Introduction
Hepatocellular carcinoma (HCC) is one of the most
common and rapidly fatal malignant tumors worldwide. Its invasion
and metastasis are the main factors that influence the prognosis.
Previous studies have indicated that the invasion and metastasis of
HCC are the combined results of multiple genes. Hypoxia-inducible
factor 1α (HIF-1α) is a transcription regulation factor that is
closely associated with the development of malignant tumors. Its
high expression in human malignant tumors had been confirmed
widely. Tumor oxygen condition and gene variation have been found
to synergistically regulate the expression levels and activity of
HIF-1α (1). Matrix
metalloproteinases (MMPs) can damage the degradation balance of the
extracellular matrix, and thereby promote cancer cells to break
through the histological barrier, invade the adjacent tissues and
metastasize to distant tissues (2).
Matrix metalloproteinase 2 (MMP2) is capable of degrading the
majority of components of the extracellular matrix. It is widely
believed that the effect of MMP2 on the extracellular matrix is
closely associated with tumor invasion and metastasis (3–9). In
the present study, quantitative polymerase chain reaction (qPCR)
and immunohistochemistry were used to detect the expression levels
of MMP2 and HIF-1α in HCC tissues; to analyze the association
between the expression levels of MMP2 and HIF-1α and the clinical
pathological characteristics of HCC; and to analyze their effect on
the survival period of patients with HCC. This study has
contributed to investigating the pathogenesis of HCC, and provides
guidance for the clinical diagnosis and prognosis determination of
HCC in the future.
Materials and methods
Common data
From January 2011 to June 2012, a total of 45
patients with HCC, who had undergone hepatectomy and were
pathologically diagnosed with HCC at Renmin Hospital of Wuhan
University (Wuhan, China), were enrolled in the present study. All
patients had not accepted radiotherapy and chemotherapy. A total of
45 samples of HCC tissue were collected. However, only 33
corresponding adjacent normal tissue were collected as the removal
of the adjacent normal tissue failed in 12 patients. The adjacent
tissue samples were collected at a distance of 3 cm from the tumor
tissues during surgery, and were confirmed to contain no cancer
cells by hematoxylin and eosin staining of the biopsies. Specimens
were immediately cut into two parts: One of which was rapidly
cryopreserved in liquid nitrogen at −80°C until required for qPCR;
and the other of which was fixed in 10% formalin for 24 h, then
underwent immunohistochemical examination after being embedded in
paraffin.
The patients included 34 males and 11 females, whose
age ranged from 36 to 78 years old. The histological types of all
HCC specimens were graded in terms of differentiation degree, as
follows: 12 well differentiated, 20 moderately differentiated, and
13 poor differentiated. In total, 15 patients (33.3%) had
extrahepatic metastasis and/or intrahepatic metastasis and 10
(22.2%) had lymph node metastasis. Tumor stage was determined
according to the International Union Against Cancer TNM staging
system (10). All patients accepted
conventional pharmacotherapy in the outpatient clinic, which
included physical examination, B-ultrasound, computed tomography
and tumor marker examination, as well as regular follow-up. The
final follow-up date was December 31st, 2012. In total, 41 cases
achieved complete follow-up and the remaining four were lost to
follow-up.
The study was carried out in accordance with the
Declaration of Helsinki. The Scientific Ethics Committee of Renmin
Hospital of Wuhan University approved the study (approval no. KF
01-143/03), and written informed consent was obtained from all
patients and their dependents prior to the start of the study.
Main reagents
TRIzol reagent was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA), reverse transcription kit was
purchased from Toyobo Co., Ltd. (Tokyo, Japan) and SYBR Green I
fluorochrome was obtained from Biotium, Inc. (Hayward, CA, USA).
PCR primers were synthesized by Shanghai Yingjun Life Technologies
Co., Ltd (Shanghai, China), and rabbit anti-human MMP2 polyclonal
and mouse anti-human HIF-1α monoclonal antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The MMP2
goat anti-rabbit polyclonal IgG (H-76; sc-10736) and HIF-1α goat
anti-mouse monoclonal (28b; sc-13515) secondary antibodies were
purchased from Santa Cruz Biotechnology, Inc. The SP-9000
immunohistochemistry kit and the DAB chromogenic kit (brown-yellow)
were purchased from Boster Biological Technology Co., Ltd. (Wuhan,
China).
qPCR
Total RNA was extracted using TRIzol reagent and
quantified by using an ultraviolet spectrophotometer (L-5; Shanghai
Precision Instrument Co., Ltd., Shanghai, China). cDNA was
synthesized by random primer reverse transcription. qPCR was
performed with SYBR Green I fluorescent dye technology. The
upstream primer of MMP2 was 5′-GGA ATG CCA TCC CCG ATA AC-3′ and
the downstream primer was 5′-CAG CCT AGC CAG CCA GTC GGA TTT-3′.
The upstream primer of HIF-1α was 5′-TGA AGT GTA CCC TAC CCT AAC
TAG CCG-3′ and the downstream primer was
5′-AATCAGCACCAAGCAGGTCATAG-3′. The upstream primer of β-actin was
5′-AAG GCC AGG TAA TTG TCA CG-3′, and the downstream primer was
5′-AGC AGC TCT GCA GTA CGT C-3′. The capacity of the PCR reaction
system was 20 μl. The cycling conditions were as follows:
Initialization for 4 min at 94°C, followed by denaturation at 95°C
for 15 sec, annealing at 55°C for 30 sec and extension at 75°C for
45 sec. This was repeated for 45 working cycles. Finally, the
specificity of the PCR products was confirmed by drawing
dissolution curves. The results of the qPCR were analyzed by the
2−ΔΔCt method. The normal liver tissue was taken as a
calibration sample in this experiment.
