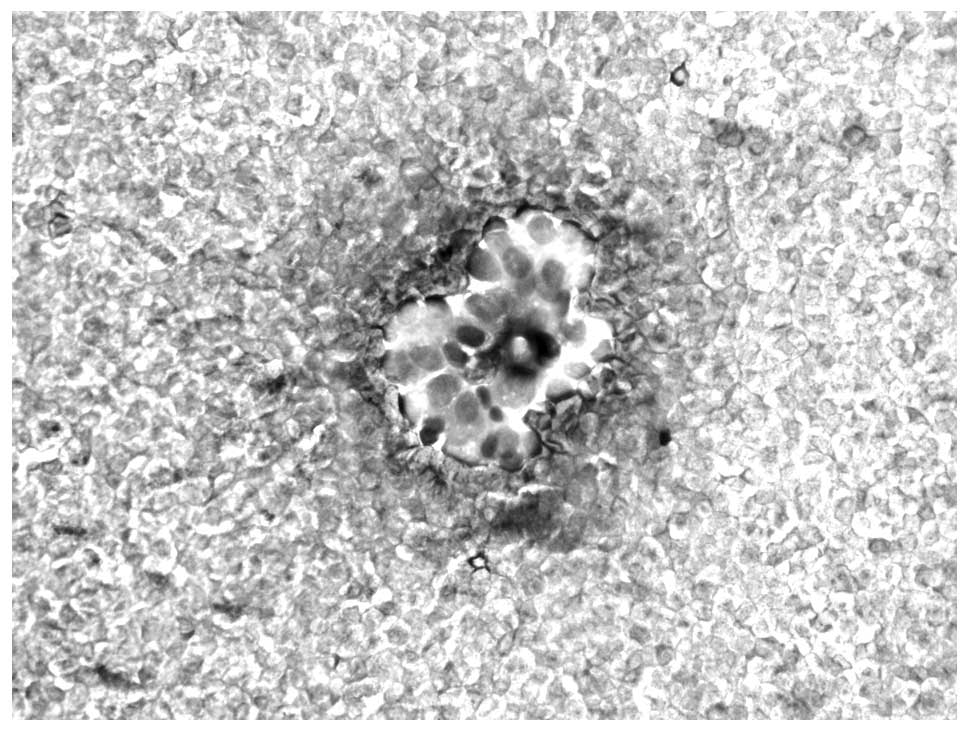
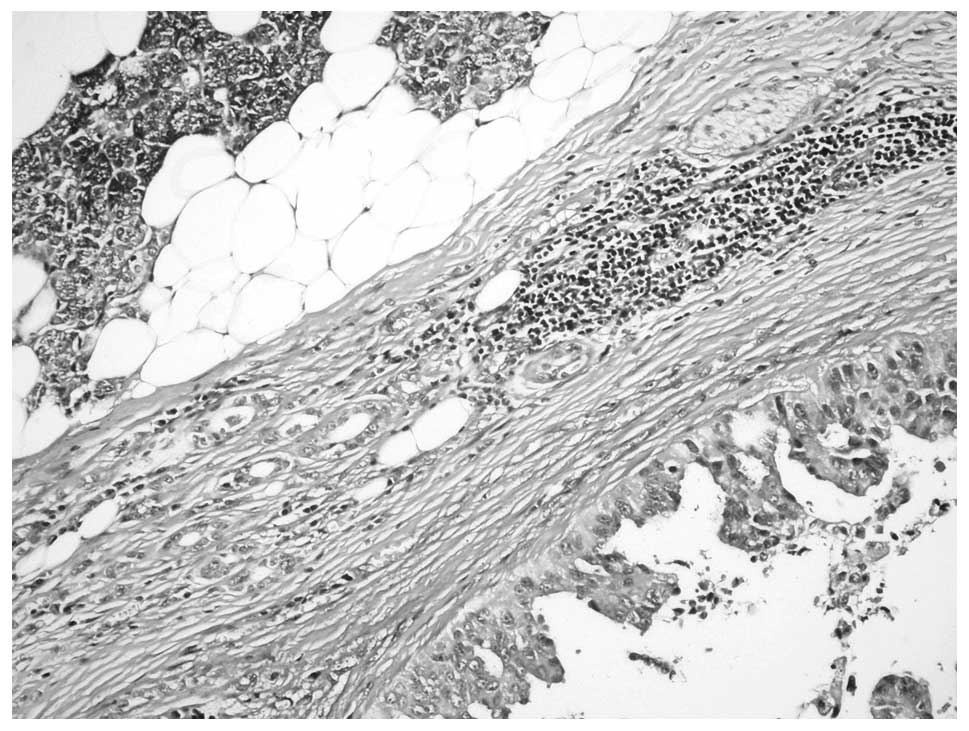
Introduction
Micropapillary carcinoma (MPC) is an uncommon
morphology typically observed within the context of the borderline
serous carcinoma of the ovary (1).
