Introduction
Statins as a pharmacological inhibitor of
3-hydroxy-3-methylglutaryl-CoAreductase are widely used in the
treatment of hypercholesterolemia in humans. Various statins have
been shown to exert several beneficial antineoplastic properties,
including antiproliferative effects on tumor cells, the inhibition
of tumor growth, the induction of cell differentiation and
apoptosis and the inhibition of the angiogenesis and metastasis of
malignant cells, such as breast cancer, leukemia, prostate cancer
and colon cancer cells (1–6). Studies that analyzed the use of
atorvastatin and fluvastatin in the NB4 acute promyelocytic
leukemia (APL) cell line found that the drugs are potent inducers
of cell differentiation and apoptosis, establishing the fact that
statins demonstrate potent antileukemic properties in vitro
and indicating the possibility that statins in combination with
all-trans retinoic acid (ATRA) could be effective in overcoming
ATRA resistance in the leukemic cells (6). Nuclear factor-κB (NF-κB) as a nuclear
factor is widely distributed in cells, and acts through regulating
cytokines, chemotactic factors, growth factors, adhesion molecules
and the gene expression of immunological receptors, and
participating in cell differentiation, immunoreaction,
inflammation, cell apoptosis and tumor growth in vivo.
Therefore, inhibiting the activation of the NF-κB signal
transduction pathway probably potentiated a novel therapeutic
strategy to treat immune disease, inflammation and tumors (7). Inducible drug resistance is a major
barrier to effective cancer therapy, and the activation of NF-κB
may aid in the development of chemoresistance (8). In fact, chemotherapeutic agents can
themselves activate NF-κB, resulting in the eventual resistance of
the tumor cells to the therapy (8).
Several studies have shown that, in UCN-01-treated cells,
simvastatin suppressed the activation of NF-κB and potentiated the
apoptosis induced by doxorubicin, paclitaxel and 5-fluorouracil
(9), acting via a Ras
farnesylation-associated mechanism to create signaling
perturbations, particularly the prevention of Ras and ERK1/2
activation, culminating in the synergistic induction of cell death
(10). However, the cytotoxic
potency of simvastatin against NB4 cells and the changes in the
NF-κB signaling pathway are not well clarified.
Therefore, the present study focused on the changes
in the expression of the genes involved in the NF-κB signaling
pathways in NB4 cells treated with simvastatin. The possible
anti-leukemia mechanism of simvastatin is also discussed.
Materials and methods
Reagents
Simvastatin was obtained as a sodium salt from Merck
Chemical Ltd., (Darmstadt, Germany) and dissolved in 99.5% ethanol
to obtain a 1-mM stock solution kept at −20°C and later diluted in
media prior to use in culture. The RNeasy® MinElute™
purified kit was purchased from Qiagen Ltd. (Hilden, Germany). The
2× SuperArray PCR master mix and 96-Well RT2 Profiler™
PCR Array (catalog no. PAHS-058A) were purchased from SABioscience
Ltd. (Qiagen Ltd.).
Cell culture and treatment
The human promyelocytic leukemia NB4 cell line
(kindly gifted by the Jiangsu Institute of Hematology, Suzhou,
Jiangsu, China) was cultured in RPMI 1640 (Gibco Ltd., Paisley, UK)
supplemented with 10% heat-inactivated fetal calf serum (Gibco Ltd,
Invitrogen Life Technologies, Carlsbad, CA, USA), 100 U/ml
penicillin and 100 μg/ml streptomycin in a humidified 5%
CO2 atmosphere at 37°C. Exponentially growing cells were
used for all experiments. Simvastatin was diluted with RPMI 1640
medium to the final concentrations of 15 μM (15SV), 10 μM (10SV)
and 5 μM (5SV) for further treatment. The number of cells was
determined by counting in a Burker chamber (Haimen Tianlong
Experimental Equipment Factory, Haimen, China), and the final NB4
cell concentration was 2×105 cells/ml. For the
dose-response studies, the NB4 cells were seeded at
2×105 cells/ml in 6-well plastic plates and treated with
15SV, 10SV or 5SV for a total treatment time of 72 h, taking NB4
cells without any treatment as normal controls. The cells of the
different groups at 24 h, 48 h, and 72 h post-incubation were
collected for further detection.
