology
Service of the State Cancer Institute ‘Arturo Beltran Ortega’
(Acapulco, Guerrero) who were diagnosed between August, 2005 and
August, 2010 via bone marrow aspiration based on the
French-American-British morphological criteria, cytochemical
staining properties and subclassified as T- or B-lineages as
previously described (5), were
included in the present study. Multiagent chemotherapeutic
protocols used were 96091, 96092 or CIE-10:C9.1.0 of the State
Cancer Institute ‘Arturo Beltran Ortega’, as previously described
(5,6). This study and the informed consent
protocol were approved by the institutional review board of the
Cancer Institute. Complete remission, relapse and poor outcomes
were as previously defined (5,7). Risk
classification was as follows: Low risk, individuals aged between
one and nine years old presenting with a white blood cell (WBC)
count of <50,000/mm3; and high risk, individuals aged
less than one or more than nine years old with a WBC count of
>50,000/mm3 (5,7). The
controls included 100 healthy individuals (4–10×103
leukocytes/mm3) without a family history of leukemia.
Collectively, the subjects in the two groups were between one and
18 years old, included males and females, and were residents of
Guerrero, Mexico. Patients provided written informed consent.
Specimen collection
A bone marrow and/or blood sample was collected from
the 170 participants and placed in tubes with anticoagulant.
Leukocytes were purified from the whole blood sample by a selective
osmotic lysis of erythrocytes; the leukocyte genomic DNA was
extracted using the phenol-chloroform technique, as described
previously (8).
Genotyping
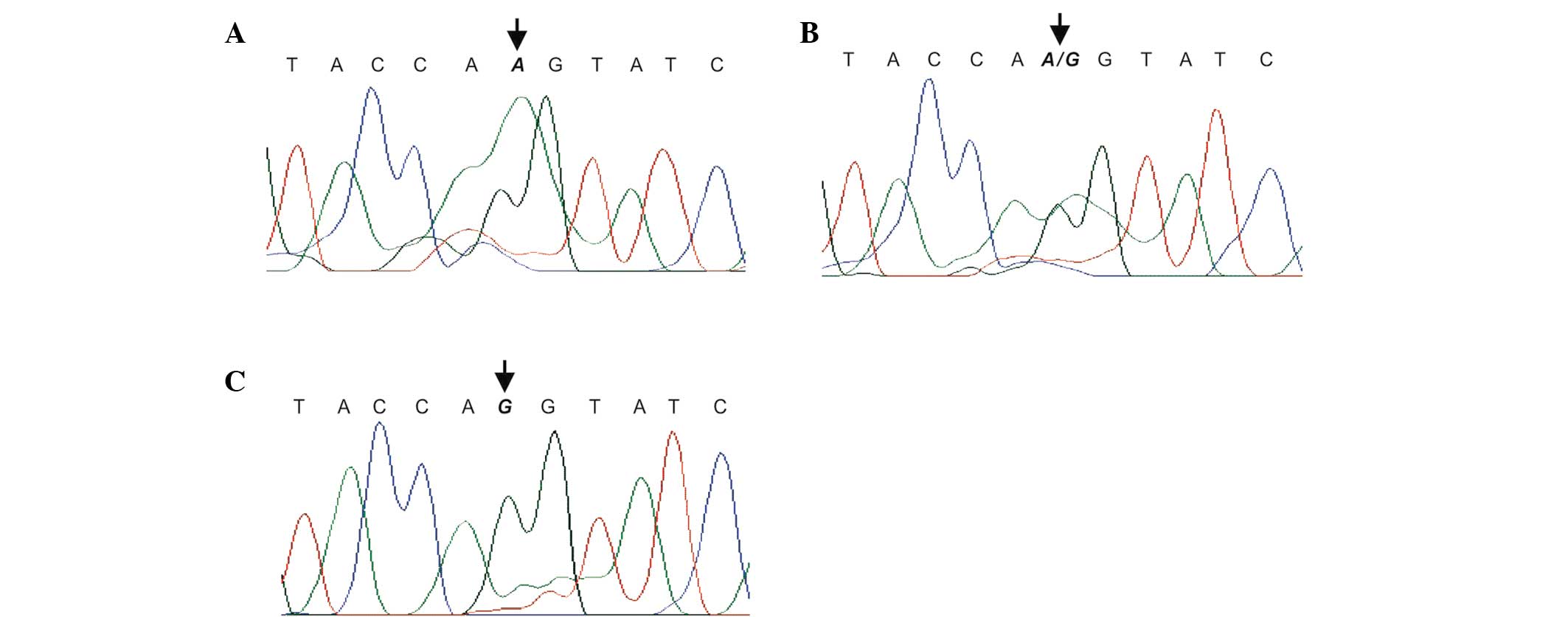
The A22G polymorphism (rs10760502) was detected by
polymerase chain reaction (PCR) and sequencing using forward
(5′-ACCTGCGCGCCGCTCTATTC-3′) and reverse
(5′-GCTGGCCCGCCTGATACCTG-3′) primers, according to previously
established protocols (9). The PCR
products were sequenced using the ABI PRISM 310 Genetic analyzer
(PE Applied Biosystems, Foster City, CA, USA) and sequence data
were analyzed using SeqManII software (DNASTAR, Inc., Madison, WI,
USA) (Fig. 1).