In addition, similar morphological characteristics of MPC are
rarely detected in other non-ovarian sites, such as the breast,
urinary bladder, lung, parotid glands and colon (2–7). In
anatomical locations other than the ovaries, MPC exhibits an
aggressive course and generally accompanies the primary neoplasm of
the organ of its origin (2).
Histologically, MPC has a typical appearance,
characterized by cells with eosinophilic cytoplasm that exhibits a
nest-like arrangement in artifactual spaces. The appearance is
reminiscent of enlarged angiolymphatic vessels and is associated
with a more aggressive clinical course and a higher rate of lymph
node metastasis compared with the typical carcinomas of the organ
of origin. Regardless of the accompanying tumor, the majority of
MPC must always be documented in the pathological diagnosis.
Although less well characterized than its
histological features, the cytological features of MPC have also
been well defined. This is predominantly due to the increasing use
of fine needle aspiration (FNA) biopsy, particularly in the
parenchymal organs, which allows earlier diagnosis of this
aggressive tumor (8–10). The early diagnosis of MPC with FNA
biopsy may also be useful in uncommon sites, such as the parotid
gland and pericardium.
In parenchymal organs, such as the breast, lung and
parotid glands, where FNA has a major diagnostic role, it is
important to be aware of MPC as a disease entity and to document
its presence. Special care must be provided when evaluating lymph
node and serous surface aspirations, due to their high tendency
toward angiolymphatic spread and metastasis.
The current study assessed the cytological
characteristics and emphasized the diagnostic value of the
cytological examination of MPC in a total of eight cases with
histologically confirmed diagnoses of pure MPC occurring at
different sites, including the breast, urinary bladder, pericardium
and parotid gland. The patient provided written informed
consent.
Case report
Introduction to cases
A total of eight cases evaluated at the Department
of Pathology, Istanbul Education and Research Hospital (Istanbul,
Turkey) between 2005 and 2012 were included. Of these, two were
originally from other centers, but were referred to the Department
of Pathology, Istanbul Education and Research Hospital for
consultation (cases 1 and 2). All cytological and histological
materials were reassessed by two of the authors.
The diagnosis of MPC was based on assessment
according to the following criteria of the most common findings:
Tight clusters; three-dimensional cell aggregates with high-grade
nuclear features; formation of morula, cell balls and staghorn
structures; and single cells with columnar configuration and
eccentric nuclei.
Case 1
A 74-year-old male presented to the clinic with a
painful parotid mass. The FNA revealed malignant cytology, not
otherwise specified (NOS). A right radical parotidectomy was
performed. The tumor was 1.2 cm in diameter and exhibited irregular
borders, and no tumor was identified in the surgical borders. The
diagnosis of in situ ductal MPC and pure invasive MPC was
determined, with a pathological stage of pT2. The patient did not
accept additional treatment and, to date, has survived for 14
months, with neck lymph node metastases.
Case 2
A 60-year-old-male presented to the clinic with
breathing difficulties and tachycardia. Pericardial aspiration led
to a diagnosis of malignant cytology, NOS, but differentiation
between mesothelioma and MPC was not possible. Computed tomography
revealed a 2-cm mass in the right lung and bronchoscopic biopsy was
performed. The diagnosis of pure MPC was determined. The patient
received two cycles of chemotherapy; however, widespread metastases
were found 4 months following the diagnosis and, subsequently, the
patient succumbed to the disease.
Case 3
A 52-year-old female presented to the clinic with a
painless mass and skin dimpling on the upper-outer quadrant of the
right breast. Modified radical mastectomy and axillary dissection
were performed. The tumor was 3.4 cm in diameter. The diagnosis of
pure MPC was determined and showed widespread intralymphatic tumor
thrombi. MPC metastasis was present in six axillary lymph nodes and
the pathological stage was pT3A. A total of six cycles of
chemotherapy and radiotherapy were administered. At present, the
patient is alive 14 months following the mastectomy, but has bone
metastasis.