MTT Assay
Cell proliferation was assessed using a methyl
thiazolyl tetrazolium (MTT) assay. Briefly, the NB4 cells of the
various groups with or without the indicated doses of simvastatin
were seeded in 96-well flat-bottomed plates (100 ml/well; Falcon;
Corning Inc., Corning, NY, USA) at a final concentration of
2×105 cells/ml for the time indicated. At 24, 48 and 72
h post-incubation, the NB4 cells were incubated with 5 mg/ml MTT
for 4 h at 37°C and then the medium was removed, the cells were
solubilized in dimethyl sulfoxide and the absorbance was measured
at 570 nm. All samples were run in triplicate. Background
absorbance was corrected by subtracting the absorbance values from
the wells with media alone (controls). The cell growth inhibition
rate was calculated according to the following formula: Cell growth
inhibition rate (%) = [1 - (absorbance of experimental group -
absorbance of blank group) / (absorbance of negative group -
absorbance of blank group)].
Observation of morphological changes to
NB4 cells
The NB4 cells (2×105/ml) of the various
groups were harvested at 24, 48 and 72 h post-incubation, washed
once in phosphate-buffered saline (PBS), centrifuged at 500 × g on
glass slides in a cytospin apparatus (Wescor Inc., Logan, UT, USA),
and then fixed and subsequently stained with Wright-Giemsa solution
(Nanjing, China). NB4 cell morphology was observed by
microscope.
Flow cytometric analysis of NB4 cell
apoptosis
Apoptosis assays were performed using an Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit
(Beyotime Institute of Biotechnology, Shanghai, China) following
the manufacturer’s instructions, and early apoptosis was evaluated
by cytofluorometry (FACScabilur, BD Biosciences, Franklin Lakes,
NJ, USA). Following 24, 48 and 72 h of incubation, the NB4 cells of
the various groups were collected and transferred to 5-ml plastic
tubes, washed twice with cold PBS, stained with Annexin V-FITC and
propidium iodide, and then analyzed by FACScabilur. Samples were
run in duplicate with 10,000 events counted per sample. The
apoptotic rate was expressed as the mean of three independent
experiments.
Human NF-κB signaling pathway detection