Statistical analysis
Continuous data are presented as the means ±
standard deviation. Categorical data were compared by the
χ2 or Fisher’s exact tests. Univariate logistic
regression analysis for the association between the risk of relapse
and A22G genetic polymorphism, gender and other clinical
characteristics were tested, and those factors that were
significant in the univariate analysis were included in a second
multivariate logistic analysis. The log-rank test and Kaplan-Meier
curves were used to analyze the effects of the A22G genetic
polymorphism and relapse of ALL on overall survival (OS). OS was
defined as the time elapsed between the date of initial diagnosis
and either death or the time of the last follow-up. The
Hardy-Weinberg equilibrium (HWE) was used to determine the genetic
equilibrium in the healthy group. P<0.05 was considered to
indicate a statistically significant difference. All the
statistical analyses were performed using SPSS software, version
21.0 (SPSS, Inc., Chicago, IL, USA) and STATA software, version 9.2
(StataCorp, College Station, TX, USA).
Results
Clinical characteristics
The clinical characteristics of the study population
have been previously reported (5,7).
Briefly, the 70 patients with ALL were aged between 1.0 and 18
years (mean age ± SD, 7.65±4.67 years), including 45 (64.29%) males
and 25 (35.71%) females. Of these, 18 patients (25.71%) were aged
between one and nine years old, and 52 patients (72.29%) were aged
less than one year or more than nine years at the time of initial
diagnosis. The median follow-up time was 38 months and the longest
follow-up was six years, which occurred in only two patients. The
relapse rate of patients with ALL was 68.57%.
The control group included 100 healthy individuals
aged between 1.0 and 18 years old (mean ± SD, 9.99±5.49 years) with
a normal leukocyte count (4–10×103
leukocytes/mm3; median 8,000 leukocytes/mm3).
In this group, 53 healthy individuals (53%) were male and 47 (47%)
were female.
Association of A22G polymorphism in FPGS
with the risk of ALL
The genotype distribution and allele frequency of
A22G polymorphism in 70 patients with ALL and 100 healthy
individuals were determined. As shown in Table I, the genotype distribution of A22G
polymorphism supported that expected by the HWE in healthy
individuals. When the genotype frequencies were compared between
the cases and controls, a statistically significant association
with ALL was found (P<0.05). The homozygous variant, G/G [odds
ratio (OR)=3.88; 95% confidence interval (CI): 2.50–6.03] and the
heterozygote variant, A/G (OR=1.37; 95% CI: 1.26–48.95) were risk
factors for ALL (Table I).
 | Table IGenotype distribution and allele
frequency of the A22G polymorphism in the FPGS gene, and
association with the risk of ALL. |
Table I
Genotype distribution and allele
frequency of the A22G polymorphism in the FPGS gene, and
association with the risk of ALL.
| A22G polymorphism
(rs10760502) | ALL cases (%)
(n=70) | Controls (%)
(n=100) | P-value | OR | 95% CI | P-value | P-value HWE |
|---|
| Genotypes |
| A/A | 19 (27.14) | 66 (66.00) | <0.001a | 1.00 | | | 0.086c |
| A/G | 38 (54.29) | 27 (27.00) | | 1.37 | 1.26–48.95 | <0.001b | |
| G/G | 13 (18.57) | 7 (7.00) | | 3.88 | 2.50–6.03 | <0.001b | |
| A/A | 19 (27.14) | 66 (66.00) | <0.001a | 1.00 | | | |
| A/G+G/G | 51 (72.86) | 34 (34.00) | | 5.21 | 2.67–10.18 | <0.001b | |
| Alleles |
| A | 76 (54.29) | 159 (79.50) | <0.001a | 1.00 | | | |
| G | 64 (45.71) | 41 (20.50) | | 1.92 | 1.22–3.01 | 0.004b | |
Risk of relapse based on genotypes and
other clinical characteristics
A logistic regression analysis showed that those
individuals with the genotype 22A/G were 1.78-fold (95% CI:
1.56–5.63; P=0.323) more likely to relapse during treatment, while
individuals with genotype 22G/G were 2.42-fold (95% CI: 1.99–11.76;
P=0.272) more likely to relapse compared with individuals with
genotype 22A/A (Table II).