Case 4
A 48-year-old male presented to the clinic with a
painless mass at the midline upper quadrant of the left breast. FNA
with ultrasonography was performed and the diagnosis of malignant
cytology, MPC was determined. Modified radical mastectomy and
axillary dissection were performed. The tumor was 2 cm in diameter.
The diagnosis of pure MPC was determined and widespread
intralymphatic tumor thrombi were observed. MPC metastasis was
present in seven axillary lymph nodes together with tumor invasion
of the chest wall. The pathological stage was pT3B. A total of six
cycles of chemotherapy and radiotherapy were administered.
Currently, the patient is alive 18 months following the mastectomy,
but has recurrence on the chest wall.
Case 5
A 53-year-old female presented with a painless mass
in the upper quadrant midline of the left breast. FNA with
ultrasonography revealed malignant cytology, NOS. Modified radical
mastectomy and axillary dissection were performed. The diagnosis of
pure MPC was determined. The tumor was 1.1 cm in diameter and
widespread intralymphatic tumor thrombi were observed.
Micropapilloma carcinoma metastasis was present in eight axillary
lymph nodes and the pathological stage was pT3B. The patient
received chemotherapy and radiotherapy; however, widespread
metastases in the body were later found. The patient succumbed to
the disease 54 months later.
Case 6
A 72-year-old male patient presented with a
complaint of hematuria. Cystoscopy revealed a solid ulcerated mass
of 1 cm in diameter on the right lateral wall and a washout
cytology sample was obtained. The diagnosis of malignant cytology,
high grade urothelial carcinoma was determined and the mass was
excised. Histological evaluation revealed a pure MPC with
widespread lymphatic invasion, with a pathological stage of pT2.
Radical cystectomy was performed and the pathological stage was
found to be pT3N1. The patient received chemotherapy for 6 months
and, to date, is alive with pelvic recurrence.
Case 7
A 61-year-old male patient presented with hematuria.
The cell block and urine cytology revealed malignant cytology, high
grade urothelial carcinoma. Cystoscopy showed a solid mass in the
trigone of 1 cm in diameter, which was resected by transurethral
resection. Histopathological evaluation revealed a pure MPC and the
pathological stage was pT2. Radical cystectomy was performed and
the pathological stage was found to be pT3N1. In addition, MPC and
widespread lymphatic invasion were present. The patient received
six cycles of chemotherapy, but was identified to have widespread
metastases in the body. The patient succumbed to the disease 54
months following the cystectomy.
Case 8
A 58-year-old male presented with hematuria.
Cystoscopy showed a flat lesion of 1.5 cm in diameter on the right
lateral wall and a washout cytology specimen was obtained. The
diagnosis of malignant cytology, MPC was determined and the mass
was excised. On histopathological evaluation, the diagnosis of
in situ MPC was determined and six cycles of bacille
Calmette-Guérin treatment were administered. To date, the patient
is alive and has been without disease for 6 months.
Results
The clinical and cytological findings are summarized
in Tables I and II. Five cases were male and three were
female; the age range was between 48 and 74 years, with a mean age
of 60 years. The average follow-up period was 20 months (range,
6–54 months). During this period, three patients succumbed to the
disease; currently, four patients are alive with disease, and one
is disease-free.
 | Table IClinical findings. |
Table I
Clinical findings.
| Case, n | Age,
years/Gender | Primer location | First clinical
presentiation | Tumor size,
cm/Stagea | Treatment | Disease outcome |
|---|
| 1 | 74/M | Right parotid | Parotid mass | 1.2/pT2 | Radical
paroidectomi | Alive with disease at
14 months |
| 2 | 60/M | Lung | Pericardial | 2/pT4 | CTh | Mortality at four
effusion months |
| 3 | 52/F | Right breast | Breast mass | 3.4/pT3 | MRM, axillary
dissection, CTh and RTh | Alive with disease at
14 months |
| 4 | 48/F | Left breast | Breast mass | 2/pT3 | MRM, axillary
dissection, CTh and RTh | Alive with recurrence
at 18 months |
| 5 | 53/F | Left breast | Breast mass | 1.1/pT3 | MRM, axillary
dissection, CTh and RTh | Mortality at 54
months |
| 6 | 72/M | Urinary bladder | Hematuria | 1/pT3 | Cystectomy and
CTh | Alive with recurrence
at eight months |
| 7 | 61/M | Urinary bladder | Hematuria | 2/pT3 | Cystectomy and
CTh | Mortality at 43
months |
| 8 | 58/M | Urinary bladder | Hematuria | 1.5/pTa | BCG | Alive at six
months |
 | Table IICytological and histological findings
of MPC. |
Table II
Cytological and histological findings
of MPC.
