Introduction
Esophageal squamous cell carcinoma (ESCC) is the
dominant type of esophageal cancer worldwide (1). However, the incidence of esophageal
adenocarcinoma (EADC) has been rapidly increasing in the Western
world over the last 50 years, particularly in western males
(2,3). The etiology of the increase in the
incidence of EADC remains obscure and has prompted further
investigation into this clinical issue.
The rapid increase of EADC in Western countries has
occurred in parallel with an increased prevalence of
gastroesophageal reflux disease (GERD) (4,5). Diet
and lifestyle alterations in the Western world have been associated
with an increased prevalence of obesity and hiatal hernias, which
are known risk factors for GERD and esophageal cancer (5,6). A
previous study proposed that EADC develops via a sequence of events
into GERD (7). Specifically,
gastroduodenal content reflux from GERD induces
inflammation-mediated hyperplasia and metaplasia, and subsequently
dysplasia and EADC.
Studies have also determined that duodeno-esophageal
or duodeno-gastro-esophageal reflux induces the sequential
development of EADC in surgical rat models (8–10).
These cancerous changes occur without the use of any exogenous
carcinogens. The rat model demonstrated the histopathological
sequence of events from GERD to EADC as an
inflammation-metaplasia-dysplasia-adenocarcinoma (ADC) sequence.
Furthermore, a recent study has established a correlation between
the quantity of reflux and the likelihood of developing EADC and
ESCC (11).
The pathogenesis of reflux-induced duodenoesophageal
carcinoma and the molecular changes in gene expression, which
drives esophageal carcinogenesis in rats, have recently been
addressed (12). The potential role
of histone deacetylase (HDAC) 1 and metastasis-associated gene
(MTA) 1 in esophageal carcinogenesis remains unclear and has not
been investigated in depth.
MTA is a newly discovered family of cancer
progression-associated genes and their encoded products (13). The expression of MTA1 and its
encoded protein, MTA1, have been found to correlate with the
malignant properties of numerous human cancers, including cancer of
the esophagus (14), breast
(15), pancreas (16), colon (17), stomach (18), liver (19) and prostate (20).
Histone acetyltransferase (HAT)- and HDAC-induced
alterations of the chromatin structure have been implicated in the
regulation of gene transcription, as well as in the process of
carcinogenesis (21,22).
Chromatin histone and non-histone proteins are the
protein targets for HDAC deacetylation via nucleosome remodeling
and histone deacetylation (NuRD) complexes containing MTA proteins.
The p53 tumor suppressor protein was the first non-histone protein
reported to be deacetylated by MTA protein-containing NuRD
complexes (23). The HDAC1/MTA1
complexes exert deacetylation activity against p53 protein in human
non-small cell carcinoma and human hepatoma cells. In addition, the
complexes have been found to inhibit p53-induced apoptosis by
attenuating the transactivation function of p53 (18,24).
To improve the understanding of esophageal
carcinogenesis in humans, animal models mimicking this tumorigenic
process are particularly powerful tools. The use of experimental
animal models is an effective method to understand the
developmental mechanisms underlying carcinogenesis. The current
study utilized a surgically induced rat reflux model of esophageal
carcinogenesis. The rat surgical reflux model provided the
opportunity to record the expression of proteins encoded by the
HDAC1 and MTA1 genes in each stage of carcinogenesis,
and to observe the effects on cell proliferation and
carcinogenesis. In addition, the model was advantageous as it
enabled examination of the expression of the HDAC1 and
MTA1 genes in all stages of esophageal carcinogenesis,
including squamous hyperplasia, squamous dysplasia, squamous cell
carcinoma (SCC), Barrett’s esophagus, ADC and adenosquamous
carcinoma (ASC). By improving the understanding of the expression
of HDAC1 and MTA1 in esophageal carcinogenesis, targeted esophageal
cancer chemotherapy may be developed.
The aim of the present study was to assess HDAC1 and
MTA1 expression in a surgical rat model of esophageal
carcinogenesis.
Materials and methods
Experimental animals
In total, 50 Wistar male rats, weighing ~250 g, were
used in the present study. The animals were housed three per cage
and maintained at a constant room temperature of 22±3°C, in 55±5%
humidity under a 12-h light-dark cycle. The rats were fed standard
solid chow (CRF-1; Charles River Laboratories Japan, Inc.,
Yokohama, Japan) and tap water that was free of carcinogens. The
study was approved by the Institutional Animal Care and Use
Committee of the Graduate School of Medical Science, Kanazawa
University (AP-111868; Kanazawa, Japan).
Surgical procedures
Following a 24-h fast, an upper abdominal incision
was made under diethyl ether inhalation anesthesia. The surgical
procedures were performed to induce duodenoesophageal reflux
following total gastrectomy of each rat as previously reported
(10).
Specimen extraction
The animals were sacrificed by diethyl ether
inhalation and the abdomen was opened. A ligature was placed around
the afferent and efferent jejunal loop proximal to the
esophagojejunal anastomosis. The esophagus was ligated at the level
of the thyroid cartilage via a thoracotomy. The esophagus and
anastomosed jejunum were subsequently removed.