by RT2 Profiler PCR Array
The untreated NB4 cells and those treated with 15SV
were collected at 48 h post-incubation from three repeated
experiments for further NF-κB signaling pathway detection. Total
RNA was extracted from the NB4 cells using the TRIzol one-step
procedure according to manufacturer’s instructions (Invitrogen Life
Technologies), and RNA cleanup was then also performed according to
the manufacturer’s instructions (RNeasy MinElute; Qiagen Ltd.).
cDNA was converted using Superscript III reverse transcriptase.
Quantitative PCR was performed according to the RT2
Profiler PCR Array instructions under the following conditions:
95°C for 10 min, then 95°C for 15 sec and 60°C for 1 min. The ΔCt
value for each pathway-focused gene was calculated in each
treatment group and the ΔΔCt method was used to analyze the
data.
Statistical methods
Statistical analyses were performed with SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA). All
experiments were performed three times in each individual sample,
and the results were presented as the mean value of the three. The
Student’s t-test was used to compare the means between two groups
and one-way analysis of variance was used to compare the means
among more than two different groups. P<0.05 was considered to
indicate a significant difference.
Results
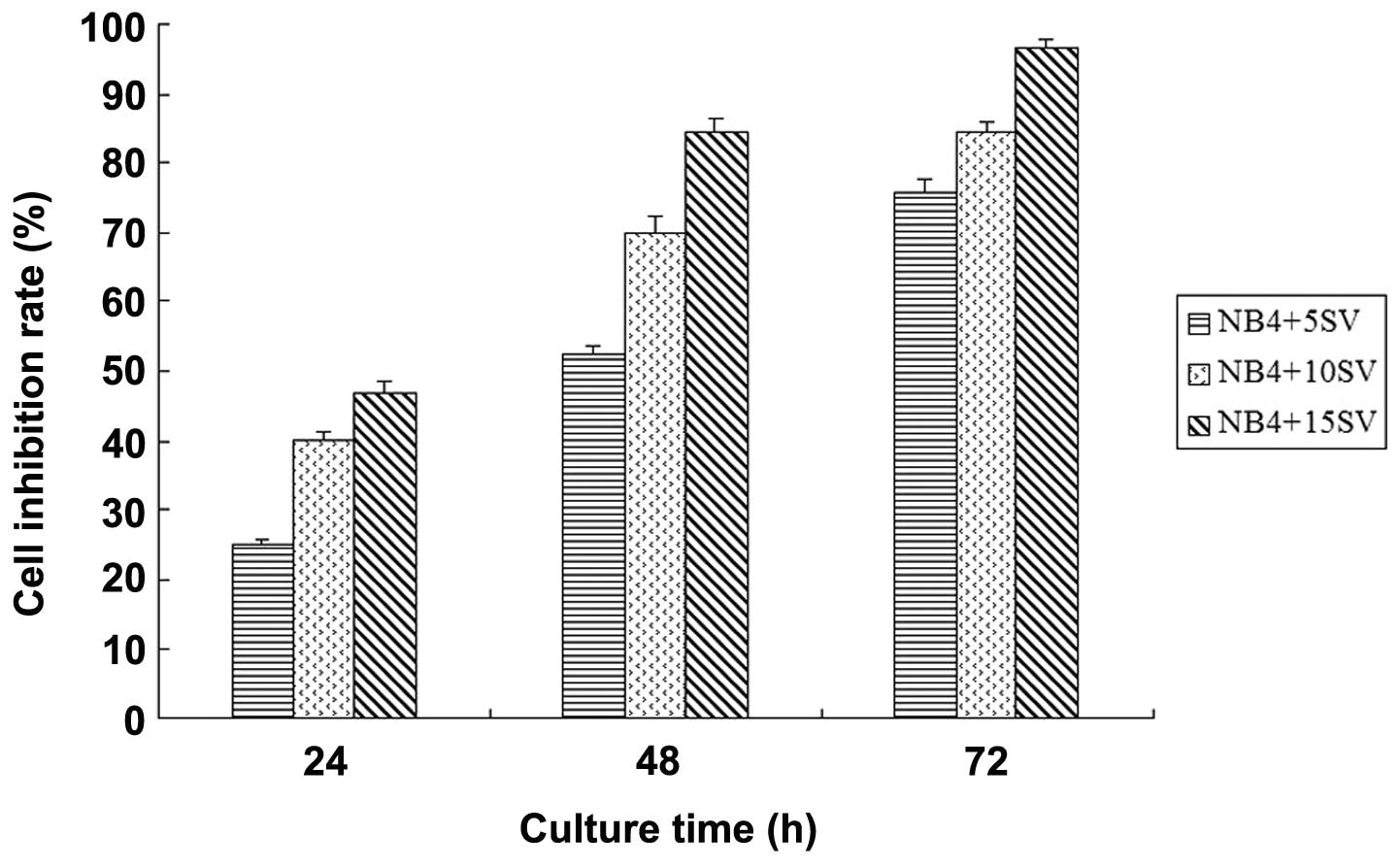
Simvastatin inhibits NB4 cell growth
Univariate analysis of variance of the MTT results
revealed that when treated with simvastatin, the NB4 cell growth
inhibition rates gradually increased with time (F=6.638, P=0.03)
and dose (F=14.111, P=0.004), indicating that simvastatin
potentially inhibits NB4 cell proliferation in a time and
dose-dependent manner (Fig. 1).
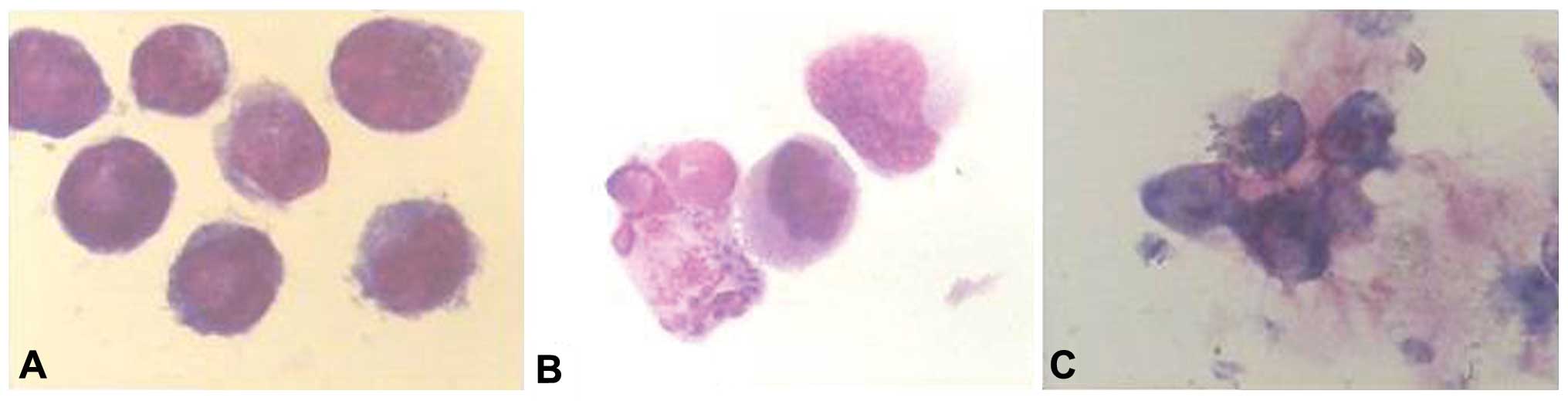
Morphological changes to NB4 cells
treated with simvastatin
The NB4 cells stained by Wright-Giemsa solution
exhibited karyorrhexis, petal-like nuclei and apoptotic body
formation with increased cytoplasm at 24 and 48 h post-incubation
when treated with simvastatin at the various concentrations. At 72
h post-incubation with simvastatin, the majority of the NB4 cells
manifested karyorrhexis (Fig.