Immunohistochemistry
Immunohistochemistry was conducted according to the
manufacturer’s instructions of the SP-9000 kit (Boster Biological
Technology Co., Ltd.). Self-tissue controls were taken as positive
control, and the negative control included phosphate-buffered
saline instead of the primary antibody. The evaluation standard was
as follows: All cells were counted in 10 randomly selected high
power fields, and semi-quantitative results were evaluated on the
basis of the degree of staining and the percentage of stained
cells. The paraffin sections were dewaxed for antigen repair and
following elimination of the endogenous peroxidase activity, the
sections were blocked with goat serum fluid. Next, the unlabeled
primary antibody was added and incubated for 1–2 h. The labeled
secondary antibody was then added and incubated for 15 min,
followed by the horseradish peroxidase labeled streptavidin for 15
min. The DAB chromogenic kit was used to stain the tissue sections,
which was followed by restaining with hematoxylin. The sections
were observed under light microscope, with the positive cells
showing brown-yellow staining. The degree of cytoplasmic staining
was scored as follows: 0, no or negligible staining; 1, pale yellow
staining; 2, brown-yellow staining; 3, brown staining.
Additionally, the percentage of positively stained cells was scored
as follows: 0, <5% of total cells; 1, 5–25%, of total cells; 2,
>25–50% of total cells; and 3, >50% of total cells. The sum
of the two scores was regarded as the final result; −, a total
score of 0 or 1; +, a total score of 2; ++ a total score of 3–4;
and +++, a total score of >5 (4). Samples with final scores of − or +
were classified as the negative group, while those with scores of
++ were classified as the positive group.
Statistical analysis
The different groups were compared according to the
baseline characteristics. Measurement data between two groups were
compared by t-tests and among multiple groups by analysis of
variance. Correlation analysis was performed by linear regression.
Results of the immunohistochemical staining were analyzed by
χ2 test and Spearman’s correlation analysis. The
Kaplan-Meier method was used for survival analysis. SPSS 15.0
software (SPSS Inc, Chicago, IL, USA) was used for all data
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of MMP2 and HIF-1α mRNA in HCC
tissues
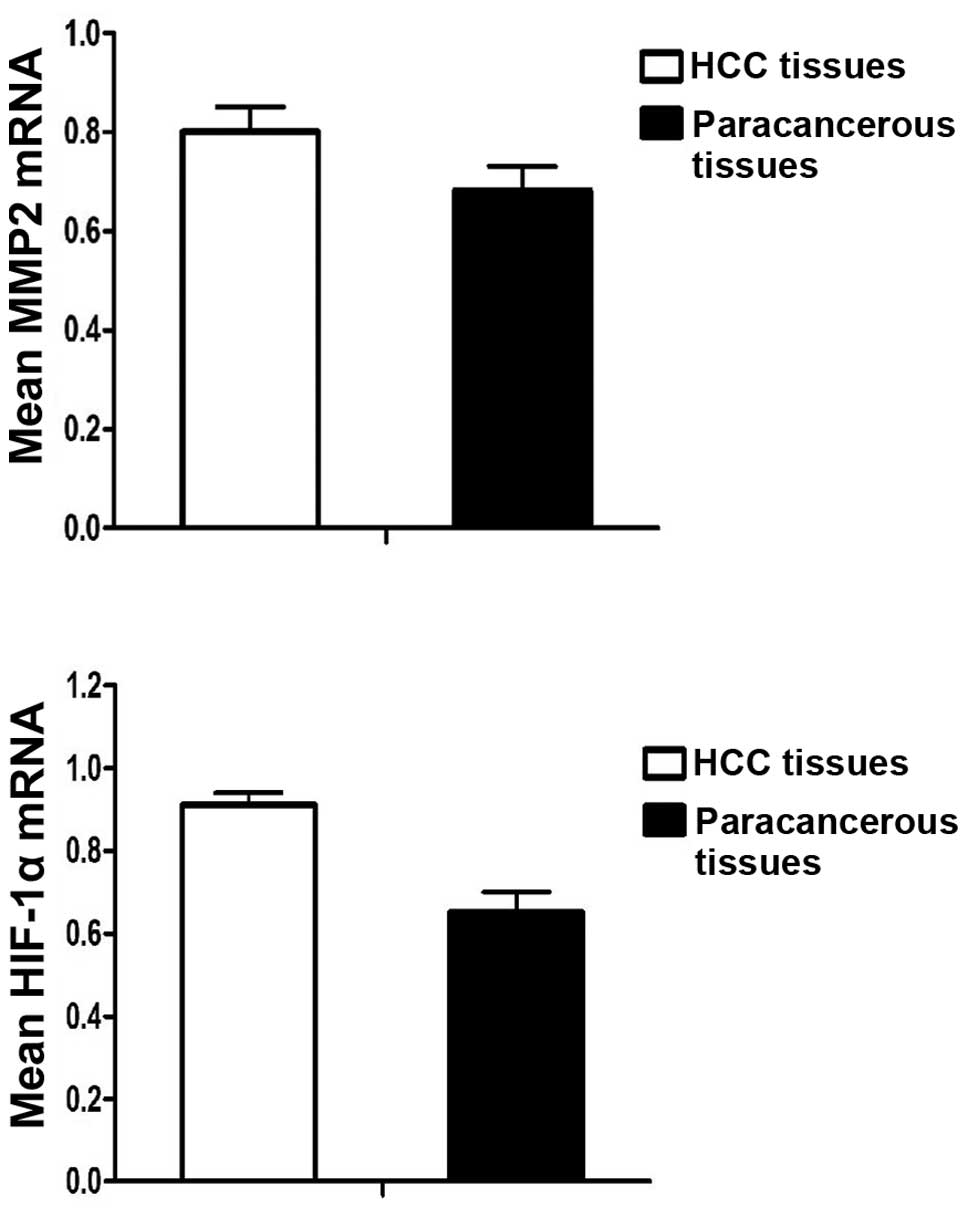
The average expression levels of MMP2 mRNA were
0.80±0.19 in the 45 HCC tissues, but 0.68±0.15 in the paracancerous
tissues. The expression levels of MMP2 mRNA in the HCC tissues were
significantly higher than those in the paracancerous tissues
(P=0.001). The expression levels of HIF-1α mRNA were 0.91±0.011 in
the HCC tissues, but 0.65±0.19 in the paracancerous tissues. The
expression levels of HIF-1α mRNA in the HCC tissues were
significantly higher than those in the paracancerous tissues
(P<0.001) (Table I and Fig. 1).