Individuals aged less than one or >10 years old with >50,000
leukocytes/mm3 (high risk) were 1.68-fold (95% CI:
1.36–3.72; P=0.05) more likely to have relapsed compared with
individuals aged between two and nine years old with <50,000
leukocytes/mm3 (low risk) (Table II).
 | Table IIAssociation between A22G polymorphism
in the FPGS gene and clinical characteristics with the risk of ALL
recurrence. |
Table II
Association between A22G polymorphism
in the FPGS gene and clinical characteristics with the risk of ALL
recurrence.
| | Univariate
analysis | Multivariate
analysis |
|---|
| |
|
|
|---|
| Characteristics | ALL cases (%) | OR | 95% CI | P-valuea | OR | 95% CI | P-valuec |
|---|
| Gender |
| Female | 25 (35.71) | 1.00 | | | | | |
| Male | 45 (64.29) | 1.38 | 0.49–3.92 | 0.540 | | | |
| Risk at
diagnosis |
| Low risk | 18 (25.71) | 1.00 | | | | | |
| High risk | 52 (74.29) | 7.64 | 1.90–30.73 | 0.004b | 1.68 | 1.36–3.72 | 0.05b |
| A22G genotypes
(rs10760502) |
| A/A | 19 (27.14) | 1.00 | | | | | |
| G/A | 38 (54.29) | 1.78 | 1.56–5.63 | 0.323 | 1.81 | 1.57–5.74 | 0.049b |
| G/G | 13 (18.57) | 2.42 | 1.99–11.76 | 0.272 | 2.44 | 2.40–11.82 | 0.017b |
The following variables were included in the
multivariate analysis: Number of leukocytes at diagnosis, age and
A22G polymorphism genotypes, in order to determine whether the A22G
polymorphism genotypes predicted the risk of relapse independently.
Patients with the genotype 22A/G or 22G/G (OR=1.81; 95% CI:
1.57–5.74; P=0.049 and OR=2.4; 95% CI: 2.40–11.82; P=0.017,
respectively), were two independent prognostic markers for the risk
of relapse compared with the other variables (Table II).
Association between A22G polymorphism and
survival of patients with ALL
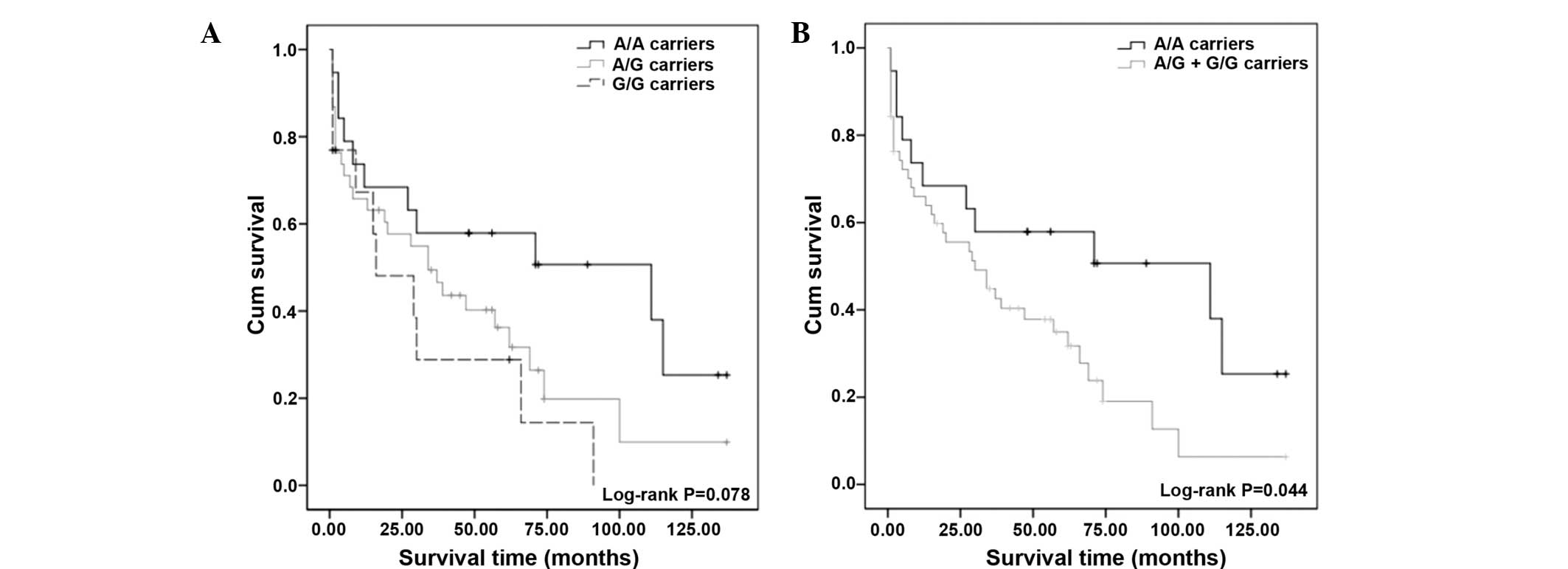
The Kaplan-Meier survival curves showed no
significant association between the FPGS A22G polymorphism and
survival, although a reduction in survival after six years of
follow-up among A/G and G/G carriers compared with the wild-type
genotype was observed (log-rank test; P=0.078) (Fig. 2A). However, a log-rank test for the
combined genotypes, 22A/G + 22G/G vs. A/A (Fig. 2B), showed a significant association
between the genotype-dependent effects for the survival of patients
with ALL (log-rank test; P=0.044) and increased survival was
observed in those patients with genotype 22A/A (Fig. 2).
Discussion
Agents that target the folate pathway, such as MTX,
are an effective treatment for several hematologic malignancies and
solid tumors. MTX is a structural analogue of folic acid that
inhibits multiple enzymes in the folate pathway and requires
polyglutamation by FPGS for activation. Patients with ALL have