Pathological assessment
The excised organs were washed with 10% formalin,
spread and pinned on a cork plate with the mucosal side facing
upwards. Following fixation of the organs with 10% formalin
solution for at least 24 h, the esophagus was cut into slices along
the longitudinal axis at 3-mm intervals and embedded in paraffin.
Next, 5 μm-thick sections of each embedded paraffin block were
prepared for histological analysis with hematoxylin and eosin
staining.
Immunohistochemistry
For the immunohistochemical staining, the Dako
Envision system (Dako, Carpinteria, CA, USA), which uses dextran
polymers conjugated with horseradish peroxidase, was employed to
avoid any endogenous biotin contamination. The sections were
deparaffinized in xylene and rehydrated in a graded ethanol series.
The endogenous peroxidase was blocked by immersing the sections in
3% H2O2 in 100% methanol for 20 min at room
temperature. Antigen retrieval was achieved by microwaving the
sections at 95°C for 10 min in 0.001 M citrate buffer (pH 6.7).
After blocking the endogenous peroxidase, the sections were
incubated with Protein Block Serum-Free (Dako) at room temperature
for 10 min to block the non-specific staining. The sections were
incubated for 2 h at room temperature with 1:100 diluted mouse
antibodies against monoclonal MTA1 (D40D1; Cell Signaling
Technology, Inc., Beverly, MA, USA) and polyclonal HDAC1 (ab19845;
Abcam, Cambridge, MA, USA). Peroxidase activity was detected with
the enzyme substrate, 3-amino-9-ethylcarbazole and for the negative
controls, the sections were incubated with Tris-buffered saline
(Wako Pure Chemical Industries, Tokyo, Japan) without the primary
antibodies. The samples with ≥10% of tumor cells were slightly
counterstained with Mayer hematoxylin and considered to show
positive staining. Immunohistochemical staining for MTA1/HDAC1 was
scored by two of the authors as positive or negative in the
epithelium that was under examination. An estimation of the
immunohistochemical expression of the markers was determined by
counting ≥100 cells in random high-power fields. The frequency of
positive cells for each antibody was reported semi-quantitatively
as follows: (−), No reaction; (+), mild with <30% of positive
cells; (++), moderate with 30–60% of positive cells; and (+++),
marked with >60% of positive cells. A positive expression was
defined as the staining of >30% of the cancer cells (++ or
+++).
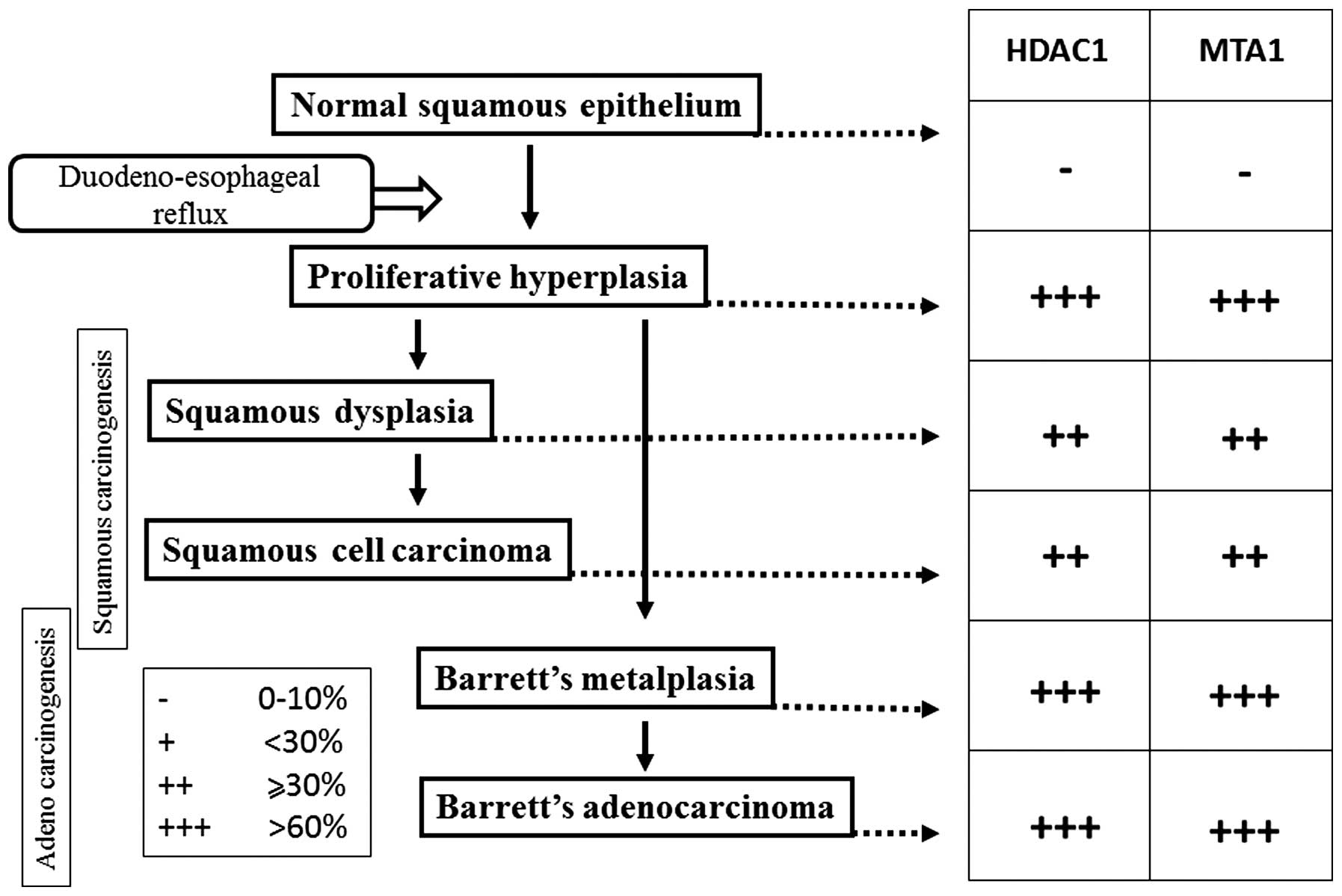
