Introduction
Thymic carcinoma is a rare cancer with a poor
prognosis (5-year survival rate, 20–30%; 2-year survival rate, 50%)
due to rapid extension in comparison with thymoma (1). Thymic carcinoma was first reported by
Shimosato et al, and later, was classically distinguished
from type C thymoma (2). A total of
13 subtypes have been defined according to the classification of
the World Health Organization (WHO) (3). Due to the presence of thymic
non-organotypia, no symptomatic paraneoplastic syndrome appears, so
patients are typically diagnosed with progressive disease when they
initially present with symptoms associated with tumor extension. In
terms of prognostic factors, staging and grade of atypia are
significant (4,5). Definitive clinical management and
treatment have usually been determined with a high level of
evidence for common cancers, whereas clinical entities are
unavailable for rare cancers, such as thymic carcinoma, due to the
lack of large-scale clinical trials. Treatment options are thus
selected by the individual physician and appear to be determined
according to the prognosis.
The present study reports a rare case of long-term
survival for 10 years in a patient with metastatic thymic carcinoma
who could be treated using chemotherapy and radiotherapy for local
control with palliative intent. Patient provided written informed
consent.
Case report
A 65-year-old male presented to the Tokyo
Metropolitan Cancer and Infectious disease Center Komagome Hospital
(Tokyo, Japan) with a dry cough and mild hemoptysis. The patient
had been treated with chemotherapy since 2002 for advanced thymic
squamous cell carcinoma, and showed solitary cerebral and pulmonary
metastases in the right lower lobe (RLL) on presentation. At the
time of the initial diagnosis, a specimen was acquired from the
primary site using computed tomography-guided biopsy. First-line
chemotherapy was comprised of cisplatin (80 mg/m2 on day
one, every 28 days) and irinotecan (60 mg/m2 on days
one, eight and 15, every 28 days). The patient subsequently
underwent irradiation of the primary site and pulmonary metastasis
in the RLL with a total dose of 60 Gy over 6 months, followed by
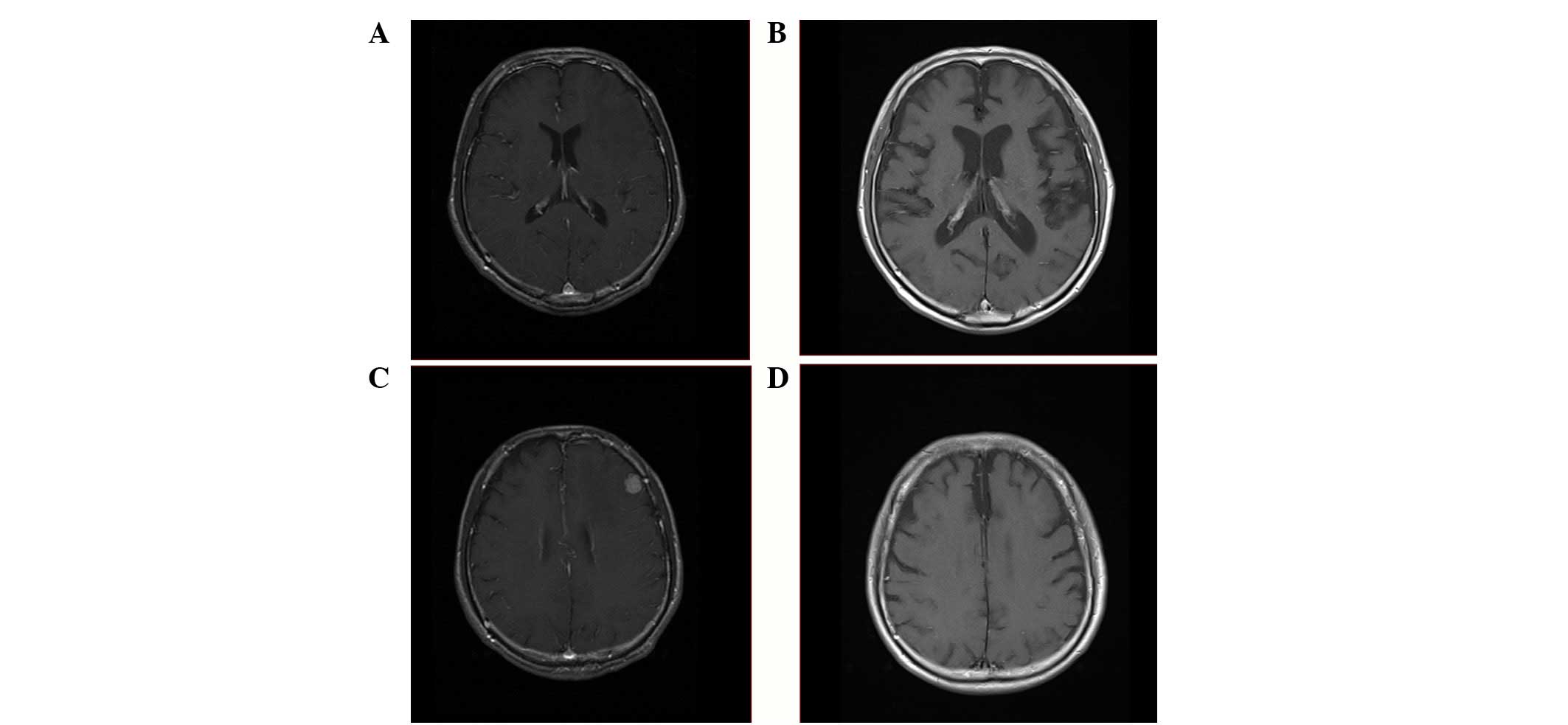
whole-brain irradiation (WBI) for the solitary brain metastasis
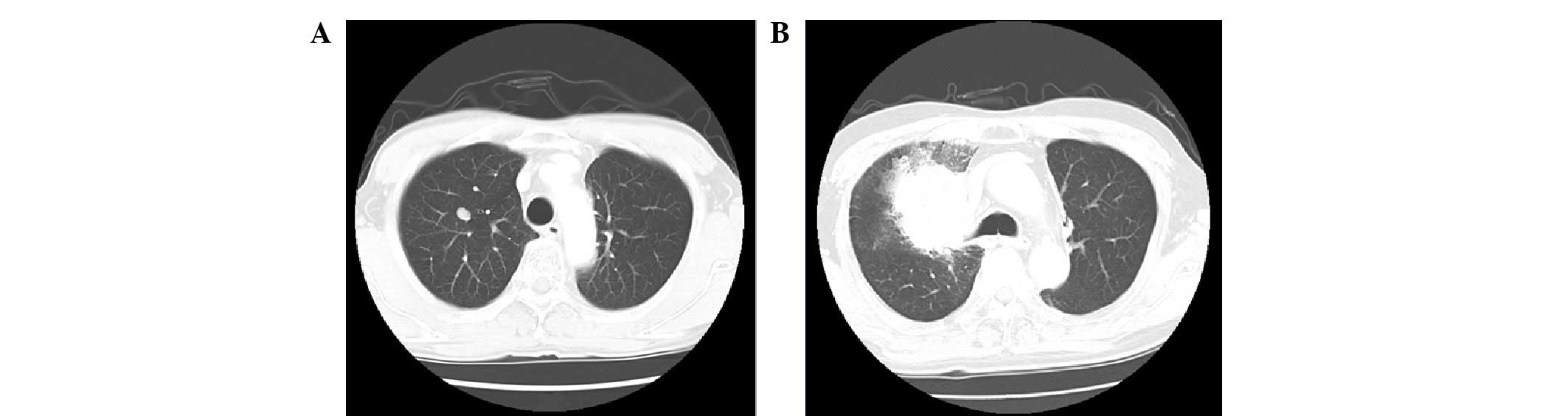
with a dose of 40 Gy two years into therapy (Fig. 1). In terms of the clinical course,
cognitive function gradually declined due to WBI, and cerebral
atrophy was shown on head imaging (Fig.