different responses to the same therapy, such as MTX resistance and
subsequent relapse of ALL (10).
Leil et al (4) reported that the A22G polymorphism
present in the FPGS gene affected individuals of Mexican-American
descent and other populations conferring resistance to MTX.
Additionally, Wessels et al (11) reported a high frequency of A22G
polymorphism in patients with rheumatoid arthritis (11). Moreover, a number of studies have
been conducted investigating the role of FPGS polymorphisms
in cancer (12,13). Our data suggests that the A22G
polymorphism is significantly associated with the risk of ALL
(P<0.05) (Table I). However,
there have been no studies evaluating the association of the A22G
polymorphism with the risk of relapse and survival in patients with
ALL in the literature to date.
In this study, patients with ALL predominantly
showed the heterozygous A/G genotype (54.29%) of the A22G
polymorphism (Table I), which is
similar to the findings in the study by Wessels et al
(11) on patients with rheumatoid
arthritis (49%) (11); however,
these results are in contrast to the findings of Leil et al
(4), in African-American (15.00%),
Caucasian-American (37.50%), Han Chinese-American (3.30%) and
Mexican-American (32.50%) populations (4). These data suggest that the A22G
polymorphism is found more frequently in patients with enzymatic
activity of FPGS.
Wessels et al (11) and Van der Straaten et al
(14), found no significant
associations between the A22G polymorphism and MTX responses in
patients with rheumatoid arthritis. Thus, whether the A22G
polymorphism affects or presents a risk in specific disease therapy
has not been clearly determined. However, our findings show a
significant difference (P<0.05) in the frequency of A22G
genotypes between children with and without relapse. Carriers of
the G22G genotypes were more likely to relapse (OR=2.42; 95% CI:
1.99–11.76; P=0.272) compared with those with the AA genotype
(Table II), suggesting a role for
the A22G polymorphism in the risk of relapse of ALL. In the
multivariate analysis, OR estimates for patients with 22G/G
genotype retained their significance (OR=2.44; 95% CI: 2.40–11.82;
P=0.017) in the presence of other prognostic factors, which also
affected ALL outcome (age, WBC and risk classes) (Table II). A second aim of this study was
to investigate the effect of the polymorphism on survival. The
survival rate of G allele carriers of the A22G polymorphism was
lower than that of patients carrying the A allele (Fig. 2). During follow-up, a reduction in
survival among G allele carriers compared to patients with the
wild-type genotype was observed (Fig.
2).