Definition of pathological findings
The histological findings in the esophagus were
classified into the following four categories: i) Proliferative
squamous hyperplasia (PHP), a condition characterized by a
thickened epithelium to twice that of a normal epithelium, with
acanthosis, elongation of the papillae and parakeratosis, as well
as thickening of the basal layer of the squamous epithelium and
preservation of a stratified appearance; ii) squamous dysplasia,
characterized by an epithelium composed of dysplastic squamous
cells with large and polymorphic nuclei with deeply stained
chromatin and an increased number of mitotic figures, which may
involve the lamina propria of the epithelium, however, do not
invade the submucosal layer; iii) Barrett’s metaplasia (BM),
presents replacement of the esophageal squamous epithelium with
columnar-lined epithelium comprised of gastric and/or intestinal
cells; and iv) carcinoma, defined as cellular and structural
atypism with epithelial invasion into the submucosal layer. ADC
consists of dysplastic glandular cell growth, with atypia and
invasiveness, and exhibits two types of histology: Tubular or
papillary ADC; and mucinous ADC. SCC is a type of squamous cell
dysplasia with marked cellular and structural atypism, which may be
divided into well- and poorly-differentiated types according to the
presence or absence of cancer pearls, respectively.
Results
Histological findings
In total, 40/50 rats survived following the surgery
and were used in the present study. Immunohistochemistry was
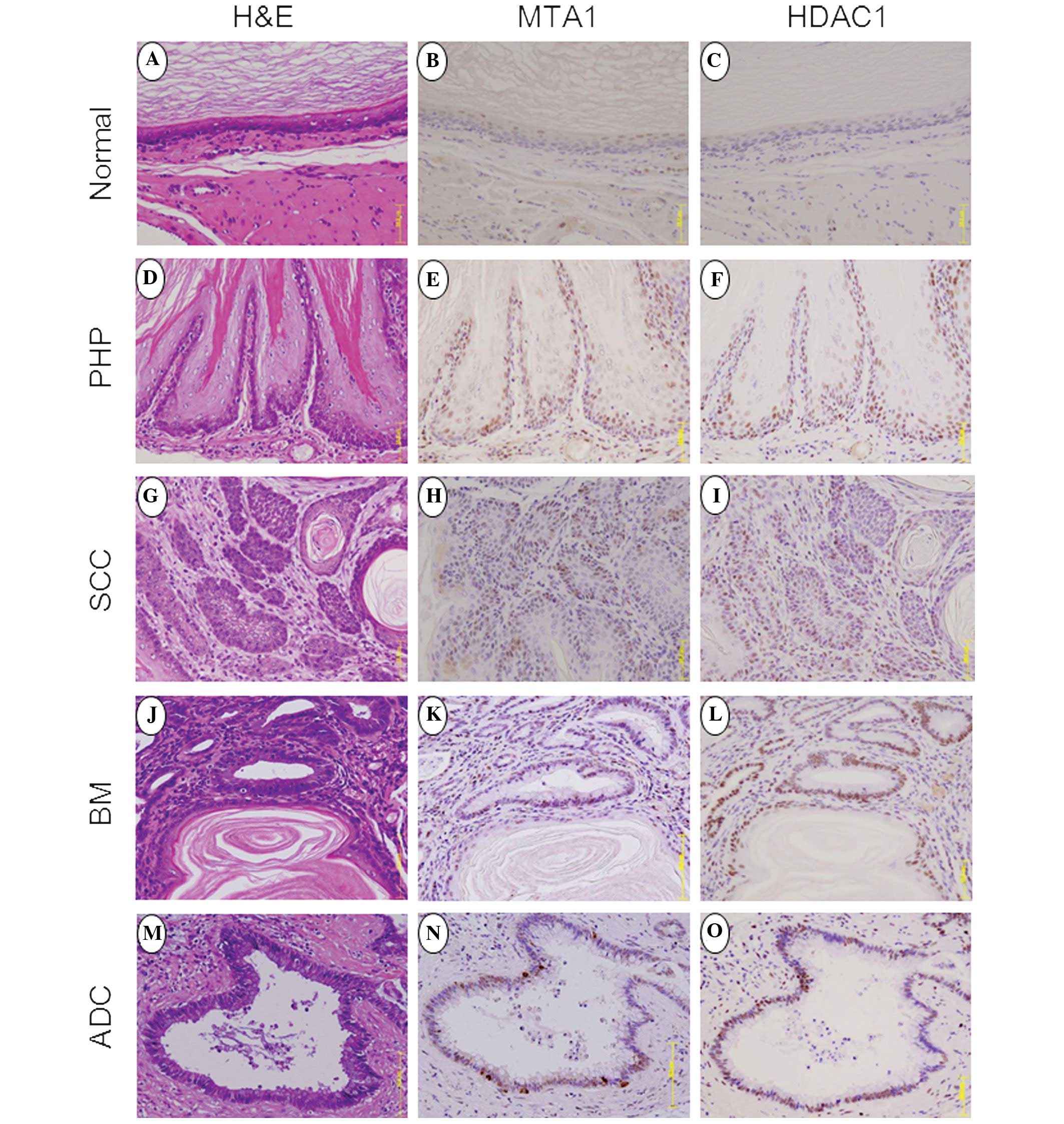
performed (Fig. 1) and demonstrated
normal esophageal epithelium in the upper esophagus at 20 weeks
post-surgery (Fig. 1A), as well as
squamous PHP (Fig. 1D), dysplasia
and BM. At 30–50 weeks following surgery, 13/35 (37%) rats had
developed esophageal cancer. In addition, SCC (Fig. 1G) was observed in 4/35 (11%) of the
rats. By contrast, dysplastic changes, including BM (Fig. 1J) and ADC (Fig. 1M), were observed within 30 weeks and
increased sequentially to 100 and 40%, respectively at 40–50 weeks
(Table I).
 | Table IOutcome and histological
findings. |
Table I
Outcome and histological
findings.
| Postoperative
week |
|---|
|
|
|---|
| 20 | 30 | 40 | 50 |
|---|
| Rats examined,
n | 5 | 10 | 10 | 15 |
| Histology, n
(%) |
| Proliferative
hyperplasia | 5 (100) | 10 (100) | 10 (100) | 15 (100) |
| Squamous
dysplasia | 1 (20) | 5 (50) | 6 (60) | 6 (40) |
| Squamous cell
