Introduction
In the European population, renal cell carcinoma
(RCC) accounts for 2% of all new cancer cases and 25% of patients
with RCC have metastases at initial presentation (1). The majority of cases of metastatic RCC
(mRCC) are refractory for chemotherapy, and immunotherapy using
interferon and/or interleukin (IL) has been effective for only a
small proportion of patients with mRCC. Recently, molecular
targeted therapies have been widely used and the therapeutic
strategy for mRCC has changed markedly as randomized control trials
have demonstrated the efficacy and safety of these drugs for the
treatment of mRCC (2–4). Three tyrosine kinase inhibitors,
sorafenib, sunitinib and axitinib, as well as two mammalian target
of rapamycin inhibitors, everolimus and temsirolimus, have been
approved for the treatment of patients with mRCC in Japan, and the
clinical outcomes of these agents have been reported (5–9).
One of the most well-established classification
systems for patients with mRCC is the Memorial Sloan Kettering
Cancer Center (MSKCC) system reported by Motzer et al
(10) in 1999 and modified in 2002
(11). In the modified
classification system, patients are classified into three groups
based on the following five risk factors for short survival: Low
Karnofsky performance status (<80%), low hemoglobin levels
(<the lower limit of normal), high serum lactate dehydrogenase
levels (>1.5-fold the upper limit of normal), high corrected
calcium levels (>10 mg/dl) and short time from diagnosis to the
initiation of targeted therapy (<1 year). Internal and external
validation (11,12) in numerous clinical studies in Europe
and America has found this risk classification system to be useful
for analyzing the prognosis of patients with mRCC. Although the
MSKCC risk classification system is useful, between 53 and 70% of
all patients with mRCC have been classified into the
intermediate-risk group (11–13).
Thus, patients with better prognoses may be included in same group
as those with worse prognoses. To predict the prognosis of each
patient, an additional factor which classifies the intermediate
risk group into two subgroups is required.
Previous studies have demonstrated that there are
several prognostic factors for advanced RCC other than the five
used in the MSKCC risk classification system, including serum
C-reactive protein (CRP) levels (14–18),
metastasis status (12,19,20)
and previous treatments (21–23).
Thus, the present study aimed to investigate the impact of these
factors and other factors on the prognosis of patients with
intermediate-risk mRCC receiving molecular targeted therapy.
Patients and methods
Patients
A total of 146 patients underwent molecular targeted
therapy at the Institute of Biomedical and Health Sciences
(Hiroshima University, Hiroshima, Japan) and other hospitals in the
Hiroshima prefecture between 2007 and 2011. Six of the patients
were excluded from the present study, as their pre-treatment serum
CRP levels were not known. The remaining 140 patients were
retrospectively classified into favorable-, intermediate- and
poor-risk groups (groups F, I and P, respectively). Group I was
further classified according to age, metastasis status, prior
nephrectomy, choice of first-line drug treatment and pre-treatment
serum CRP levels. The overall survival (OS) rate of the patients in
each subgroup was then compared with that in groups F and P. The
study was approved by the ethics committee of Hiroshima University
(Hiroshima, Japan).
Statistical analysis
In each group, the OS rate from the initiation of
molecular targeted therapy to the date of mortality was determined
using the Kaplan-Meier method, and differences between groups were
analyzed using the log-rank test. χ2 analysis was used
for categorical variables. For multivariate analyses, the Cox
proportional-hazards regression model was used. All statistical
analyses were performed using the StatView 5.0 software package
(SAS Institute, Inc., Cary, NC, USA) and P<0.05 was considered
to indicate a statistically significant difference.
Results
The cohort included in the present study consisted
of 140 consecutive patients who underwent molecular targeted
therapy for mRCC. The characteristics of the patients are shown in
Table I. In total, 118 (84.3%)
patients were male. Twenty-two patients (15.7%) were classified
into group F, 95 (67.9%) were classified into group I and 23
(16.4%) were classified into the group P. In group I, the
percentage of patients with two or more metastatic organs was
significantly reduced (P=0.0001) compared with that in group P, and
the percentage of patients with pre-treatment CRP levels >0.3
mg/dl was also significantly reduced (P=0.0025) compared with that
in group P. Furthermore, in group I, the percentage of patients who
had previously undergone nephrectomy was significantly increased
(P<0.0001) compared with that in group P.