| | Cytological
findings | | |
|---|
| |
| | |
|---|
| Case, n | Sampling method | Background and single
cells | Cell clusters | Cytological
diagnosis | Histological
diagnosis |
|---|
| 1 | FNA | A few isolated
high-grade malignant cells | Three-dimensional
solid epithelial aggregates and monolayer sheets | Malignant cytology,
NOS | Micropapillary
carcinoma with in situ ductal carcinoma |
| 2 | Pericardial
aspiration | Isolated malignant
cells and high-grade nuclear features | Three-dimensional
aggregates | Malignant cytology,
NOS | Micropapillary
carcinoma |
| 3 | FNA | Single cells with a
columnar configuration and eccentric nuclei and high-grade nuclear
features | Small cell
groups | Malignant cytology
and micropapillary carcinoma | Micropapillary
carcinoma with in situ ductal |
| 4 | FNA | Isolated malignant
cells, high-grade nuclear features and apocrine-like cells | Three-dimensional
aggregates and small cell groups | Malignant cytology
and micropapillary carcinoma | Micropapillary
carcinoma with in situ ductal |
| 5 | FNA | Isolated malignant
cells, and high-grade nuclear features | Small cell
groups | Malignant cytology,
NOS | Micropapillary
carcinoma with in situ ductal |
| 6 | Urine (washout
material) | Isolated malignant
cells, high-grade nuclear features and eccentric nuclei | Three-dimensional
aggregates and morula-like structures | Malignant cytology
and urothelial carcinoma | Micropapillary
carcinoma |
| 7 | Urine and cell
block | Isolated malignant
cells, high grade nuclear features and irregular nuclear
contours | Cell clusters with
angulated borders and scallopes | Malignant cytology
and urothelial carcinoma | Micropapillary
carcinoma |
| 8 | Urine (washout
material) | Isolated malignant
cells, high grade nuclear features and irregular nuclear
contours | Cell clusters with
angulated borders and scallopes | Malignant cytology
and possible micropapillary carcinoma | In situ
micropapillary carcinoma |
The locations of the MPCs were as follows: Three in
the breast, three in the bladder, one in the parotid and one in the
lung (with pericardial effusion). The mean tumor diameter was 1.9
cm, ranging between 1.1 and 3.4 cm.
Each case revealed different cytological MPC
parameters; however, all parameters were detected in total.
Three-dimensional aggregates, high-grade nuclear features, cell
clusters with angulated or scalloped borders, and single cells with
columnar configuration and eccentric nuclei were the most common
findings (Figs. 1 and 2).