2).
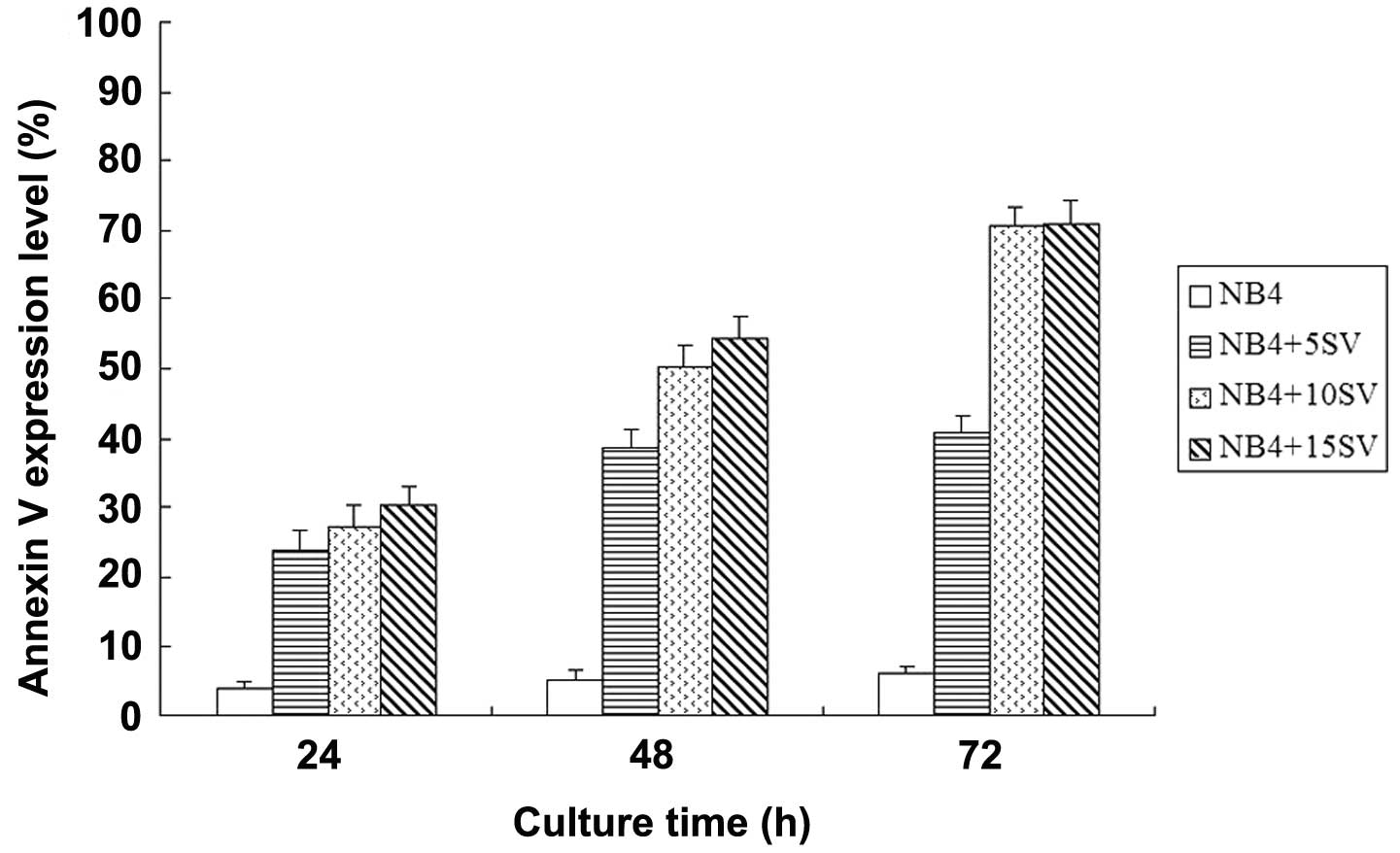
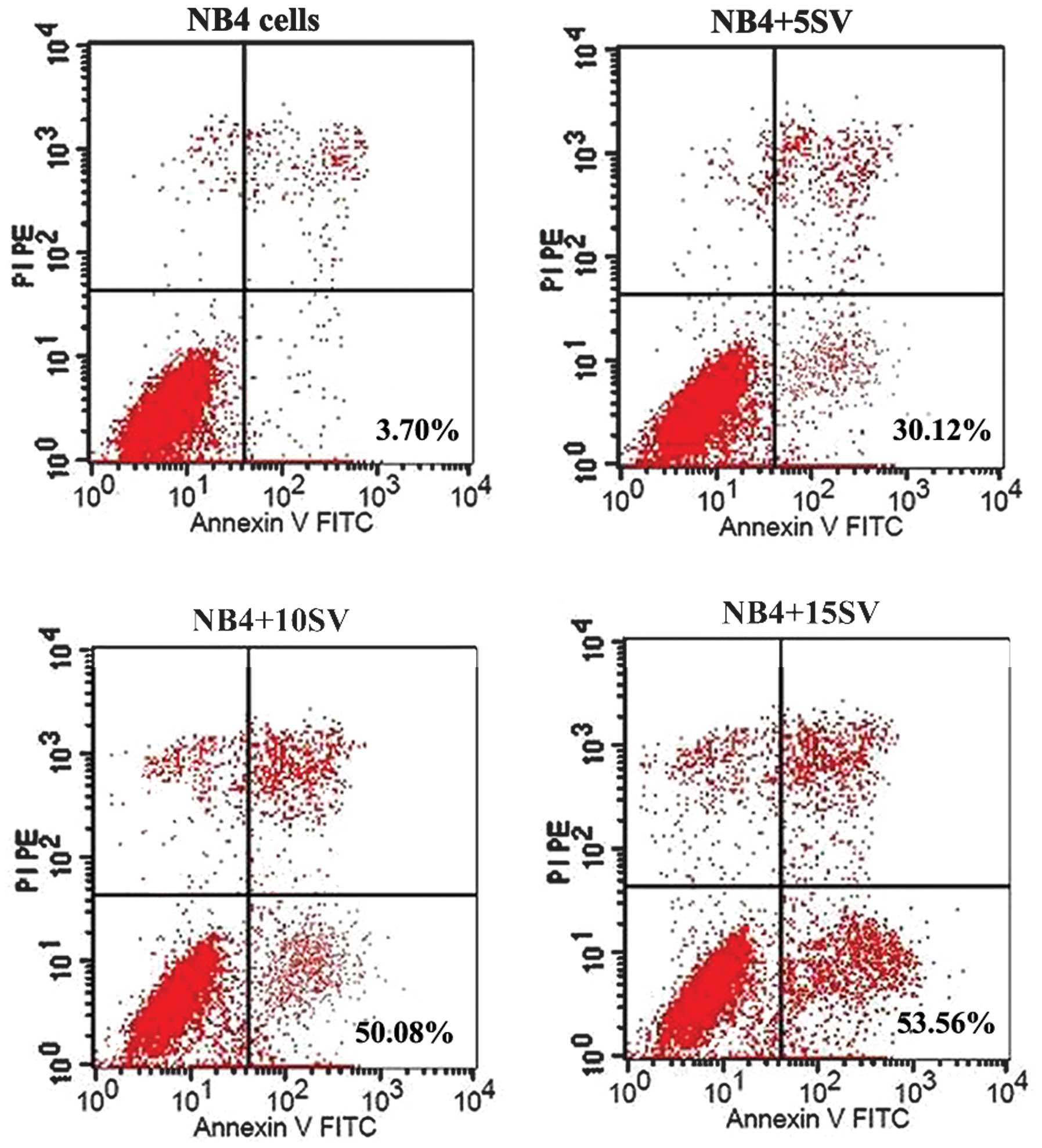
Simvastatin induces NB4 cell apoptosis in
a time- and dose-dependent manner
When treated with simvastatin, the Annexin V
expression levels of the NB4 cells increased in a time- (F=6.909,
P=0.028) and dose-dependent (F=14.431, P=0.004) manner, and the
15SV group exhibited the highest level of apoptosis promotion, with
the Annexin V expression levels of 70.49±2.68 and 70.72±3.43% at 48
and 72 h post-incubation respectively, indicating that simvastatin
potentially promotes NB4 cell apoptosis (Figs. 3 and 4). Further t-tests showed that there were
no statistical differences (P>0.05) in the Annexin V expression
levels at 48 and 72 h in the 5SV group, therefore, untreated NB4
cells and those treated with 15SV were used at 48 h post-incubation
for human NF-κB signaling pathway detection by RT2
Profiler™ PCR Array.
Expression of NF-κB signaling pathway
involves genes in NB4 cells treated with simvastatin
Table I shows the
changes in the mRNA expression levels of the 84 genes involved in
the NF-κB signaling pathway. Fold-change (2−ΔΔCt) is
measured as the level of normalized gene expression
(2−ΔCt) in the test sample divided by the level of
normalized gene expression (2−ΔCt) in the control
sample. Fold-regulation represents the fold-change results in a
biologically meaningful way. When fold-change is >1, positive
regulation or upregulation is indicated, and the fold-regulation is
equal to the fold-change. However, when the fold-change is <1,
negative regulation or downregulation is indicated, and the
fold-regulation is the negative inverse of the fold-change.