carcinoma | 0 (0) | 1 (10) | 1 (10)a | 2 (13)a |
| Barrett’s
metaplasia | 2 (40) | 7 (70) | 10 (100) | 15 (100) |
|
Adenocarcinoma | 0 (0) | 1(10) | 4 (40) | 6 (40) |
MTA1 expression
A high positive expression of MTA1 was identified in
the basal layer of PHP (Fig. 1E),
but not in the normal epithelium at 20 weeks (Fig. 1B). At 30 weeks, BM showed a high
positive expression of MTA1 (Fig.
1K). ADC-associated BM showed a high positive expression of
MTA1 at 30–50 weeks post-surgery (Fig.
1N). By contrast, SCC demonstrated marginally reduced
expression of MTA1 at 30–50 weeks post-surgery (Fig. 1H). The MTA1 immunohistochemistry
staining showed nuclear expression of MTA1 in all of the stages of
squamous carcinogenesis, including PHP, squamous dysplasia and SCC,
and adenocarcinogenesis, including BM and ADC, however, this was
not observed in the normal squamous epithelium (Fig. 2).
HDAC1 expression
Similarly, the positive expression of HDAC1 was
found in PHP (Fig. 1F), BM
(Fig. 1L), ADC (Fig. 1O) and SCC (Fig. 1I), but not identified in the normal
squamous epithelium (Fig. 1C).
Furthermore, the HDAC1 immunohistochemical staining showed nuclear
expression of HDAC1 in all of the stages of squamous
carcinogenesis, including PHP, squamous dysplasia and SCC, and
adenocarcinogenesis, including BM and ADC, however, this was not
observed in the normal squamous epithelium (Fig. 2).
Discussion
The incidence of GERD-induced esophageal carcinoma
is rising in the USA and the Western world (4,5). We
have pioneered the use of a rat reflux model of esophageal
carcinoma, which is based on surgically inducing
duodenogastroesophageal reflux akin to GERD in humans without the
use of a carcinogen (8–10). The model has been successfully used
to investigate reflux-induced esophageal carcinogenesis. While the
correlation between reflux and esophageal carcinoma has been
investigated in a number of studies, the molecular mechanisms
underlying esophageal carcinogenesis remains poorly understood
(8–10).
Numerous molecular alterations leading to the
development of esophageal carcinoma have been reported (12). Chronically inflamed tissue results
in the activation of multiple signaling pathways that lead to
inflammation and tumorigenesis. A number of these factors, such as
NF-κB and Egr-1, have been shown to have additive or synergistic
effects on the activation of a number of inflammation-associated
genes, particularly those that are associated with the neoplastic
transformation (25).