 | Table IThe expression levels of MMP2 and
HIF-1α mRNA in HCC and paracancerous tissues
(2−ΔΔCt). |
Table I
The expression levels of MMP2 and
HIF-1α mRNA in HCC and paracancerous tissues
(2−ΔΔCt).
| Group | n | MMP2 | HIF-1α |
|---|
| HCC | 33 | 0.80±0.19 | 0.91±0.11 |
| Paracancerous | 33 | 0.68±0.15 | 0.65±0.19 |
| P-value | | 0.001 | <0.001 |
Expression of MMP2 and HIF-1α protein in
HCC tissues
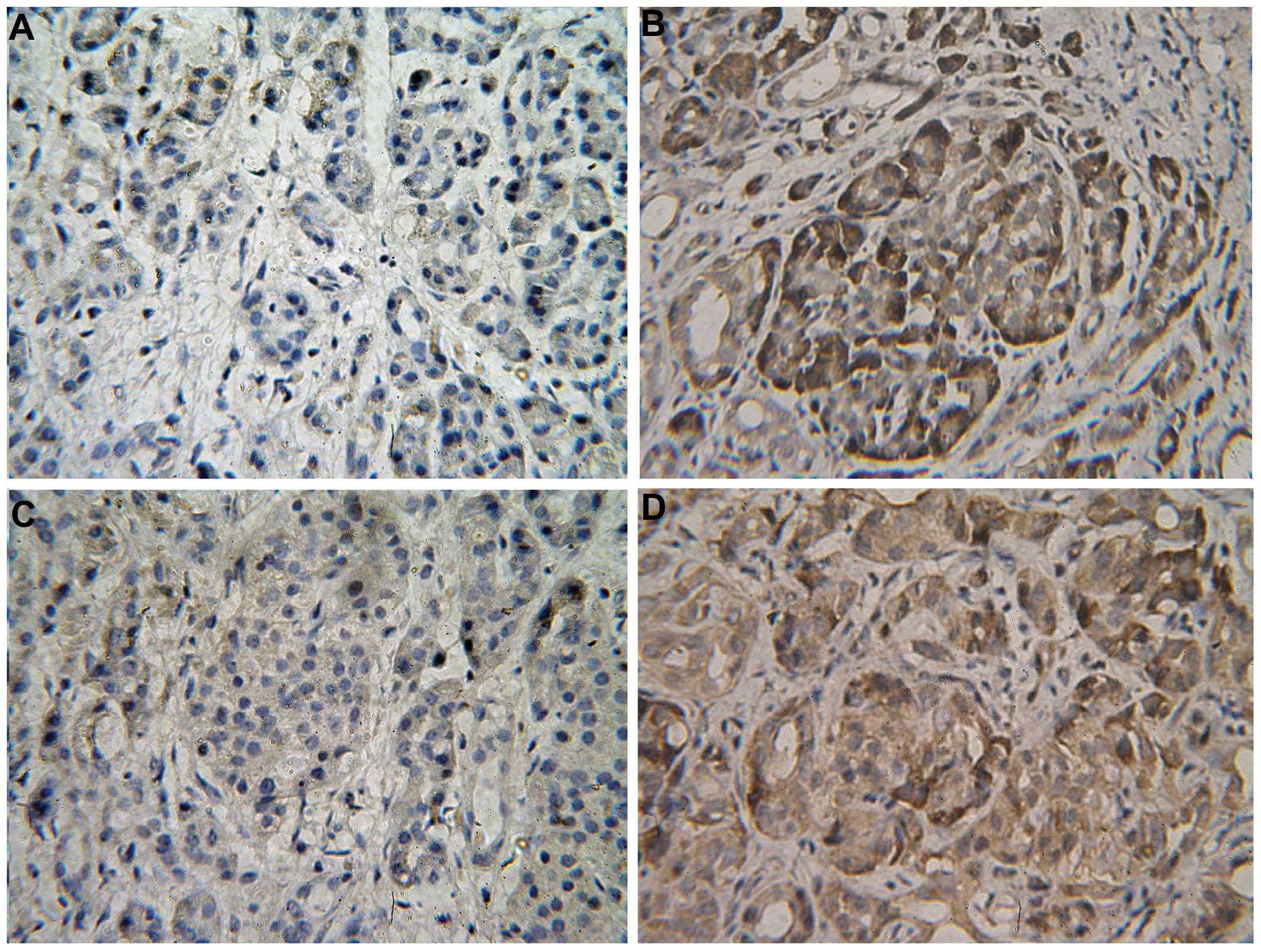
MMP2 protein was identified to be expressed in the
cytoplasm and cell membrane, while HIF-1α protein was found to be
expressed in the cytoplasm and nucleolus (Fig. 2). The two proteins appeared as brown
or buffy particles by immunohistochemical staining. There were 28
cases of positive expression of MMP2 protein among 45 HCC tissues,
and 32 of HIF-1α. The positive expression rate of MMP2 and HIF-1α
protein was 62.2 and 71.1%, respectively. However, only 8 cases
demonstrated positive expression of MMP2 protein and only 10
demonstrated positive expression of HIF-1α protein, among the 33
paracancerous tissues. The positive expression rate of MMP2 and
HIF-1α protein in the paracancerous tissues was 24.2 and 30.3%
(Table II). The expression levels
of HIF-1α and MMP2 in the HCC tissues were significantly higher
than those in the paracancerous tissues (P<0.05).