Table I shows that, among the 84
genes, the expression levels of 11 genes changed with a
fold-difference of 1.5 to 2.0, and the expression levels of 45
genes manifested fold-change values of >2.0. Of the 56
differently-expressed genes, 9 manifested upregulation, including
the inhibitory κB (IκB) family genes, BCL3, IκBα, caspase 8 and
IFNβ; 47 manifested downregulation, including the IκB kinase (IKK)
family genes, the NF-κB family genes, pro-inflammatory factors such
as IL-1, IL-6, IL-8 and TNF, cellular adhesion molecule ICAM/LFA
and the toll-like receptor (TLR) pathway, which mediated immune
response-associated genes such as TLR family, MYD88 and IL-1
receptor-associated kinase (IRAK)1/2. The changes in the expression
of these genes indicated that simvastatin may promote NB4 cell
apoptosis by regulating the gene expression involved in TLR and
NF-κB signaling pathways.
 | Table IDifferentially-expressed genes
involved in the NF-κB signaling pathway of the NB4 cells treated
with 15SV at 48h post-incubation. |
Table I
Differentially-expressed genes
involved in the NF-κB signaling pathway of the NB4 cells treated
with 15SV at 48h post-incubation.
| Gene | Fold-change of up- or
downregulation |
|---|
| AGT | −7.19087 |
| AKT1 | −1.34598 |
| ATF1 | 1.932655 |
| BCL10 | 1.015843 |
| BCL3a | 3.1638 |
| CFB | 1.611881 |
| BIRC2 | 1.168396 |
| NOD1 | 1.352533 |
| CASP1a | −2.34227 |
| CASP8a | 2.42342 |
| CCL2 | −19.4145 |
| CD40 | −1.00316 |
| CFLAR | −1.08346 |
| CHUK | −1.51554 |
| CSF2 | 1.222123 |
| CSF3 | −3.36992 |
| SLC44A2 | −2.71589 |
| EDARADD | −6.09493 |
| LPAR1 | −6.09493 |
| EGR1 | −6.70121 |
| ELK1 | −1.05755 |
| F2R | −3.32772 |
| FADDa | −1.12544 |
| FASLGa | −6.09493 |
| FOS | −6.4125 |
| GJA1 | 1.139515 |
| HMOX1 | −25.0176 |
| HTR2B | 1.526521 |
| ICAM1a | −87.9626 |
| IFNA1 | 1.179248 |
| IFNB1 | 2.015843 |
| IFNG | −6.09493 |
| IKBKBa | 1.196008 |
| IKBKEa | −1.52768 |
| IKBKGa | −1.12064 |
| IL10a | −12.9526 |
| IL1Aa | −3.32951 |
| IL1Ba | −72.6281 |
| IL1R1a | −8.26226 |
| IL6a | −1.72447 |
| IL8a | −57.0666 |
| IRAK1a | 1.346327 |
| IRAK2a | −31.9603 |
| JUNa | −42.4192 |
| LFAa | −4.13282 |
| LTBR | 1.432608 |
| MALT1 | −1.72488 |
| MAP3K1a | 4.217983 |
| MYD88a | −2.82548 |
| NLRP12 | −40.5471 |
| NFKB1a | −2.77427 |
| NFKB2a | −3.9451 |
| NFKBIAa | 7.6537 |
| PPM1A | 1.634145 |
| RAF1 | −1.15277 |
| REL | −1.25596 |
| RELAa | −1.40901 |
| RELBa | −1.72021 |
| TRIM13 | 1.162488 |
| RHOA | −2.55771 |
| RIPK1 | −1.75764 |
| SLC20A1 | 2.165973 |
| STAT1 | −1.09569 |
| TBK1 | −1.2594 |
| TICAM2 | −3.75585 |
| TLR1a | −3.5425 |
| TLR2a | −6.09493 |
| TLR3a | −1.41519 |
| TLR4a | −2.27636 |
| TLR6a | −6.42164 |
| TLR7a | 1.196063 |
| TLR8a | −1.06758 |
| TLR9a | −2.31402 |
| TMED4 | 1.070532 |
| TNFa | −22.7788 |
| TNFAIP3a | −23.5035 |
| TNFRSF10A | −1.05723 |
| TNFRSF10Ba | −2.53081 |
| TNFRSF1A | 2.346345 |
| CD27 | −2.32356 |
| TNFSF10 | −3.35489 |
| TNFSF14 | −1.17296 |
| TRADD | −1.6789 |
| TICAM1 | −7.99424 |
Discussion
Atorvastatin and fluvastatin have previously been
demonstrated as potent inducers of cell differentiation and
apoptosis in the NB4 cell line (6).