 | Table ICharacteristics of 140 patients with
metastatic renal cell carcinoma who underwent molecular targeted
therapy. |
Table I
Characteristics of 140 patients with
metastatic renal cell carcinoma who underwent molecular targeted
therapy.
| MSKCC risk | |
|---|
|
| |
|---|
| Parameter | Favorable | Intermediate | Poor | Total |
|---|
| No. patients | 22 | 95 | 23 | 140 |
| Age, range
(median) | 46–81 (63) | 40–85 (64) | 39–78 (62) | 39–85 (64) |
| Gender, n (%) |
| Male | 19 (86.4) | 82 (86.3) | 17 (73.9) | 118 (84.3) |
| Female | 3 (13.6) | 13 (13.7) | 6 (26.1) | 22 (15.7) |
| Histological type, n
(%) |
| Clear | 19 (86.4) | 67 (70.5) | 7 (30.4) | 93 (66.4) |
| Non-clear | 2 (9.1) | 6 (6.3) | 3 (13.0) | 11 (7.9) |
| Unknown | 1 (4.5) | 22 (23.2) | 13 (56.5) | 36 (25.7) |
| No. metastatic
organs, n (%) |
| 1 | 11 (50.0) | 53 (55.8)a | 2 (8.7) | 66 (47.1) |
| ≥2 | 11 (50.0) | 42 (44.2) | 21 (91.3) | 74 (52.9) |
| Bone metastasis, n
(%) |
| Yes | 5 (22.7) | 24 (25.3) | 5 (21.7) | 34 (24.3) |
| No | 17 (77.3) | 71 (74.7) | 18 (78.3) | 106 (75.7) |
| Prior nephrectomy, n
(%) |
| Yes | 22 (100.0) | 77 (81.0)a | 7 (30.4) | 106 (75.7) |
| No | 0 (0.0) | 18 (19.0) | 16 (69.6) | 34 (24.3) |
| Pre-treatment CRP
levels, n (%) |
| ≤0.3 mg/dl | 16 (72.7) | 48 (50.5) | 3 (13.0) | 67 (47.9) |
| >0.3 mg/dl | 6 (27.3) | 47 (49.5)a | 20 (87.0) | 73 (52.1) |