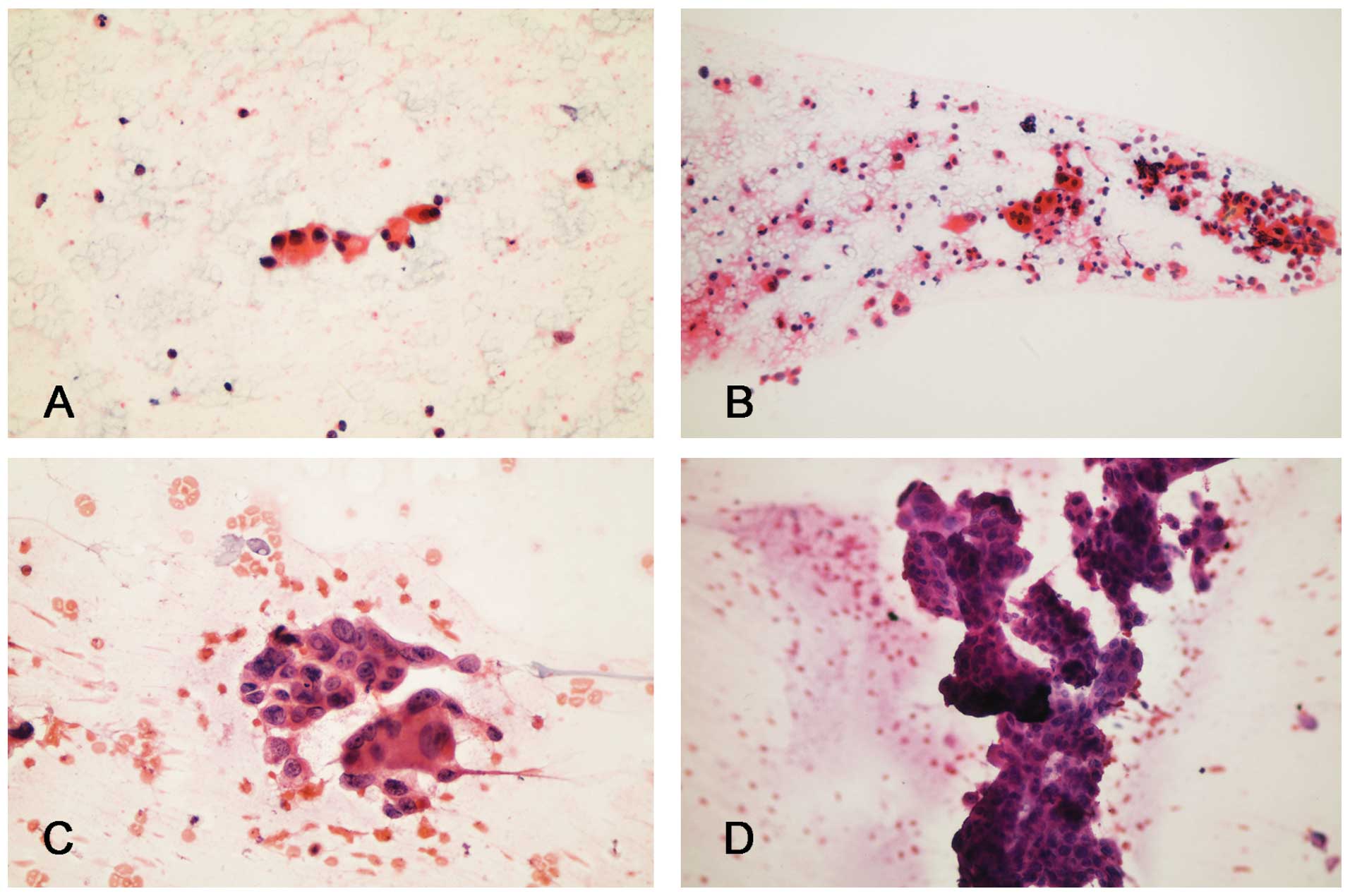 | Figure 1Cytopathology of micropapillary
carcinoma samples obtained from different cases: (A) Case 5, single
cells with columnar configuration and eccentric nuclei (stain,
Papanicolaou; magnification, ×400); (B) case 4, cohesive tumor
groups and scattered cells with apocrine-like cells (stain,
Papanicolaou; magnification, ×200); (C) case 2, cohesive tumor
groups and scattered cells with high-grade nuclear features
indicating an invasive micropapillary component (stain, H&E;
magnification, ×300); and (D) cohesive tumor cells forming large
micropapillary structures indicating an in situ
micropapillary ductal carcinoma (stain, H&E; magnification,
×200). H&E, hematoxylin and eosin. |
In seven cases, the diagnoses were confirmed by
excisional biopsy; in one patient presenting with pericardial
metastasis, confirmation was performed using bronchoscopic biopsy.
A diagnosis of invasive pure MPC was determined in seven cases,
accompanied by in situ ductal carcinoma in those patients
with lesions in the breast and parotid gland (Fig. 3). In one case with MPC in the
urinary bladder, only in situ MPC was present.
Discussion
In addition to borderline serous carcinoma of the
ovary, MPC has also been identified in other sites, such as the
stomach, colon and parotid gland, over the last two decades
(2–10). Despite its rarity, it deserves extra
attention by virtue of its aggressive course. MPC coexisting with
other morphological types of primary adenocarcinoma is more common
than pure MPC. It may also initially present as distant metastasis
due to a high rate of vascular invasion. The reverse polarity
(inside-out growth) is the typical histological characteristic of
the tumor that is likely to be essential in its pathogenesis. The
direct contact between the secretory surface and the surrounding
stroma is also likely to facilitate disease spread (2–10,12).