 | Table IIThe expression of MMP2 and HIF-1α
protein in HCC and paracancerous tissues. |
Table II
The expression of MMP2 and HIF-1α
protein in HCC and paracancerous tissues.
| | Expression, n
(%) |
|---|
| |
|
|---|
| Group | n | MMP2-positive | HIF-1α-positive |
|---|
| HCC | 45 | 28 (62.22) | 32 (71.11) |
| Paracancerous | 33 | 8 (24.24) | 10 (30.30) |
| P-value | | 0.003 | 0.001 |
Correlation between MMP2/HIF-1α protein
expression and clinicopathological features
The results showed that the expression of MMP2 and
HIF-1α protein was not associated with patient age, gender and
histological grade, but was associated with tumor size, metastasis,
capsule formation and TNM stage (P<0.05). ). In addition, the
AFP levels were not found to correlate with MMP2 protein
expression, but associated with HIF-1α protein expression. The
expression levels of MMP2 and HIF-1α mRNA and its protein were
significantly high when the tumor had a diameter >5 cm, was
intrahepatic, exhibited portal metastasis and was of TNM stage III
or IV (Tables III and IV).
 | Table IIICorrelation between MMP2 and HIF-1α
mRNA expression and clinicopathological features. |
Table III
Correlation between MMP2 and HIF-1α
mRNA expression and clinicopathological features.
| Clinicopathological
features | MMP2 mRNA | P-value | HIF-1α mRNA | P-value |
|---|
| Age, years | | 0.679 | | 0.685 |
| ≤59 | 0.86±0.18 | | 0.90±0.13 | |
| ≥60 | 0.83±0.16 | | 0.84±0.08 | |
| Gender | | 0.566 | | 0.744 |
| Male | 0.90±0.16 | | 0.88±0.11 | |
| Female | 0.80±0.17 | | 0.86±0.12 | |
| AFP level, ng/l | | 0.234 | | 0.197 |
| >400 | 0.84±0.18 | | 0.89±0.13 | |
| ≤400 | 0.75±0.16 | | 0.74±0.08 | |
| Histological
grade | | 0.418 | | 0.279 |
| High
differentiation | 0.80±0.15 | | 0.88±0.11 | |
| Middle
differentiation | 0.83±0.20 | | 0.89±0.12 | |
| Low
differentiation | 0.91±0.13 | | 0.82±0.08 | |
| Tumor diameter,
cm | | 0.033 | | 0.030 |
| ≤5cm | 0.75±0.21 | | 0.82±0.08 | |
| >5cm | 0.91±0.16 | | 0.91±0.12 | |
| Metastasis | | 0.021 | | 0.049 |
| Positive | 0.90±0.13 | | 0.90±0.12 | |
| Negative | 0.76±0.18 | | 0.82±0.08 | |
| Capsule | | 0.012 | | 0.018 |
| Positive | 0.75±0.12 | | 0.81±0.12 | |
| Negative | 0.91±0.19 | | 0.90±0.07 | |
| TNM stage | | 0.006 | | 0.016 |
| I and II | 0.75±0.19 | | 0.81±0.07 | |
| III and IV | 0.91±0.06 | | 0.91±0.03 | |
 | Table IVCorrelation with MMP2 and HIF-1α
protein expression and clinicopathological features. |
Table IV
Correlation with MMP2 and HIF-1α
protein expression and clinicopathological features.