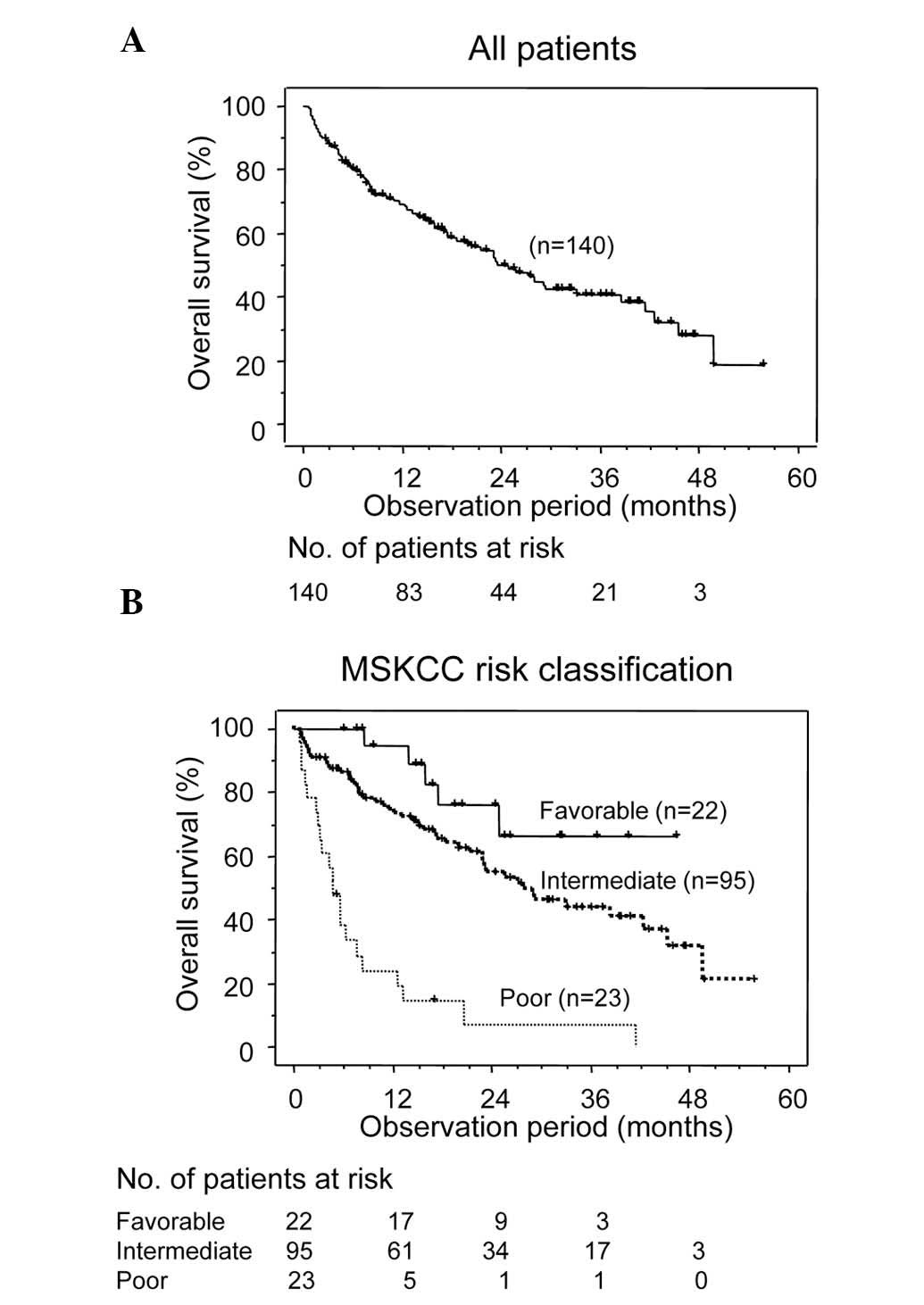
The OS curve for the entire cohort of patients is
shown in Fig. 1A. During the
follow-up period, which was a median of 15.6 months, 70/140 (50.0%)
patients succumbed due to RCC and 3/140 (2.1%) patients succumbed
due to other causes. The 1-, 2- and 3-year OS rates for the entire
cohort were 69.3, 49.9 and 40.6%, respectively. The OS curves for
the three MSKCC risk groups are shown in Fig. 1B. The 1-, 2- and 3-year OS rates for
group F (94.7, 75.9 and 66.4%, respectively) and group I (74.3,
54.4 and 43.7%, respectively) were significantly increased
(P<0.0001) compared with those for group P (19.1, 7.2 and 0%,
respectively). To elucidate the prognosis of the patients in group
I more accurately, the patients in group I were further classified
into two subgroups based on age, serum CRP levels prior to
commencing molecular targeted therapy, prior nephrectomy,
first-line drug treatment, bone metastasis and the number of
metastatic organs. When CRP levels ≤0.3 mg/dl were considered to be
normal, the 1-, 2- and 3-year OS rates of the patients in the
normal-CRP subgroup (91.3, 69.3 and 62.3%, respectively) were
significantly increased (P<0.0001) compared with those of the
patients in the high-CRP subgroup (56.5, 38.7 and 24.6%,
respectively) (Fig. 2F).