As emphasized previously, the early diagnosis of MPC
is extremely important due to its aggressive course and, therefore,
FNA and other cytological biopsy techniques have a major role in
early diagnosis and management. FNA is a relatively easy biopsy
method with a number of documented cases of breast MPC diagnosed
using this approach (10,13,14).
However, few cases of MPC in the urinary bladder have been
reported, possibly due to the inapplicability of FNA and the
availability of urine as the only cytological sample (15,16).
Although an increased number of studies examining the role of
cytological examination in the lung have been conducted compared
with those in the urinary bladder (17), to the best of our knowledge, no
cases of primary MPC in the parotid gland or MPC in the lungs
presenting with pericardial findings have been previously
reported.
MPC usually exhibits similar characteristics in all
organs, which assists in the diagnosis. General cytological
features of MPC are as follows: i) Background consists of mucin,
tumor diathesis and particularly urine smear with a dirty
background, including numerous inflammatory cells; ii) cell
clusters include tight clusters, three-dimensional cell aggregates
with high grade nuclear features, morula, cell balls, staghorn
structures and cell clusters with scalloped borders; and iii)
cytological findings comprise cells with a high nuclear/cytoplasmic
ratio, dense cytoplasm, moderate to severe nuclear atypia,
including some vacuolated cells and apocrine-like cells, scattered
single cells with columnar configuration and eccentric nuclei
In the majority of the present eight cases, the
aforementioned cytological characteristics were present. The most
frequent cytological findings were three-dimensional aggregates
with high-grade nuclear features, cell clusters with angulated or
scalloped borders, and single cells with columnar configuration and
eccentric nuclei. In addition, single apocrine-like cells were more
frequent in the breast samples.
All of the cases had pure MPC and a malignant
cytology was readily established. A diagnosis of malignancy is
easier to establish than a direct diagnosis of MPC in cytological
materials, particularly in the breast. The histological
characteristics of the neoplasm accompanied by MPC and additional
features, including the dirty mucinous background, marked nuclear
pleomorphism, discohesive cell groups, single cells, and inability
to observe the surrounding myoepithelial cells in the breast FNA,
assist in a diagnosis of malignancy. However, a diagnosis of pure
MPC may not be readily determined and requires a good knowledge of
its cytology. MPC of the bladder is uncommon; studies describing
its cytological features are rare (8,15,16).
Although its cytological features do not differ from
those of MPCs in other organs, the differential diagnosis from
high-grade urothelial carcinomas may be difficult. Three cases of
bladder MPC in the current series had a diagnosis of malignant
cytology; only the eighth case was determined as an MPC. The most
distinguishing feature of this case was the sharp, distinctive
borders of the cell clusters. This case was the micropapillary
variant of in situ urothelial carcinoma. In situ MPC
is less aggressive than its invasive counterpart; its course is
similar to that of urothelial carcinoma and may progress (18).
Parotid aspiration in the present study showed
characteristics of an in situ ductal carcinoma rather than
the typical characteristics of invasive MPC. In addition,
histological examination revealed a predominance of in situ
ductal carcinoma (solid and micropapillary). The invasive component
exclusively consisted of MPC. MPC in the parotid glands is
extremely rare with only a few previous cases reported (6,19), and
the current study presents the first report with cytological
findings. In suspected cases of ductal adenocarcinoma of the
parotid gland, the possibility of MPC must also be considered.
Furthermore, to the best of our knowledge, the
presented case of lung MPC with initial pericardial presentation
represents the first of such a case in the literature. As compared
with the pleura, the pericardium is a less frequent site of
metastasis. This patient with chest pain presented to Namık Kemal
University Medical Center (Tekirdağ, Turkey) and a pericardial
aspiration was performed. The microscopic slides were then referred
to the Department of Pathology, Istanbul Education and Research
Hospital for consultation. Initially, it was not possible to
differentiate between mesothelioma and metastasis due to the
cytological similarity between these two conditions. However,
following the detection of a mass lesion in the radiological
imaging studies, a biopsy was performed with a subsequent diagnosis
of MPC. This suggests that MPC must be included in the differential
diagnosis of less common metastasis sites, such as the
pericardium.