| MMP2 protein, n | HIF-1α protein,
n |
|---|
|
|
|
|---|
| Clinicopathological
features | Positive | Negative | P-value | Positive | Negative | P-value |
|---|
| Age, years | | | 0.751 | | | 0.655 |
| ≤59 | 15 | 9 | | 18 | 9 | |
| ≥60 | 13 | 8 | | 14 | 4 | |
| Gender | | | 0.516 | | | 0.512 |
| Male | 18 | 10 | | 20 | 6 | |
| Female | 10 | 7 | | 12 | 7 | |
| AFP level, ng/l | | | 0.121 | | | 0.012 |
| >400 | 20 | 13 | | 20 | 7 | |
| ≤400 | 8 | 4 | | 12 | 6 | |
| Histological
grade | | | 0.325 | | | 0.224 |
| High | 9 | 3 | | 10 | 2 | |
| Intermediate | 9 | 4 | | 15 | 5 | |
| Low | 10 | 3 | | 7 | 6 | |
| Tumor diameter,
cm | | | 0.024 | | | 0.039 |
| ≤5 | 6 | 9 | | 10 | 3 | |
| >5 | 22 | 8 | | 22 | 10 | |
| Metastasis | | | 0.012 | | | 0.038 |
| Positive | 19 | 8 | | 22 | 7 | |
| Negative | 9 | 9 | | 10 | 6 | |
| Capsule | | | 0.023 | | | 0.033 |
| Positive | 15 | 4 | | 13 | 8 | |
| Negative | 13 | 13 | | 19 | 5 | |
| TNM stage | | | 0.028 | | | 0.024 |
| I and II | 9 | 9 | | 10 | 9 | |
| III and IV | 19 | 8 | | 22 | 4 | |
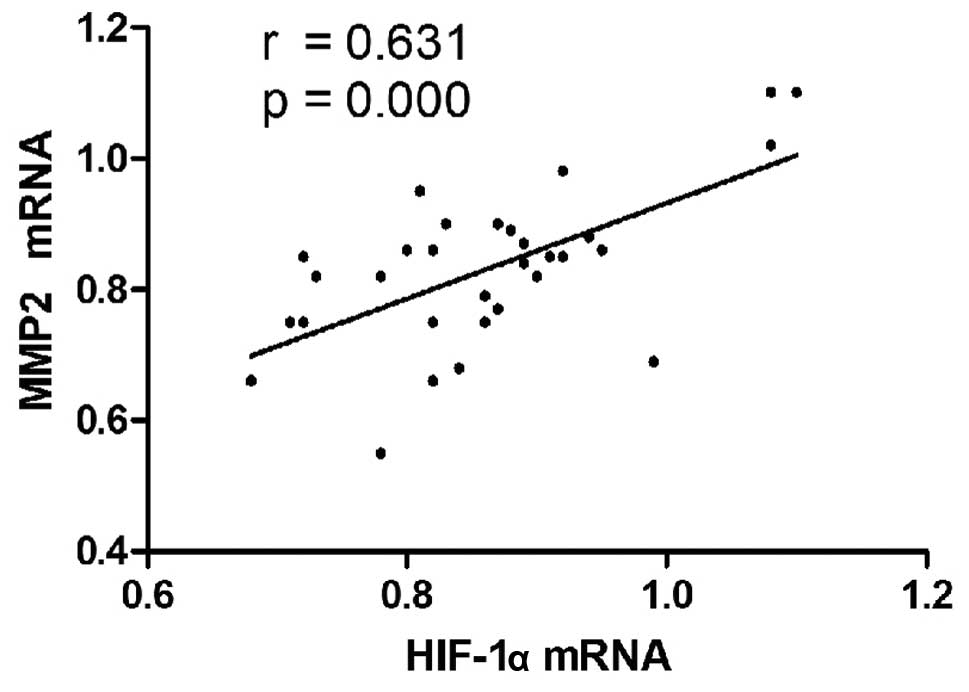
Correlation between MMP2 and HIF-1α
Among the 45 cases of HCC, Pearson’s correlation
analysis of the qPCR results showed that the mRNA levels of MMP2
and HIF-1α were positively correlated (r=0.631, P<0.001;
Fig. 3). From the
immunohistochemistry results, MMP2 and HIF-1α protein were both
expressed in 24 cases, but not in 9 cases. Spearman’s correlation
analysis of the immunohistochemistry results indicated that MMP2
and HIF-1α protein levels were also positively correlated (r=0.521,
P<0.001; Table V).
 | Table VCorrelation between MMP2 and
HIF-1α. |
Table V
Correlation between MMP2 and
HIF-1α.
| MMP2 protein | | |
|---|
|
| | |
|---|
| HIF-1α protein | Negative
(n=11) | Positive
(n=28) | rs
value | P-value |
|---|
| Negative | 9 | 4 | 0.521 | <0.001 |
| Positive | 8 | 24 | | |
Correlation among MMP2, HIF-1α and
survival data for patients with HCC
It was shown that the average survival period for
patients with positive expression of MMP2 was 15.4 months, while
that for patients with negative MMP2 expression was 23.4 months,
according to the Kaplan-Meier survival analysis. It was found that
the survival period for patients with positive MMP2 expression was
significantly longer than that for patients with negative MMP2
expression, by the log-rank test (P=0.04; Table VI). Similarly, the survival period
for patients with positive expression of HIF-1α was significantly
longer than that for patients with negative expression of HIF-1α
(P=0.009, Table VI). The average
survival period for patients with positive HIF-1α expression was
14.8 months, but this was 22.6 months for those with negative
expression of HIF-1α. The survival period for patients with both
MMP2 and HIF-1α expression was significantly shorter than that of
the other groups (P=0.226; Table
VI).