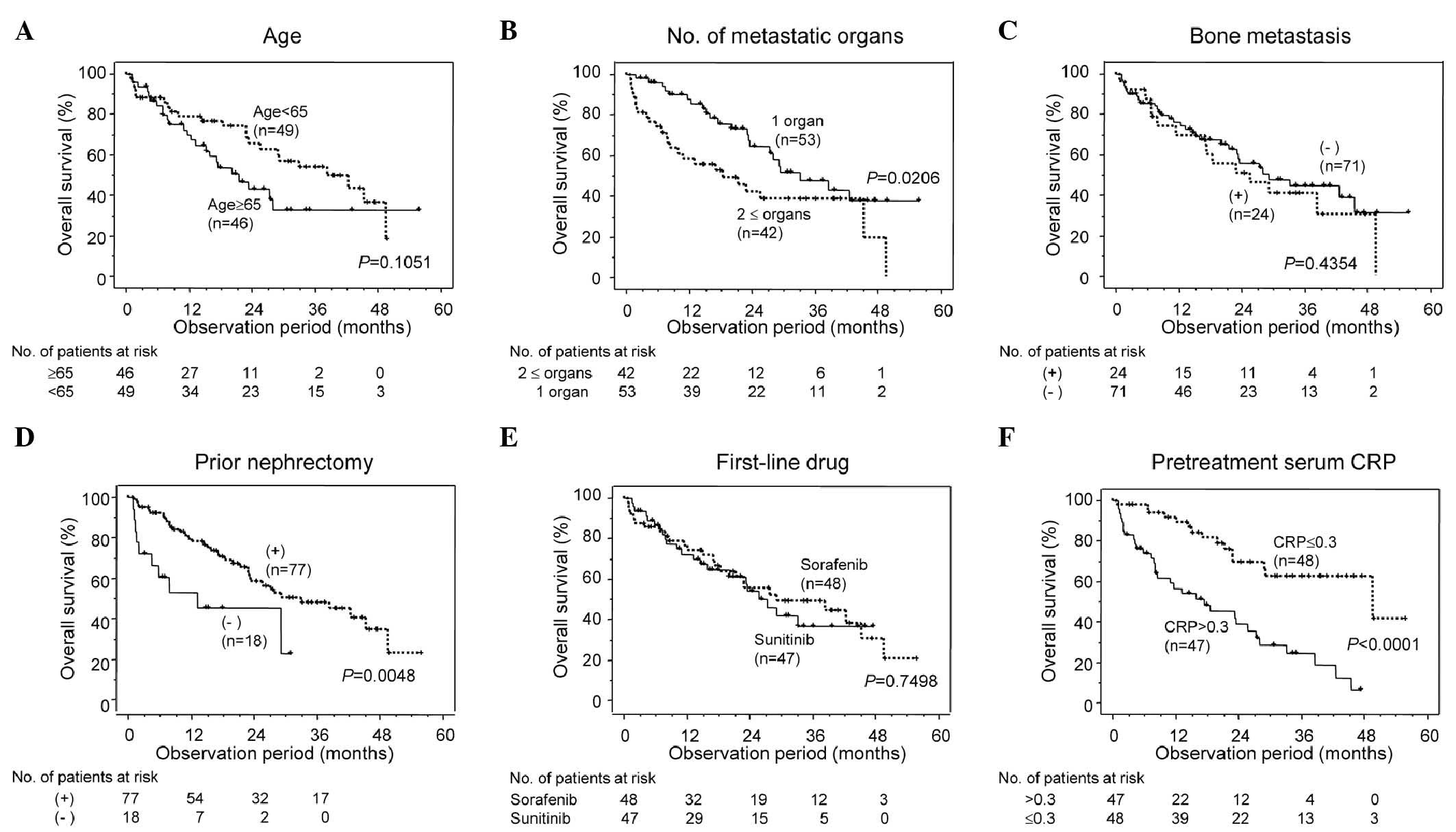
When patients in group I were classified into two
subgroups based on the five remaining factors, the OS of the
patients with two or more metastatic organs was found to be
significantly decreased (P=0.0206) compared with that of the
patients with one or no metastatic organs, and the OS of the
patients with no prior nephrectomy was significantly decreased
(P=0.0048) compared with that of those who had previously undergone
nephrectomy (Fig. 2B and D).
Multivariate analyses revealed that pre-treatment serum CRP levels
and prior nephrectomy were independent prognostic factors for OS in
the patients in group I (P<0.0001 and P=0.0313, respectively)
(Table II).
 | Table IIMultivariate analyses of the
association between various paramaters and overall survival in
patients with intermediate-risk metastatic renal cell
carcinoma. |
Table II
Multivariate analyses of the
association between various paramaters and overall survival in
patients with intermediate-risk metastatic renal cell
carcinoma.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Parameter | P-value
(log-rank) | HR | 95% CI | P-value |
|---|
| Number of metastatic
organs (1 vs. ≥2) | 0.0206 | 0.561 | 0.302–1.043 | 0.0677 |
| Bone metastasis | 0.4354 | - | - | - |
| Prior
nephrectomy | 0.0048 | 2.394 | 1.082–5.298 | 0.0313 |
| First-line drug | 0.7498 | - | - | - |
| Pre-treatment CRP
level (normal vs. abnormal) | <0.0001 | 3.898 | 2.062–7.370 | <0.0001 |
| Age at diagnosis
(years; <65 vs. ≥65) | 0.1051 | - | - | - |
The OS of the patients in the high-CRP subgroup of
group I was significantly decreased compared with that of the
patients in group F (P=0.0126), while it was significantly
increased compared with that of the patients in group P (P=0.0009).