The alterations of the chromatin structure by
HATs and HDACs are implicated in the regulation of
gene transcription, as well as in the process of carcinogenesis.
Tumors demonstrate the hyperacetylation of histone H4 and the
increased expression of HDAC1, thus implying that a certain
interaction may exist between the hyperacetylation of histone H4
and HDAC1 expression (21).
ΔNp63α, an N-terminally truncated form, which functions as a key
ESCC cell survival factor, associates with HDAC1 and
HDAC2 to form an active transcriptional repressor complex
that may be targeted to provide a therapeutic advantage. The
repression of the proapoptotic Bcl-2 family member genes, including
p53 upregulated modulator of apoptosis, by
p63/HDAC is required for the survival of ESCC cells
(26). Therefore, the
immunohistochemical determination of HDACs may aid with predicting
the response to specific HDAC inhibitors (27).
MTA is a newly discovered family of cancer
progression-associated genes (13).
MTA1, the first gene identified in this family, has been
repeatedly reported to be overexpressed along with its protein
product, MTA1, in a wide range of human cancers. Therefore, MTA1
may be one of the significant molecules in cancer progression.
Esophageal cancers have been investigated for MTA1/MTA1
overexpression and those esophageal cancer cells that were
overexpressing MTA1/MTA1 have shown significantly higher
frequencies of adventitial invasion and lymph node metastasis, as
well as higher rates of lymphatic involvement (28). Thus, the MTA1 protein may be a
useful predictor of the malignant potential of ESCC (14).
ATP-dependent chromatin-remodeling complexes open
chromatin structures and facilitate transcriptional activation. A
novel human complex, NURD, contains ATP-dependent nucleosome
remodeling activity and HDAC activity that associate with
transcriptional repression. The NuRD complexes share four core
proteins (HDAC1, HDAC2, RbAp46 and RbAp48) with the Sin3 complex.
These complexes contain the dermatomyositis-specific autoantigens,
Mi-2α/β, MTA1/2 and p66, which are functionally and physically
linked (29–33).
The MTA1 protein, a component of the NuRD complex,
possesses strong transcription-repressing activity (30). MTA2, another component of the NuRD
complex, is highly expressed in rapidly dividing cells (32). Toh et al (34) reported the physical interaction
between the MTA1 protein and HDAC1. The MTA protein family members
basic functions of chromatin remodeling and histone deacetylating
activities are exerted via NuRD complexes. While there are
additional non-histone deacetylating proteins in NuRD complexes,
the MTA proteins are likely to be the principal components. In
addition, the MTA-NuRD complexes show transcription-repression
activities (35,36). Although all MTA protein family
members are found in NuRD complexes, each MTA protein may form a
distinct NuRD complex that targets different sets of promoters
(33). Thus, the MTA-HDAC complex
is further involved in the normal transcriptional balance of the
cell (37).
HDAC, via NuRD complexes containing MTA proteins,
deacetylates, chromatin histones and non-histone proteins. The p53
tumor suppressor protein was the first non-histone protein reported
to be deacetylated by MTA protein-containing NuRD complexes
(23,37). A HDAC1 complex, which contains the
MTA2 protein mediates the deacetylation of p53. An MTA2-associated
NuRD complex is also involved, and this HDAC1/MTA2 complex
interacts with p53 in vitro and in vivo, which
significantly reduces the steady-state levels of acetylated p53.
The deacetylation of p53 causes an increase in its own degradation
through MDM2 and a reduction in p53-dependent transcriptional
activation. Eventually, this results in the repression of the
normal p53 function that mediates cell growth arrest and apoptosis
(23,38). The same phenomenon is observed
between the p53 and MTA1 complex (23,24).
Previous studies have determined that the HDAC1/MTA1 complexes
deacetylate the p53 protein and attenuate the transactivation
function of p53 in human carcinoma, thereby inhibiting p53-induced
apoptosis (18,24). The MTA proteins have been shown to
be ubiquitinated transcriptional corepressors, which function in
HDAC and are part of the NuRD complex, a nucleosome remodeling and
HDAC complex whose stability appears to be regulated by the binding
of ubiquitinated MTA1 to E3 ubiquitin ligase constitutive
photomorphogenesis protein-1 (37,39).
The MTA1 protein inhibits p53-induced apoptosis by deacetylation of
p53, which may be associated with the increased metastatic
potential of cancer cells with high MTA1 expression (23,24).
Miyatani et al (40) examined a possible association
between HDAC1 and MTA1 expression, and disease progression in the
esophageal metaplasia-dysplasia-carcinoma sequence and particularly
in low-grade dysplasia. The percentage of HDAC1- and MTA1-positive
expression in low-grade dysplasia of BM was as high as that of
cancer. Therefore, BM with increased HDAC1 and MTA1 expression is
considered to be a precancerous lesion. The results of the current
study showed that MTA1 and HDAC1 expression is already present in
PHP and BM. The positive expression rate of MTA1 and HDAC1 was
similar to that among PHP, BM and EADC. Thus, increased MTA1 and
HDAC1 expression indicates that PHP and BM may already have
malignant potential.