 | Table VICorrelation between MMP2 and HIF-1α
expression and survival data for patients with HCC. |
Table VI
Correlation between MMP2 and HIF-1α
expression and survival data for patients with HCC.
| | Survival time,
months | |
|---|
| |
| |
|---|
| Target protein | Expression | Mean | 95% CI | P-value |
|---|
| MMP2 | Positive | 15.4 | 10.9–20.1 | 0.040 |
| Negative | 23.4 | 17.3–30.5 | |
| HIF-1α | Positive | 14.8 | 12.1–19.4 | 0.009 |
| Negative | 22.6 | 17.2–27.5 | |
|
MMP2+ |
HIF-1α+ | 11.5 | 9.2–14.3 | 0.226 |
|
HIF-1α− | 17.3 | 12.3–22.4 | |
|
MMP2− |
HIF-1α+ | 18.9 | 14.3–22.3 | 0.017 |
|
HIF-1α− | 23.8 | 17.9–30.6 | |
Discussion
The invasion and metastasis of liver cancer is a
complex process in which the dissolution of the extracellular
matrix plays an important role. MMPs are a group of proteolytic
enzymes, which can break down the extracellular matrix and basement
membrane, and promote tumor invasion and metastasis. MMP2 is the
main proteolytic enzyme among the MMPs. MMP2 is a type IV
collagenase and is secreted as a zymogen, which is then
proteolytically processed to the active form, which contributes to
degradation and damage of the extracellular matrix and basement
membrane. Therefore, this promotes tumor cell infiltration of the
surrounding tissues by breaking through the basement membrane,
ultimately leading to tumor cell invasion and metastasis (6,7).
Previous studies have indicated that MMP2 is expressed in a variety
of tumor cells, and is associated with tumor cell growth, invasion
and metastasis (2,11). Sechoedl et al (12) found that MMP2 was not expressed in
normal liver cells, but MMP2 expression was significantly increased
in fibrolamellar carcinoma cells. By comparing the expression of
MMP2 in fibrolamellar carcinoma with that in HCC, it was found that
the pathogenesis and biological behavior were different in
different histological types of liver cancer. Previous studies have
shown that MMP2 expression deficiency decreases corneal
angiogenesis (13), and
MMP2−/− had increased survival times, vessel density,
invasive phenotypes and migration along blood vessels in the brain
parenchyma in a glioblastoma model (8). In the present study, the expression
levels of MMP2 mRNA and protein were examined by qPCR and
immunohistochemistry, respectively. It was found that the
expression levels of MMP2 mRNA and protein in HCC tissues were
significantly higher than those in paracancerous tissues, and were
not associated with patient age or gender. However, MMP2 mRNA and
protein levels were positively correlated with AFP levels, clinical
TNM stage, tumor size and metastasis. Survival analysis showed that
the survival time of patients with negative MMP2 expression was
significantly longer than that of patients with positive MMP2
expression. Therefore, the upregulation of MMP2 protein expression
in the HCC tissues had produced a marked effect on the occurrence
and development of HCC. We hypothesize that activation of the MMP2
signaling pathway may promote the proliferation, invasion and
metastasis of the liver cancer cells, thus affecting the prognosis
of HCC.
HIF-1α, a signal transcription factor that is widely
expressed in human cells under a hypoxic environment, is important
in tumorigenesis, development, invasion, metastasis and apoptosis
(14). Studies have shown that
HIF-1α is expected to be an important indicator that contributes to
predicting tumor diagnosis and recurrence, as well as in monitoring
tumor invasion and metastasis (15–18).
Due to the lack of blood supply, invasive carcinoma will encounter
hypoxia, nutrient deficiency and accumulation of metabolites
(19). Overexpression of HIF-1α in
tumor tissues has been shown to correlate with upregulation of
vascular endothelial growth factor (VEGF), stimulating angiogenesis
and poor prognosis. HIF-1α plays a key role in the VEGF signaling
pathway under the anaerobic environment, and can increase the
activity of VEGF mRNA as well as the transcriptional activity of
VEGF (9,20). The present study showed that both
HIF-1α mRNA and protein expression in HCC tissues were markedly
higher than that in paracancerous tissues. HIF-1α was not
associated with gender and age, but correlated with AFP levels,
tumor size, capsule formation, metastasis and TNM stage. This
suggested that the upregulation of HIF-1α could not only promote
tumor growth, but also enhance the ability of tumor invasion.
According to survival analysis, it was shown that there was no
significant difference in survival time between patients with
HIF-1α-positive and -negative expression at an early stage
following hepatectomy. However, the cumulative survival rate in
patients with positive HIF-1α expression was significantly lower
than that of patients with negative HIF-1α expression, which
further demonstrated that the prognosis of patients with positive
HIF-1α expression was worse than that of patients with negative
HIF-1α expression. The main reason may be that the formation of the
active HIF-1 heterodimer, which is composed of the HIF-1α and
HIF-1β subunits, regulates the transcription of genes involved in
processes such as metabolic adaptation, apoptosis resistance,
angiogenesis, invasion and metastasis when it translocates into the
nucleus and binds to a series of hypoxic response elements
(14). During the latter stage of
liver cancer, the survival rate of patients with positive
expression of HIF-1α was significantly lower than that of patients
with negative HIF-1α expression, possibly due to higher risk of
recurrence, metastasis, and resistance to radiotherapy and
chemotherapy. Thus, HIF-1α protein has the potential to be an
important reference index for estimating the recurrence, metastasis
and prognosis of patients with liver cancer.