Furthermore, OS rates were not observed to differ significantly
between the patients in group F and the patients in the normal-CRP
subgroup of group I (P=0.7556) (Figs.
1B and 2F). These data show
that the patients in group I could be divided into two subgroups
with different prognoses based on pre-treatment CRP levels.
Discussion
The present study elucidated the efficacy of using
pre-treatment CRP levels to further classify the MSKCC risk
classification’s intermediate-risk group into two subgroups with
significantly different prognoses in the molecular targeted therapy
era. Molecular targeted therapy has markedly changed the treatment
strategy for mRCC; therefore, establishing an enhanced risk
classification for patients with mRCC is important. The MSKCC risk
classification system is widely used globally. Patil et al
(19) analyzed prognostic factors
in a randomized study using sunitinib or interferon-α and
demonstrated the use of the same five factors used in the MSKCC
risk classification system. This study suggests that the MSKCC risk
classification system is useful in the molecular targeted therapy
era, even though it was based on data of patients with mRCC treated
during the cytokine therapy era. However, for Japanese patients
with mRCC, there are certain differences with regard to the
distribution of each group in the MSKCC risk classification, and an
increased OS time was reported for patients with mRCC in each group
(13). The intermediate-risk group
(group I) is the largest of the three risk groups in numerous
studies, including the present study (Table I) (10–13).
In the present study, while there were a number of significant
differences in background and OS rate between the patients in group
I compared with those in group P, there were not as many
differences between the patients in group I and group F (Table I, Fig.
1). Thus, group I may include patients with quite different
prognoses and should be divided into two subgroups in order to
determine the prognosis of the patients more accurately.
Previous studies have demonstrated several
prognostic factors for advanced RCC besides those used in the MSKCC
risk classification system. Bone metastasis has been reported to be
a predictive factor associated with poor prognosis (20) and Mekhail et al (12) demonstrated the importance of the
number and site of metastases in the study for external validation
of the MSKCC risk classification system. Furthermore, several
studies have reported the impact of cytoreductive nephrectomy as an
independent prognostic factor for OS in patients with mRCC
(21–23). Serum CRP levels have been suggested
to be one of the most important prognostic factors for advanced RCC
(14). Pre-operative serum CRP
levels have been reported to be a predictor for metastasis and
mortality following radical nephrectomy in patients with localized
RCC (15,16). In addition, in patients with mRCC,
CRP kinetics have been demonstrated to be a predictor for clinical
course (17).
In the present study, the patients in group I were
classified into two subgroups based on age at initial presentation,
serum CRP levels prior to commencing molecular targeting therapy,
previous nephrectomy, the existence of bone metastases and the
number of metastatic organs. The OS rate of the patients whose
pre-treatment CRP levels were ≤0.3 mg/dl was found to be
significantly increased compared with that of the patients whose
pre-treatment CRP levels were >0.3 mg/dl. In addition, the OS
rate of the patients who had previously undergone nephrectomy was
significantly increased compared with that of the patients who had
not undergone nephrectomy (Fig. 2).
Furthermore, high pre-treatment serum CRP levels and prior
nephrectomy were identified to be independent prognostic factors
for OS rate in patients with mRCC (Table II). CRP is one of the most
representative acute-phase reactants produced primarily in the
liver in response to inflammatory reactions through interleukin
(IL)-6 signaling (24). Elevated
CRP levels are observed during infection, cardiovascular diseases,
diabetes and malignancies (24).
Several studies have reported an association between elevated serum
CRP levels and malignant diseases other than RCC, including
colorectal cancer (25), lung
cancer (26) and urothelial cancer
(27). The mechanism through which
a systemic inflammatory response, indicated by elevated CRP levels,
reduces OS rates in patients with mRCC is still unclear. However,
certain relevant experimental data have been reported (28,29). A
previous study showed that the release of cytokines and growth
factors in an inflammatory response stimulates tumor growth
(28). Furthermore, it has been
demonstrated that renal cancer cells may produce IL-6, which may
promote the growth of renal cancer cells in an autocrine manner
(29). These reports suggest that
CRP may have an important role in the progression of RCC. Measuring
serum CRP levels is useful, simple, inexpensive and reproducible.