Bonde et al (12) established that cell lines derived
from a reflux-associated rat model of esophageal cancer exhibited
glandular features. While the histology of the xenografts expressed
a squamous phenotype when they were transplanted into nude mice.
Cytogenetic analysis also showed significant similarities between
rat and human esophageal cancers, including ESCC and EADC.
Furthermore, cytogenetic analysis of the rat model revealed a
highly aneuploid cell population with the derangement of key
chromosomes that encode a variety of oncogenes. The neoplastic
nature, gene expression profile and pathway activation of these
rodent reflux-induced tumors was found to be comparable with human
esophageal cancer. In rats with BM and EADC, the deletion and
translocation of chromosome 7 and 11, and overexpression of
important peptide mediators are considered to be significant in
carcinogenesis, including hypoxia-inducible factor (HIF) 1α, cyclin
dependent kinase 4, vascular endothelial growth factor, polo-like
kinase and the epidermal growth factor receptor.
HIF1α is a key regulator of angiogenic factors
(41) and another important
non-histone protein that is deacetylated by the HDAC1/MTA1
complexes. The MTA1 protein binds to and deacetylates HIF1α, which
increases HDAC1 expression and subsequently stabilizes HIF1α via a
positive feedback mechanism (23).
The expression of HDAC1 and MTA1 is similar in squamous
carcinogenesis and adenocarcinogenesis. Therefore, esophageal
squamous and adeno differentiation may possess a common genetic
pathway.
Histologically, the spectrum of esophageal cancer is
divided into ESCC and EADC. However, the mechanism by which each
histological type of carcinoma arises from the esophageal mucosa
remains unknown. In our previous study, the rodents received one of
the following procedures: Duodeno-forestomach reflux with reduced
exposure to duodenal contents; duodenoesophageal reflux with
increased exposure to duodenal contents; or three control
operations. The fraction of ESCC in the reduced exposure group was
significantly higher than that of the high exposure group, while
the fraction of EADC in the reduced exposure group was
significantly lower than that of the high exposure group. In brief,
high exposure to duodenal contents promotes the development of
EADC, and low exposure induces ESCC. The severity of the
duodenoesophageal reflux in rodents is associated with the
development of different histological types of esophageal carcinoma
(11). In the present study, ESCC
was shown to arise from dysplastic changes to squamous cells that
were naturally located proximal to the columnar-lined epithelium.
By contrast, EADC arose in the areas of columnar-lined epithelium
adjacent to the surgical anastomosis.
MTA1 expression levels are closely associated with
the degree of malignant potential. For example, metastatic
malignant tissue demonstrates increased expression when compared
with tissue from high-grade prostatic intraepithelial neoplasia
(42). In the present study,
squamous PHP was found to express MTA1 and HDAC1 and therefore, PHP
may have malignant potential, which chemopreventive agents could be
directed against.
The present study was not designed to completely
evaluate the esophageal cancer signaling pathway. However, the
results are consistent with those of previous studies, which
demonstrate that the MTA1/HDAC1 complexes are involved in
other malignancies. This study also demonstrates the involvement of
these complexes in esophageal cancer.
Thus, it has been proposed that the MTA and HDAC
proteins, particularly MTA1 and HDAC1, present as master
coregulatory molecules involved in esophageal carcinogenesis.
The HDAC inhibitors have been shown to be potent
inducers of growth arrest, differentiation and/or apoptotic cell
death of transformed cells and, as a result, are currently
receiving considerable attention as antitumor agents. In addition,
the HDAC inhibitors have been reported to disrupt the cell cycle in
the G2 phase, allowing cells to prematurely enter the M phase, as
well as to interfere directly with the mitotic spindle checkpoint
(43).
Kai et al (20) reported a novel pathway of chromatin
remodeling by resveratrol (Res) via the functional restriction of
the MTA1/NuRD complex; Res decreased MTA1 expression, thus
deregulating the MTA1/HDAC1 complexes leading to increased
p53 acetylation (Ac-p53), and enhanced binding to the p21 and Bax
promoters in the PCa cells. Furthermore, Res treatment on MTA1-null
background resulted in a marked increase in the apoptotic function
of Res, which indicates MTA1 as an antiapoptotic protein. Finally,
the combined treatment of Res and the HDAC inhibitor, SAHA,
cooperatively increased Ac-p53 levels and apoptosis, indicating
novel therapeutic strategies in combination with the use of HDAC
inhibitors that have already been clinically approved (20). Thus it is necessary to administer
the HDAC inhibitor and Res for the treatment of GERD patients at an
early stage.
In conclusion, the expression of HDAC1 and MTA1 may
be required to promote the development of neoplastic transformation
in cancer cells with squamous and adeno differentiation.
Furthermore, the HDAC inhibitor and administration of Res may
prevent esophageal carcinogenesis.
Abbreviations:
|
ESCC
|
esophageal squamous cell carcinoma
|
|
EADC
|
esophageal adenocarcinoma
|
|
HDAC
|
histone deacetylases
|
|
MTA
|
metastasis-associated gene
|
|
GERD
|
gastroesophageal reflux disease
|
References
|
1
|
Hongo M, Nagasaki Y and Shoji T:
Epidemiology of esophageal cancer: Orient to occident. Effects of
chronology, geography and ethnicity. J Gastroenterol Hepatol.