The association between HIF-1α and MMP2 expression
in tumor tissues has rarely been studied. It has been demonstrated
that MMP2 expression increases the translational and
transcriptional levels of HIF-1α, so HIF-1α protein expression is
enhanced (21–23). HIF-1α has been revealed to enhance
tumor invasion and metastasis through downregulating E-cadherin
(24) and upregulating MMP2
(25). Giannelli et al
(26) found that human hepatoma
cell lines with an invasive phenotype could produce and activate
MMP2, resulting in high expression levels of MMP2, which could
often migrate through the extracellular matrix substrate surface
and invade through the basement membrane in vitro.
Krishnamachary et al (24)
indicated that hypoxia failed to induce MMP2 expression in
HIF-1α−/− embryonic stem cells, but induced MMP2
overexpression in HIF-1α+/+ embryonic stem cells. MMP2
expression was restrained after interfering HIF-1α expression in
HCT116 colon cancer cells under hypoxia, by using siRNA.
Accordingly, the invasive ability of the colon cancer cells
significantly decreased. In addition, Choi et al (27) reported that MMP2 expression of liver
cancer cells was restrained after blocking HIF-1α expression by
using adenovirus shRNA to transfect hepatoma cell lines, and so the
invasive and growth ability of liver cancer cells was significantly
decreased. Therefore, the authors speculated that there may be a
connection between the MMP2 and HIF-1α signaling pathways. A
positive correlation between MMP2 and HIF-1α expression was
identified by relativity analysis in the current study. MMP2 and
HIF-1α in HCC may be interconnected, with both proteins promoting
the progress of HCC; however, the specific mechanisms of this
remain to be further explored. In conclusion, MMP2 expression may
be regulated by HIF-1α in HCC, and the hypoxic microenvironment in
HCC tissues may induce nuclear transcription factor HIF-1α
overexpression, which possibly activates MMP2 and participates in
the invasion and metastasis of the cancer cells.
Acknowledgements
The authors would like to thank Yun-gui Nie, Hong-bo
Li, Kui Wang and Kai Yang for assistance with the experiment, and
Ya-jun Yuan and Ji-lin Yuan for critically reviewing and
constructively discussing the manuscript.
References
|
1
|
Marín-Hernández A, Gallardo-Pérez JC,
Ralph SJ, Rodríguez-Enríquez S and Moreno-Sánchez R: HIF-1alpha
modulates energy metabolism in cancer cells by inducing
over-expression of specific glycolytic isoforms. Mini Rev Med Chem.
9:1084–1101. 2009.
|
|
2
|
Littlepage LE, Sternlicht MD, Rougier N,
Phillips J, Gallo E, Yu Y, Williams K, Brenot A, Gordon JI and Werb
Z: Matrix metalloproteinases contribute distinct roles in
neuroendocrine prostate carcinogenesis, metastasis, and
angiogenesis progression. Cancer Res. 6:2224–2234. 2010.
|
|
3
|
Hajitou A, Sounni NE, Devy L,
Grignet-Debrus C, Lewalle JM, Li H, Deroanne CF, Lu H, Colige A,
Nusgens BV, Frankenne F, Maron A, Yeh P, Perricaudet M, Chang Y,
Soria C, Calberg-Bacq CM, Foidart JM and Noël A: Down-regulation of
vascular endothelial growth factor by tissue inhibitor of
metalloproteinase-2: effect on in vivo mammary tumor growth and
angiogenesis. Cancer Res. 8:3450–3457. 2001.
|
|
4
|
Yamasaki M, Nagatomo T, Matsuyama T, Ikeho
Y, Kato E and Nishiyama K, Sakakibara Y, Suiko M and Nishiyama K:
Conjugated linoleic acids inhibit hypoxia inducible factor-1α
stabilization under hypoxic condition in human hepatocellular
carcinoma cells. J Oleo Sci. 9:491–496. 2012.
|
|
5
|
Birner P, Schindl M, Obermair A, Plank C,
Breitenecker G and Oberhuber G: Overexpression of hypoxia-inducible
factor 1alpha is a marker for an unfavorable prognosis in
early-stage invasive cervical cancer. Cancer Res. 17:4693–4696.
2000.
|
|
6
|
Karahan N, Güney M, Baspinar S, Oral B,
Kapucuoglu N and Mungan T: Expression of gelatinase (MMP-2 and
MMP-9) and cyclooxygenase-2 (COX-2) in endometrial carcinoma. Eur J
Gynaecol Oncol. 28:184–188. 2007.
|
|
7
|
Sakata H, Fujimura M, Watanabe M and
Tominaga T: Association of cavernous malformation within vestibular
schwannoma: immunohistochemical analysis of matrix
metalloproteinase-2 and -9. Neurol Med Chir. 47:509–512. 2007.
|
|
8
|
Du R, Petritsch C, Lu K, Liu P, Haller A,
Ganss R, Song H, Vandenberg S and Bergers G: Matrix
metalloproteinase-2 regulates vascular patterning and growth
affecting tumor cell survival and invasion in GBM. Neuro Oncol.
3:254–264. 2008.
|
|
9
|
Xiang ZL, Zeng ZC, Fan J, Tang ZY, Zeng HY
and Gao DM: Gene expression profiling of fixed tissues identified
hypoxia-inducible factor-1α, VEGF, and matrix metalloproteinase-2
as biomarkers of lymph node metastasis in hepatocellular carcinoma.