In another study, the following cytotoxic potency against HL-60 was
found: Simvastatin
(SV)>atorvastatin>cerivastatin>fluvastatin. Notably, the
all-trans retinoic acid (ATRA)-resistant HL-60 variant, HL-60-R2,
was twice as sensitive to SV compared with HL-60. These findings
indicated that simvastatin exhibits the most cytotoxic potency
against the ATRA-resistant HL-60 variant, which may overcome the
ATRA resistance to APL cells (11).
The present results showed that simvastatin inhibited NB4 cell
growth and promoted cell apoptosis in a time- and dose-dependent
manner, as found in the results of a previous study (11). It was also found that the expression
levels of 56 genes involved in the NF-κB signaling pathways were
changed in the NB4 cells treated with 15SV at 48 h post-incubation,
and it was hypothesized that the proapoptotic mechanism may be
associated with the changes in the gene expression levels involved
in the NF-κB signaling pathway regulated by simvastatin. With
regard to the underlying proapoptotic dose of simvastatin, it has
previously been reported that combining tipifarnib and simvastatin
at dose of 5 and 50 μM, respectively, exhibited a synergistic
apoptosis effect in KG1 and TF-1 cells (12). The present study found that 15SV
manifested clear anti-leukemia effects on the NB4 cells, avoiding
the side-effects caused by high-dose simvastatin.
The expression of a wide range of genes that are
involved in numerous processes, including the inflammatory and
immune responses of the cell, cell growth and development, is
regulated by the eukaryotic NF-κB transcription factor family. The
involvement of NF-κB-mediated signal transduction has been
indicated in the inflammatory response, autoimmune diseases,
tumorigenesis, apoptosis and in the regulation of viral
replication. NF-κB transcription factor activation occurs in
response to a range of signals, including, pathogens, cytokines,
injuries and other stressful conditions. NF-κB protein activation
is strictly regulated, and inappropriate NF-κB signaling pathway
activation has been associated with chronic inflammation,
autoimmunity and a number of cancer types (13–15).
Due to its critical role in cell survival, cell adhesion,
inflammation, differentiation and cell growth, NF-κB has been
indicated to be involved in carcinogenesis.
TLRs are a class of proteins that are required for
the host defense against infection. TLRs play a key role in
auto-immunity, and are considered to be important recognition and
signal transduction receptors (16). MyD88 is a TLR domain-containing
cytoplasmic protein. Evidence indicates that all of the TLRs, with
the probable exception of TLR3, utilize this pathway. MyD88
interacts with IRAK-4, via their respective death domains. IRAK-4
then recruits IRAK-1 to the complex, leading to its phosphorylation
and activation (17). IRAK-1 and
IRAK-4 then dissociate from the complex and interact with TNF
receptor-associated factor-6, which in turn recruits transforming
growth factor-β-activated kinase-1 (TAK-1)-binding protein-1
(TAB-1) and TAB-2 to the complex. This leads to the phosphorylation
and activation of the kinase, TAK-1 (18). TAK-1 then activates kinases upstream
of p38 and JNK, and the IKK complex, leading to NF-κB activation
and the induction of proinflammatory cytokine expression, including
that of IL-1, IL-6, IL-12 and TNF-α. Therefore, the TLR/NF-κB
signaling pathway upregulates inflammatory cytokine expression,
which activates NF-κB, resulting in cell apoptosis inhibition and
subsequent cell immortalization (18). The present results revealed that
when treated with 15SV, the mRNA expression levels of the IKK and
NF-κB family genes in NB4 cells were all downregulated, however,
the expression levels of the IκB family genes, which inhibit NF-κB
transcriptional activity, were upregulated, indicating that
simvastatin promotes NB4 cell apoptosis through the inhibition of
cell transcription mediated by NF-κB. Additionally, the present
study found that the mRNA expression levels of the pro-inflammatory
factors (IL-1, IL-6, IL-8 and TNF), the TLR signaling
pathway-associated genes (TLR family genes, MYD88 and IRAK1/2), and
the cell adhesion molecules (ICAM/LFA) were all downregulated,
indicating that simvastatin also induces NB4 cell apoptosis through
the inhibition of the expression of genes involved in the
inflammation signal transduction pathway. This subsequently
suppresses cell transcriptional activity. The activation of NF-κB
can suppress cell apoptosis through regulating the gene expression
of the IAPs family, the Bcl-2 family, the RAF family, JNK, FLIP and
A20, however, the manner by which these proteins suppress cell
apoptosis is not fully understood (8). Thus, suppression of NF-κB activation
in cancer cells, and the subsequent induction of cell apoptosis may
provide an additional target for the treatment of immune disease,
inflammation and malignant tumors.