24:729–735. 2009.
|
|
2
|
Wheeler JB and Reed CE: Epidemiology of
esophageal cancer. Surg Clin North Am. 92:1077–1087. 2012.
|
|
3
|
Bollschweiler E, Wolfgarten E, Gutschow C
and Hölscher AH: Demographic variations in the rising incidence of
esophageal adenocarcinoma in white males. Cancer. 92:549–555.
2001.
|
|
4
|
Reid BJ, Li X, Galipeau PC and Vaughan TL:
Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new
synthesis. Nat Rev Cancer. 10:87–101. 2010.
|
|
5
|
Engel LS, Chow WH, Vaughan TL, et al:
Population attributable risks of esophageal and gastric cancers. J
Natl Cancer Inst. 95:1404–1413. 2003.
|
|
6
|
Lagergren J, Bergström R, Lindgren A and
Nyrén O: Symptomatic gastroesophageal reflux as a risk factor for
esophageal adenocarcinoma. N Engl J Med. 340:825–831. 1999.
|
|
7
|
Jankowski JA, Harrison RF, Perry I,
Balkwill F and Tselepis C: Barrett’s metaplasia. Lancet.
356:2079–2085. 2000.
|
|
8
|
Miwa K, Segawa M, Takano Y, et al:
Induction of oesophageal and forestomach carcinomas in rats by
reflux of duodenal contents. Br J Cancer. 70:185–189. 1994.
|
|
9
|
Miwa K, Sahara H, Segawa M, et al: Reflux
of duodenal or gastro-duodenal contents induces esophageal
carcinoma in rats. Int J Cancer. 67:269–274. 1996.
|
|
10
|
Miyashita T, Ohta T, Fujimura T, et al:
Duodenal juice stimulates oesophageal stem cells to induce
Barrett’s oesophagus and oesophageal adenocarcinoma in rats. Oncol
Rep. 15:1469–1475. 2006.
|
|
11
|
Miyashita T, Miwa K, Fujimura T, et al:
The severity of duodeno-esophageal reflux influences the
development of different histological types of esophageal cancer in
a rat model. Int J Cancer. 132:1496–1504. 2013.
|
|
12
|
Bonde P, Sui G, Dhara S, et al:
Cytogenetic characterization and gene expression profiling in the
rat reflux-induced esophageal tumor model. J Thorac Cardiovasc
Surg. 133:763–769. 2007.
|
|
13
|
Nawa A, Nishimori K, Lin P, et al: Tumor
metastasis-associated human MTA1 gene: its deduced protein
sequence, localization, and association with breast cancer cell
proliferation using antisense phosphorothioate oligonucleotides. J
Cell Biochem. 79:202–212. 2000.
|
|
14
|
Toh Y, Ohga T, Endo K, et al: Expression
of the metastasis-associated MTA1 protein and its relationship to
deacetylation of the histone H4 in esophageal squamous cell
carcinomas. Int J Cancer. 110:362–367. 2004.
|
|
15
|
Toh Y, Pencil SD and Nicolson GL: Analysis
of the complete sequence of the novel metastasis-associated
candidate gene, mta1, differentially expressed in mammary
adenocarcinoma and breast cancer cell lines. Gene. 159:97–104.
1995.
|
|
16
|
Iguchi H, Imura G, Toh Y and Ogata Y:
Expression of MTA1, a metastasis-associated gene with histone
deacetylase activity in pancreatic cancer. Int J Oncol.
16:1211–1214. 2000.
|
|
17
|
Higashijima J, Kurita N, Miyatani T, et
al: Expression of histone deacetylase 1 and metastasis-associated
protein 1 as prognostic factors in colon cancer. Oncol Rep.
26:343–348. 2011.
|
|
18
|
Nicolson GL, Nawa A, Toh Y, Taniguchi S,
Nishimori K and Moustafa A: Tumor metastasis-associated human MTA1
gene and its MTA1 protein product: role in epithelial cancer cell
invasion, proliferation and nuclear regulation. Clin Exp
Metastasis. 20:19–24. 2003.
|
|
19
|
Moon WS, Chang K and Tarnawski AS:
Overexpression of metastatic tumor antigen 1 in hepatocellular
carcinoma: Relationship to vascular invasion and estrogen
receptor-alpha. Hum Pathol. 35:424–429. 2004.
|
|
20
|
Kai L, Samuel SK and Levenson AS:
Resveratrol enhances p53 acetylation and apoptosis in prostate
cancer by inhibiting MTA1/NuRD complex. Int J Cancer.
126:1538–1548. 2010.
|
|
21
|
Toh Y, Yamamoto M, Endo K, et al: Histone
H4 acetylation and histone deacetylase 1 expression in esophageal
squamous cell carcinoma. Oncol Rep. 10:333–338. 2003.
|
|
22
|
Sun WJ, Zhou X, Zheng JH, et al: Histone
acetyltransferases and deacetylases: molecular and clinical
implications to gastrointestinal carcinogenesis. Acta Biochim
Biophys Sin (Shanghai). 44:80–91. 2012.
|
|
23
|
Toh Y and Nicolson G: MTA1 of the MTA
(metastasis-associated) gene family and its encoded proteins:
molecular and regulatory functions and role in human cancer
progression. Atlas Genet Cytogenet Oncol Haematol. 15:303–315.