Clin Cancer Res. 16:5463–5472. 2011.
|
|
10
|
Varotti G, Ramacciato G, Ercolani G, Grazi
GL, Vetrone G, Cescon M, Del Gaudio M, Ravaioli M, Ziparo V, Lauro
A and Pinna A: Comparison between the fifth and sixth editions of
the AJCC/UICC TNM staging systems for hepatocellular carcinoma:
multicentric study on 393 cirrhotic resected patients. Eur J Surg
Oncol. 31:760–767. 2005.
|
|
11
|
Nishida Y, Miyamori H, Thompson EW, Takino
T, Endo Y and Sato H: Activation of matrix metalloproteinase-2
(MMP-2) by membrane type 1 matrix metalloproteinase through an
artificial receptor for proMMP-2 generates active MMP-2. Cancer
Res. 21:9096–9104. 2008.
|
|
12
|
Schoedel KE, Tyner VZ, Kim TH,
Michalopoulos GK and Mars WM: HGF, MET, and matrix-related
proteases in hepatocellular carcinoma, fibrolamellar variant,
cirrhotic and normal liver. Mod Pathol. 1:14–21. 2003.
|
|
13
|
Kato T, Kure T, Chang JH, Gabison EE, Itoh
T, Itohara S and Azar DT: Diminished corneal angiogenesis in
gelatinase A-deficient mice. FEBS Lett. 2:187–190. 2001.
|
|
14
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 10:721–732. 2003.
|
|
15
|
Hao J, Song X, Song B, Liu Y, Wei L, Wang
X and Yu J: Effects of lentivirus-mediated HIF-1alpha knockdown on
hypoxia-related cisplatin resistance and their dependence on p53
status in fibrosarcoma cells. Cancer Gene Ther. 7:449–455.
2008.
|
|
16
|
Burrows N, Resch J, Cowen RL, von
Wasielewski R, Hoang-Vu C, West CM, Williams KJ and Brabant G:
Expression of hypoxia-inducible factor 1 alpha in thyroid
carcinomas. Endocr Relat Cancer. 1:61–72. 2010.
|
|
17
|
Seeber LM, Horrée N, van der Groep P, van
der Wall E, Verheijen RH and van Diest PJ: Necrosis related
HIF-1alpha expression predicts prognosis in patients with
endometrioid endometrial carcinoma. BMC Cancer. 10:3072010.
|
|
18
|
Samulitis BK, Landowski TH and Dorr RT:
Inhibition of protein synthesis by imexon reduces HIF-1alpha
expression in normoxic and hypoxic pancreatic cancer cells. Invest
New Drugs. 1:89–98. 2009.
|
|
19
|
Milosevic M, Fyles A, Hedley D and Hill R:
The human tumor microenvironment: invasive (needle) measurement of
oxygen and interstitial fluid pressure. Semin Radiat Oncol.
3:249–258. 2004.
|
|
20
|
Vumbaca F, Phoenix KN, Rodriguez-Pinto D,
Han DK and Claffey KP: Double-stranded RNA-binding protein
regulates vascular endothelial growth factor mRNA stability,
translation, and breast cancer angiogenesis. Mol Cell Biol.
2:772–783. 2008.
|
|
21
|
Jing SW, Wang YD, Kuroda M, Su JW, Sun GG,
Liu Q, Cheng YJ and Yang CR: HIF-1α contributes to hypoxia-induced
invasion and metastasis of esophageal carcinoma via inhibiting
E-cadherin and promoting MMP-2 expression. Acta Med Okayama.
5:399–407. 2012.
|
|
22
|
Mimeault M and Batra SK: Hypoxia-inducing
factors as master regulators of stemness properties and altered
metabolism of cancer- and metastasis-initiating cells. J Cell Mol
Med. 1:30–54. 2013.
|
|
23
|
Nurwidya F, Takahashi F, Minakata K,
Murakami A and Takahashi K: From tumor hypoxia to cancer
progression: the implications of hypoxia-inducible factor-1
expression in cancers. Anat Cell Biol. 2:73–78. 2012.
|
|
24
|
Krishnamachary B, Berg-Dixon S, Kelly B,
Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P
and Semenza GL: Regulation of colon carcinoma cell invasion by
hypoxia-inducible factor 1. Cancer Res. 63:1138–1143. 2003.
|
|
25
|
Xie T, Yuan XL, Yu SY, Yang B and Dong LL:
Interference of HIF-1alpha by RNA reduces the expression of matrix
metalloproteinase-2 in human cervical carcinoma HeLa cells. Ai
Zheng. 6:600–605. 2008.(In Chinese).
|
|
26
|
Giannelli G, Bergamini C, Fransvea E,
Marinosci F, Quaranta V and Antonaci S: Human hepatocellular
carcinoma (HCC) cells require both alpha3beta1 integrin and matrix
metalloproteinases activity for migration and invasion. Lab Invest.
4:613–627. 2001.
|
|
27
|
Choi SH, Shin HW, Park JY, Yoo JY, Kim do
Y, Ro WS, Yun CO and Han KH: Effects of the knockdown of hypoxia
inducible factor-1alpha expression by adenovirus-mediated shRNA on
angiogenesis and tumor growth in hepatocellular carcinoma cell
lines. Korean J Hepatol. 3:280–287. 2010.
|