To the best of our knowledge, this study is the
first to evaluate the effects of the A22G polymorphism in patients
with ALL. Our data generates a novel hypotheses regarding the role
of FPGS A22G polymorphism in the risk and relapse of ALL and its
effects on the survival of patients with ALL. Further independent
studies are required to clarify whether the associations reported
in this study which just escaped statistical significance,
presumably due to the limited sample size, can be corroborated. It
is of interest to determine the association of FPGS variants with
the folate pathway, which plays a role as an important target for
anticancer therapeutics. These investigations may result in novel
therapeutic regimens to correlate individual genetic variations
with response to antifolate therapy and efficacy in patients with
unfavorable FPGS genotypes.
Acknowledgements
The authors would like to thank the patients and
their parents for their collaboration, as well as M.Sc. Monica
Virginia Saavedra Herrera, Dra. Ana Betha Rivera Ramirez and to Dr.
Marco Antonio Teran Porcayo (during tenure) of the State Cancer
Institute ‘Arturo Beltran Ortega’ for their contribution of
biological material and facilitating access to clinical data. This
study was supported by the National Council of Science and
Technology (CONACYT, Mexico) fellowship awarded to M.Sc. Carlos
Alberto Rangel Rodriguez (August, 2009 to July, 2011).
References
|
1
|
Daniel-Cravioto A, Gonzalez-Bonilla CR,
Mejia-Arangure JM, et al: Genetic rearrangement MLL/AF4 is most
frequent in children with acute lymphoblastic leukemias in Mexico
City. Leuk Lymphoma. 50:1352–1360. 2009.
|
|
2
|
Mejia-Arangure J, Bonilla M, Lorenzana R,
et al: Incidence of leukemias in children from El Salvador and
Mexico City between 1996 and 2000: population-based data. BMC
Cancer. 5:332005.
|
|
3
|
Database of Deaths National Institute of
Statistics (INEGI)/Health Secretariat (SSA). Directorate General of
Health Information; Mexico: February. 2006, Available at:
www.inegi.org.mx/urisimplewww.inegi.org.mx/. Accessed
April, 2008
|
|
4
|
Leil TA, Endo C, Adjei AA, et al:
Identification and characterization of genetic variation in the
folylpolyglutamate synthase gene. Cancer Res. 67:8772–8782.
2007.
|
|
5
|
Gómez-Gómez Y, Organista-Nava J,
Saavedra-Herrera MV, et al: Survival and risk of relapse of acute
lymphoblastic leukemia in a Mexican population is affected by
dihydrofolate reductase gene polymorphisms. Exp Ther Med.
3:665–672. 2012.
|
|
6
|
Insurance-Popular. Secretary of
Health/Seguro Popular. http://www.seguro-popular.gob.mx/.
Accessed May, 2009
|
|
7
|
Leyva-Vázquez MA, Organista-Nava J,
Gómez-Gómez Y, et al: Polymorphism G80A in the reduced folate
carrier gene and its relationship to survival and risk of relapse
in acute lymphoblastic leukemia. J Investig Med. 60:1064–1067.
2012.
|
|
8
|
Merante F, Raha S, Reed J and Proteau G:
The Simultaneous Isolation of RNA and DNA from Tissues and Cultured
Cells. Methods Mol Biol. 58:3–9. 1996.
|
|
9
|
Organista-Nava J, Gómez-Gómez Y,
Saavedra-Herrera MV, et al: Polymorphisms of the gamma-glutamyl
hydrolase gene and risk of relapse to acute lymphoblastic leukemia
in Mexico. Leuk Res. 34:728–732. 2010.
|
|
10
|
Dulucq S, St-Onge G, Gagné V, et al: DNA
variants in the dihydrofolate reductase gene and outcome in
childhood ALL. Blood. 111:3692–3700. 2008.
|
|
11
|
Wessels JA, van der Kooij SM, le Cessie S,
et al: A clinical pharmacogenetic model to predict the efficacy of
methotrexate monotherapy in recent-onset rheumatoid arthritis.
Arthritis Rheum. 56:1765–1775. 2007.
|
|
12
|
Lee KM, Lan Q, Kricker A, et al:
One-carbon metabolism gene polymorphisms and risk of non-Hodgkin
lymphoma in Australia. Hum Genet. 122:525–533. 2007.
|
|
13
|
Lim U, Wang SS, Hartge P, et al:
Gene-nutrient interactions among determinants of folate and
one-carbon metabolism on the risk of non-Hodgkin lymphoma: NCI-SEER
case-control study. Blood. 109:3050–3059. 2007.
|
|
14
|
van der Straaten RJ, Wessels JA, de
Vries-Bouwstra JK, et al: Exploratory analysis of four
polymorphisms in human GGH and FPGS genes and their effect in
methotrexate-treated rheumatoid arthritis patients.
Pharmacogenomics. 8:141–150. 2007.
|