Thus, serum CRP levels may be a powerful biomarker for mRCC.
One limitation of the present study is that it is
retrospective; therefore, patient selection bias may exist. For
example, the patients enrolled in the present study included those
in whom mRCC was first diagnosed and those in whom mRCC recurred
following radical nephrectomy. In the present study, ‘prior
nephrectomy’ refers to both cytoreductive nephrectomy for mRCC and
radical nephrectomy prior to recurrence. Further prospective
investigations are required to confirm the potential of
pre-treatment serum CRP levels to be used to predict prognosis in
patients with intermediate-risk mRCC. In conclusion, the present
study demonstrated the role of serum CRP levels as a prognostic
factor in patients with mRCC in the intermediate-risk group, prior
to being treated with molecular targeted therapy. Further
classifying the patients with different prognoses in the
intermediate-risk group may help determine the prognosis of
patients with mRCC more accurately. Further studies are required to
establish an enhanced strategy for the use of novel molecular
targeted agents, based on more accurate risk classification,
involving the consideration of patient CRP levels.
Acknowledgements
The authors would like to thank Dr Koji Mita, Dr
Yuhei Takahiro, Dr Yoshikatsu Kobukata, Dr Tateki Yoshino, Dr
Hiroyuki Moriyama, Dr Hiroshi Masumoto, Dr Seiji Fujiwara, Dr
Yuichi Kadonishi, Dr Mitsuru Nakahara, Dr Koji Ueda, Dr Yasuhisa
Hasegawa, Dr Masami Mizutani, Dr Kenichiro Fukuoka, Dr Masanobu
Shigeta and Dr Hideki Mochizuki for their help with the data
collection.
References
|
1
|
Vogl UM, Zehetgruber H, Dominkus M, et al:
Prognostic factors in metastatic renal cell carcinoma:
metastatectomy as independent prognostic variable. Br J Cancer.
95:691–698. 2006.
|
|
2
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Sunitinib versus interferon alfa in metastatic renal-cell
carcinoma. New Engl J Med. 356:115–124. 2007.
|
|
3
|
Motzer RJ, Escudier B, Oudard S, et al:
Efficacy of everolimus in advanced renal cell carcinoma: a
double-blind, randomized, placebo-controlled phase III trial.
Lancet. 372:449–456. 2008.
|
|
4
|
Hudes G, Carducci M, Tomczak P, et al:
Temsirolimus, interferon alfa, or both for advanced renal-cell
carcinoma. N Engl J Med. 356:2271–2281. 2007.
|
|
5
|
Uemura H, Shinohara N, Yuasa T, et al: A
phase II study of sunitinib in Japanese patients with metastatic
renal cell carcinoma: insights into the treatment, efficacy and
safety. Jpn J Clin Oncol. 40:194–202. 2010.
|
|
6
|
Tomita Y, Shinohara N, Yuasa T, et al:
Overall survival and updated results from a phase II study of
sunitinib in Japanese patients with metastatic renal cell
carcinoma. Jpn J Clin Oncol. 40:1166–1172. 2010.
|
|
7
|
Akaza H, Tsukamoto T, Murai M, Nakajima K
and Naito S: Phase II study to investigate the efficacy, safety,
and pharmacokinetics of sorafenib in Japanese patients with
advanced renal cell carcinoma. Jpn J Clin Oncol. 37:755–762.
2007.
|
|
8
|
Tsukamoto T, Shinohara N, Tsuchiya N, et
al: Phase III trial of everolimus in metastatic renal cell
carcinoma: subgroup analysis of Japanese patients from RECORD-1.
Jpn J Clin Oncol. 41:17–24. 2011.
|
|
9
|
Fujisaka Y, Yamada Y, Yamamoto N, et al: A
phase 1 clinical study of temsirolimus (CCI-779) in Japanese
patients with advanced solid tumors. Jpn J Clin Oncol. 40:732–738.
2010.
|
|
10
|
Motzer RJ, Mazumdar M, Bacik J, Berg W,
Amsterdam A and Ferrara J: Survival and prognostic stratification
of 670 patients with advanced renal cell carcinoma. J Clin Oncol.