2). Pulmonary metastasis in the RUL was histologically
confirmed as thymic squamous cell carcinoma following
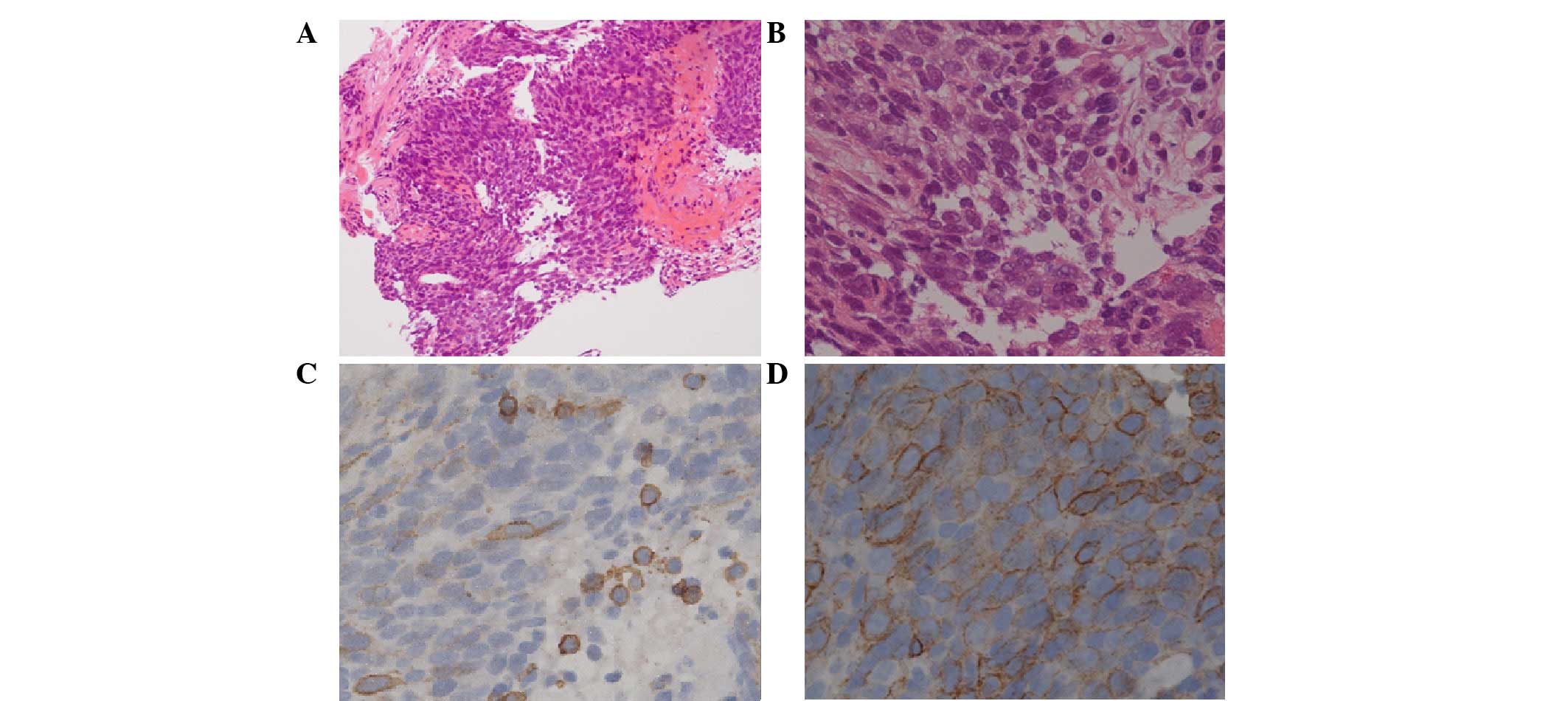
transbronchial biopsy (Fig. 3).
Histologically, the tumor was composed of highly atypical
epithelioid cells, with nuclear atypia and eosinophilic cytoplasms
in the fibrotic stroma (Fig. 3A and
B). Immunohistochemically, the tumor cells were positive for
CD5 and c-Kit (Fig. 3C and D). The
patient was treated with nine lines of chemotherapy and palliative
radiotherapy for local control of the brain metastasis and two
pulmonary metastases. The patient then underwent second-line
chemotherapy comprised of two cycles of carboplatin (area under the
curve 6 on day one, every 21 days)/paclitaxel (80 mg/m2
on days one, eight and 15, every 21 days). In the fourth year,
pulmonary metastasis in the RUL was targeted and irradiated once at
a dose of 60 Gy, but recurrence subsequently developed. Following
completion of radiation therapy, the patient received six lines of
chemotherapy in 4 years, comprised of four cycles of gemcitabine
(800 mg/m2 on days one and eight, every 21 days) and
vinorelbine (25 mg/m2 on days one and eight, every 21
days), two cycles of docetaxel (60 mg/m2 on day one,
every 21 days), 12 cycles of S-1 (80 mg/m2 on days one
to 14, every 21 days), four cycles of amrubicin (35
mg/m2 on days one to three, every 21–28 days), eight
cycles of irinotecan (100 mg/m2 on days one, eight and
15, every 28 days), three cycles of pemetrexed (500
mg/m2 on day one, every 21 days), and lastly, the
combination of four cycles of doxorubicin 40 mg/m2,
cisplatin (50 mg/m2 on day one, every 21 days),
vincristine (0.6 mg/m2 on day three, every 21 days) and
cyclophosphamide (700 mg/m2 on day four, every 21 days)
(ADOC). The patient succumbed to carcinomatous lymphangiosis in the
10th year of treatment following the initiation of first-line
chemotherapy.