2011.
|
|
24
|
Moon HE, Cheon H and Lee MS:
Metastasis-associated protein 1 inhibits p53-induced apoptosis.
Oncol Rep. 18:1311–1314. 2007.
|
|
25
|
Abdel-Latif MM, Duggan S, Reynolds JV and
Kelleher D: Inflammation and esophageal carcinogenesis. Curr Opin
Pharmacol. 9:396–404. 2009.
|
|
26
|
Ramsey MR, He L, Forster N, Ory B and
Ellisen LW: Physical association of HDAC1 and HDAC2 with p63
mediates transcriptional repression and tumor maintenance in
squamous cell carcinoma. Cancer Res. 71:4373–4379. 2011.
|
|
27
|
Langer R, Mutze K, Becker K, et al:
Expression of class I histone deacetylases (HDAC1 and HDAC2) in
oesophageal adenocarcinomas: an immunohistochemical study. J Clin
Pathol. 63:994–998. 2010.
|
|
28
|
Toh Y, Kuwano H, Mori M, Nicolson GL and
Sugimachi K: Overexpression of metastasis-associated MTA1 mRNA in
invasive oesophageal carcinomas. Br J Cancer. 79:1723–1726.
1999.
|
|
29
|
Tong JK, Hassig CA, Schnitzler GR,
Kingston RE and Schreiber SL: Chromatin deacetylation by an
ATP-dependent nucleosome remodelling complex. Nature. 395:917–921.
1998.
|
|
30
|
Xue Y, Wong J, Moreno GT, Young MK, Côté J
and Wang W: NURD, a novel complex with both ATP-dependent
chromatin-remodeling and histone deacetylase activities. Mol Cell.
2:851–861. 1998.
|
|
31
|
Wade PA, Gegonne A, Jones PL, Ballestar E,
Aubry F and Wolffe AP: Mi-2 complex couples DNA methylation to
chromatin remodelling and histone deacetylation. Nat Genet.
23:62–66. 1999.
|
|
32
|
Zhang Y, Ng HH, Erdjument-Bromage H,
Tempst P, Bird A and Reinberg D: Analysis of the NuRD subunits
reveals a histone deacetylase core complex and a connection with
DNA methylation. Genes Dev. 13:1924–1935. 1999.
|
|
33
|
Bowen NJ, Fujita N, Kajita M and Wade PA:
Mi-2/NuRD: multiple complexes for many purposes. Biochim Biophys
Acta. 1677:52–57. 2004.
|
|
34
|
Toh Y, Kuninaka S, Endo K, et al:
Molecular analysis of a candidate metastasis-associated gene, MTA1:
possible interaction with histone deacetylase 1. J Exp Clin Cancer
Res. 19:105–111. 2000.
|
|
35
|
Kumar R, Wang RA and Bagheri-Yarmand R:
Emerging roles of MTA family members in human cancers. Semin Oncol.
30(Suppl 16): S30–S37. 2003.
|
|
36
|
Manavathi B, Peng S, Rayala SK, et al:
Repression of Six3 by a corepressor regulates rhodopsin expression.
Proc Natl Acad Sci USA. 104:13128–13133. 2007.
|
|
37
|
Toh Y and Nicolson GL: The role of the MTA
family and their encoded proteins in human cancers: molecular
functions and clinical implications. Clin Exp Metastasis.
26:215–227. 2009.
|
|
38
|
Luo J, Su F, Chen D, Shiloh A and Gu W:
Deacetylation of p53 modulates its effect on cell growth and
apoptosis. Nature. 408:377–381. 2000.
|
|
39
|
Li DQ, Ohshiro K, Reddy SD, et al: E3
ubiquitin ligase COP1 regulates the stability and functions of
MTA1. Proc Natl Acad Sci USA. 106:17493–17498. 2009.
|
|
40
|
Miyatani T, Kurita N, Mikami C, et al:
Malignant potential of Barrett’s esophagus: special reference to
HDAC-1 and MTA-1 expression. Hepatogastroenterology. 58:472–476.
2011.
|
|
41
|
Yoo YG, Na TY, Seo HW, et al: Hepatitis B
virus X protein induces the expression of MTA1 and HDAC1, which
enhances hypoxia signaling in hepatocellular carcinoma cells.
Oncogene. 27:3405–3413. 2008.
|
|
42
|
Hofer MD, Kuefer R, Varambally S, et al:
The role of metastasis-associated protein 1 in prostate cancer
progression. Cancer Res. 64:825–829. 2004.
|
|
43
|
Taddei A, Roche D, Bickmore WA and
Almouzni G: The effects of histone deacetylase inhibitors on
heterochromatin: implications for anticancer therapy? EMBO Rep.
6:520–524. 2005.
|