In summary, the use of simvastatin in vitro
inhibits human acute promyelocytic leukemia NB4 cell proliferation
and induces apoptosis in a time- and dose-dependent manner. The
mechanism behind this may be associated with the regulation of the
expression of genes involved in the TLR-mediated inflammatory
response and NF-κB signaling pathways.
Acknowledgements
The authors are grateful to Dr Zixing Chen and Dr
Jiannong Cen for their assistance in analyzing the PCR array data
and in the statistical analysis. This study was supported by the
135 Opening Project of Jiangsu Province, China (KF200947).
References
|
1
|
Sassano A and Platanias LC: Statins in
tumor suppression. Cancer Lett. 260:11–19. 2008.
|
|
2
|
Hindler K, Cleeland CS, Rivera E and
Collard CD: The role of statins in cancer therapy. Oncologist.
11:306–315. 2006.
|
|
3
|
Sivaprasad U, Abbas T and Dutta A:
Differential efficacy of 3-hydroxy-3-methylglutaryl CoA reductase
inhibitors on the cell cycle of prostate cancer cells. Mol Cancer
Ther. 5:2310–2316. 2006.
|
|
4
|
Campbell MJ, Esserman LJ, Zhou Y, et al:
Breast cancer growth prevention by statins. Cancer Res.
66:8707–8714. 2006.
|
|
5
|
Martirosyan A, Clendening JW, Goard CA and
Penn LZ: Lovastatin induces apoptosis of ovarian cancer cells and
synergizes with doxorubicin: potential therapeutic relevance. BMC
Cancer. 10:1032010.
|
|
6
|
Sassano A, Katsoulidis E, Antico G, et al:
Suppressive effects of statins on acute promyelocytic leukemia
cells. Cancer Res. 67:4524–4532. 2007.
|
|
7
|
Escárcega RO, Fuentes-Alexandro S,
García-Carrasco M, Gatica A and Zamora A: The transcription factor
nuclear factor-kappa B and cancer. Clin Oncol (R Coll Radiol).
19:154–161. 2007.
|
|
8
|
Bharti AC and Aggarwal BB: Nuclear
factor-kappa B and cancer: its role in prevention and therapy.
Biochem Pharmacol. 64:883–888. 2002.
|
|
9
|
Ahn KS, Sethi G and Aggarwal BB: Reversal
of chemoresistance and enhancement of apoptosis by statins through
down-regulation of the NF-kappaB pathway. Biochem Pharmacol.
75:907–913. 2008.
|
|
10
|
Dai Y, Khanna P, Chen S, Pei XY, Dent P
and Grant S: Statins synergistically potentiate
7-hydroxystaurosporine (UCN-01) lethality in human leukemia and
myeloma cells by disrupting Ras farnesylation and activation.
Blood. 109:4415–4423. 2007.
|
|
11
|
Tomiyama N, Matzno S, Kitada C, Nishiguchi
E, Okamura N and Matsuyama K: The possibility of simvastatin as a
chemotherapeutic agent for all-trans retinoic acid-resistant
promyelocytic leukemia. Biol Pharm Bull. 31:369–374. 2008.
|
|
12
|
van der Weide K, de Jonge-Peeters SD,
Kuipers F, de Vries EG and Vellenga E: Combining simvastatin with
the farnesyltransferase inhibitor tipifarnib results in an enhanced
cytotoxic effect in a subset of primary CD34+ acute
myeloid leukemia samples. Clin Cancer Res. 15:3076–3083. 2009.
|
|
13
|
Toubi E and Shoenfeld Y: Toll-like
receptors and their role in the development of autoimmune diseases.
Autoimmunity. 37:183–188. 2004.
|
|
14
|
Bassères DS and Baldwin AS: Nuclear
factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic
initiation and progression. Oncogene. 25:6817–6830. 2006.
|
|
15
|
Courtois G and Gilmore TD: Mutations in
the NF-kappaB signaling pathway: implications for human disease.
Oncogene. 25:6831–6843. 2006.
|
|
16
|
Pasare C and Medzhitov R: Toll-like
receptors: linking innate and adaptive immunity. Adv Exp Med Biol.
560:11–18. 2005.
|
|
17
|
Suzuki N, Suzuki S, Duncan GS, et al:
Severe impairment of interleukin-1 and Toll-like receptor
signalling in mice lacking IRAK-4. Nature. 416:750–756. 2002.
|
|
18
|
Ninomiya-Tsuji J, Kishimoto K, Hiyama A,
Inoue J, Cao Z and Matsumoto K: The kinase TAK1 can activate the
NIK-I kappaB as well as the MAP kinase cascade in the IL-1
signalling pathway. Nature. 398:252–256. 1999.
|