17:2530–2540. 1999.
|
|
11
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002.
|
|
12
|
Mekhail TM, Abou-Jawde RM, Boumerhi G, et
al: Validation and extention of the Memorial Sloan-Kettering
prognostic factors model for survival in patients with previously
untreated metastatic renal cell carcinoma. J Clin Oncol.
23:832–841. 2005.
|
|
13
|
Naito S, Yamamoto N, Takayama T, et al:
Prognosis of Japanese metastatic renal cell carcinoma patients in
the cytokine era: a cooperative group report of 1463 patients. Eur
Urol. 57:317–325. 2010.
|
|
14
|
Wu Y, Fu X, Zhu X, et al: Prognostic role
of systemic inflammatory response in renal cell carcinoma: a
systemic review and meta-analysis. J Cancer Res Clin Oncol.
137:887–896. 2011.
|
|
15
|
Johnson TV, Abbasi A, Owen-Smith A, et al:
Absolute preoperative C-reactive protein predicts metastasis and
mortality in the first year following potentially curative
nephrectomy for clear cell renal cell carcinoma. J Urol.
183:480–485. 2010.
|
|
16
|
Komai Y, Saito K, Sakai K and Morimoto S:
Increased preoperative serum C-reactive protein level predicts a
poor prognosis in patients with localized renal cell carcinoma. BJU
Int. 99:77–80. 2006.
|
|
17
|
Saito K, Tatokoro M, Fujii Y, et al:
Impact of C-reactive protein kinetics on survival of patients with
metastatic renal cell carcinoma. Eur Urol. 55:1145–1153. 2009.
|
|
18
|
Saito K and Kihara K: C-reactive protein
as a biomarker for urological cancers. Nat Rev Urol. 8:659–666.
2011.
|
|
19
|
Patil S, Figlin RA, Hutson TE, et al:
Prognostic factors for progression-free and overall survival with
sunitinib targeted therapy and with cytokine as first-line therapy
in patients with metastatic renal cell carcinoma. Ann Oncol.
22:295–300. 2011.
|
|
20
|
Beuselinck B, Oudard S, Rixe O, et al:
Negative impact of bone metastasis on outcome in clear-cell renal
cell carcinoma treated with sunitinib. Ann Oncol. 22:794–800.
2011.
|
|
21
|
Choueiri TK, Xie W, Kollmannsberger C, et
al: The impact of cytoreductive nephrectomy on survival of patients
with metastatic renal cell carcinoma receiving vascular endothelial
growth factor targeted therapy. J Urol. 185:60–66. 2011.
|
|
22
|
Polcari AJ, Gorbonos A, Milner JE and
Flanigan RC: The role of cytoreductive nephrectomy in the era of
molecular targeted therapy. Int J Urol. 16:227–233. 2009.
|
|
23
|
Crispen PL and Blute ML: Role of
cytoreductive nephrectomy in the era of targeted therapy for renal
cell carcinoma. Curr Urol Rep. 13:38–46. 2012.
|
|
24
|
Heikkilä K, Ebrahim S and Lawlor DA: A
systemic review of the association between circulating
concentrations of C reactive protein and cancer. J Epidemiol
Community Health. 61:824–833. 2007.
|
|
25
|
Canna K, McMillan DC, McKee RF, McNicol
AM, Horgan PG and McArdle CS: Evaluation of a cumulative prognostic
score based on the systemic inflammatory response in patients
undergoing potentially curative surgery for colorectal cancer. Br J
Cancer. 90:1707–1709. 2004.
|
|
26
|
McKeown DJ, Brown DJ, Kelly A, Wallace AM
and McMillan DC: The relationship between circulating
concentrations of C-reactive protein, inflammatory cytokines and
cytokine receptors in patients with non-small-cell lung cancer. Br
J Cancer. 91:1993–1995. 2004.
|
|
27
|
Yoshida S, Saito K, Koga F, et al:
C-reactive protein level predicts prognosis in patients with
muscle-invasive bladder cancer treated with chemotherapy. BJU Int.
101:978–981. 2008.
|
|
28
|
Abramovitch R, Marikovsky M, Meir G and
Neeman M: Stimulation of tumour growth by wound-derived growth
factors. Br J Cancer. 79:1392–1398. 1999.
|
|
29
|
Miki S, Iwano M, Miki Y, et al:
Interleukin-6 (IL-6) functions as an in vitro autocrine growth
factor in renal cell carcinomas. FEBS Lett. 250:607–610. 1989.
|