It is important to recognize the well-defined
cytological characteristics of MPC to determine a precise
diagnosis. A malignant condition with high metastatic potential,
such as MPC, may occur in extremely rare sites, including the
parotid glands, or initially present on serous surfaces, such as
the pericardium.
References
|
1
|
Gilks CB, Alkushi A, Yue JJ, et al:
Advanced-stage serous borderline tumors of the ovary: a
clinicopathological study of 49 cases. Int J Gynecol Pathol.
22:29–36. 2003.
|
|
2
|
Nassar H: Carcinomas with micropapillary
morphology: clinical significance and current concepts. Adv Anat
Pathol. 11:297–303. 2004.
|
|
3
|
Amin MB, Ro JY, el-Sharkawy T, et al:
Micropapillary variant of transitional cell carcinoma of the
urinary bladder. Histologic pattern resembling ovarian papillary
serous carcinoma. Am J Surg Pathol. 18:1224–1232. 1994.
|
|
4
|
Amin MB, Tamboli P, Merchant SH, et al:
Micropapillary component in lung adenocarcinoma: a distinctive
histologic feature with possible prognostic significance. Am J Surg
Pathol. 26:358–364. 2002.
|
|
5
|
Kim MJ, Hong SM, Jang SJ, et al: Invasive
colorectal micropapillary carcinoma: an aggressive variant of
adenocarcinoma. Hum Pathol. 37:809–815. 2006.
|
|
6
|
Nagao T, Gaffey TA, Visscher DW, et al:
Invasive micropapillary salivary duct carcinoma: a distinct
histologic variant with biologic significance. Am J Surg Pathol.
28:319–326. 2004.
|
|
7
|
Ide Y, Horii R, Osako T, et al:
Clinicopathological significance of invasive micropapillary
carcinoma component in invasive breast carcinoma. Pathol Int.
61:731–736. 2011.
|
|
8
|
Sakuma T, Furuta M, Minura A, et al: Urine
cytology of micropapillary carcinoma of the urinary bladder. Diagn
Cytopathol. 39:852–856. 2011.
|
|
9
|
Duncan LD, Jacob S and Atkinson S: Fine
needle aspiration cytologic findings of micropapillary carcinoma in
the lung: a case report. Acta Cytol. 51:605–609. 2007.
|
|
10
|
Kelten EC, Akbulut M and Duzcan SE:
Diagnosis dilemma in cytologic features of micropapillary carcinoma
of the breast. Acta Cytol. 53:463–66. 2009.
|
|
11
|
Edge S, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual. 7th edition. Springer; New York, NY:
2010
|
|
12
|
Nassar H, Pansare V, Zhang H, et al:
Pathogenesis of invasive micropapillary carcinoma: role of MUC1
glycoprotein. Mod Pathol. 17:1045–1050. 2004.
|
|
13
|
Lui PC, Lau PP, Tse GM, et al: Fine needle
aspiration cytology of invasive micropapillary carcinoma of the
breast. Pathology. 39:401–405. 2007.
|
|
14
|
Bayramoglu H, Zekioglu O, Erhan Y, Ciris M
and Ozdemir N: Fine needle aspiration biopsy of invasive
micropapillary carcinoma of the breast: A report of five cases.
Diagn Cytopathol. 27:214–217. 2002.
|
|
15
|
Heymann JJ, Saqi A, Turk AT and Crapanzano
J: Micropapillary urothelial carcinoma: cytologic features in a
retrospective series of urine specimens. Cytojournal. 10:42013.
|
|
16
|
Ylagan LR and Humpbrey PA: Micropapillary
variant of transitional cell carcinoma of the urinary bladder: a
report of three cases with cytologic diagnosis in urine specimens.
Acta Cytol. 45:599–604. 2011.
|
|
17
|
Rudomina DE, Lin O and Moreira AL:
Cytologic diagnosis of pulmonary adenocarcinoma with micropapillary
pattern: does it correlate with the histologic findings? Diagn
Cytopathol. 37:333–339. 2009.
|
|
18
|
Amin A and Epstein JI: Noninvasive
micropapillary urothelial carcinoma: a clinicopathologic study of
18 cases. Hum Pathol. 43:2124–2148. 2012.
|
|
19
|
Yamamoto H, Uryu H, Segawa Y and
Tsuneyoshi M: Aggressive invasive micropapillary salivary duct
carcinoma of the parotid gland. Pathol Int. 58:322–326. 2008.
|