Discussion
The patient in the present study presented with
thymic carcinoma with distant metastasis at the time of the initial
diagnosis (stage IVb according to the Masaoka-Koga staging system)
and was a long-term survivor who was treated for 10 years with
chemotherapy and radiotherapy for local control with palliative
intent. Local irradiation enabled control of the primary site and
pulmonary metastasis in the RLL. Although the brain metastasis was
also well controlled with WBI, cognitive function declined as a
result of this treatment.
Thymic carcinoma is classified as a type C thymoma
with strong atypia according to the 1999 WHO classification, on the
basis of the classification by Müller-Hermelink et al
(6). In the 2004 WHO
classification, thymic carcinoma was categorized separately from
thymoma. Histological subtypes of thymic carcinoma include squamous
and lymphoepithelioma-like carcinoma, comprising 60–70% of cases
(3). A diagnosis can be reached
using immunohistochemical staining for cluster of differentiation
(CD)5 and c-kit, with c-kit expression appearing more frequently in
thymic carcinoma (75%) than in thymoma (2%) (7). The expression of insulin-like growth
factor 1 in thymic epithelial tumors is associated with prognosis
in these patients (8). As there is
a loss of thymic organo-specific characteristics that induce
CD4/CD8 double-positive T cells, as seen in myasthenia gravis and
pure red cell aplasia, symptomatic paraneoplastic syndrome does not
appear. Patients with thymic carcinoma are thus usually diagnosed
with progressive disease subsequent to presenting with symptoms
associated with tumor extension.
As first-line chemotherapy for advanced thymic
carcinoma, cisplatin and anthracycline-based chemotherapies, such
as ADOC (9) and the combination of
cisplatin, Adriamycin and cyclophosphamide (10), are applied in the clinical setting
based on Einhorn’s protocol for germ cell tumors. Only a
prospective phase II trial with carboplatin and paclitaxel for
unresectable stages has been performed, indicating the efficacy of
platinum-based doublet chemotherapy (11). With regard to second-line
chemotherapies, evidence for efficacious regimens has not been
presented and almost all reported series have included only small
numbers of patients (12). In the
present case, ad hoc treatment with singlet chemotherapy or local
treatment with radiotherapy were compatible with long-term
survival. Each treatment period was not particularly long, but
progression-free intervals were modest. In previously reported
cases, the prognosis of stage IVb tumors, as classified in the
Masaoka-Koga Stating System of thymic carcinoma, was in the range
of 19–46 months (13). In the
present case, nine lines of chemotherapy appeared to be beneficial
for inhibiting disease progression as salvage chemotherapy,
although clinical progress is rarely indolent. We previously
documented a first-line chemotherapy response rate of 47.5% and a
median survival time of 24.5 months. The overall survival rates at
1, 2, and 5 years were recorded as 72.5, 52.5 and 17.5%,
respectively (14). In general,
thymic carcinoma demonstrates an aggressive clinical course, but
~20% of patients treated with palliative-intent chemotherapy for
advanced thymic carcinoma survive for 5 years. Oncologists should
be mindful of the fact that a substantial proportion of patients
with advanced thymic carcinoma show an indolent clinical process.
In the present case, the primary site, pulmonary metastasis at
initial diagnosis and solitary brain metastasis were locally
controlled using radiotherapy alone at a dose of 40–60 Gy. No
evidence of recurrence was demonstrated radiologically at the end
stage in this patient. In terms of the clinical process, the thymic
carcinoma was indolent and sensitive to treatment. However,
cognitive function was adversely affected by whole-brain
metastasis, with an unexpected longer survival time. Patients with
indolent thymic carcinoma may survival longer, so stereotactic
radiotherapy, such as cyberknife or γ-knife surgery, is reasonable
to minimize the effects on cognitive function.
In conclusion, thymic carcinoma is known to have a
poor prognosis and an aggressive clinical process, however,
clinically indolent patients with advanced thymic carcinoma are
occasionally encountered. Treatment modalities should thus be
prudently selected while considering the possibility of long-term
survival, although optimal management for advanced thymic carcinoma
has yet to be defined due to the rarity of this pathology. A high
level of evidence for the clinical management of rare cancers
cannot realistically be determined in large prospective clinical
studies, so longer follow-up periods for minimally invasive
treatments are warranted. Retrospective multiple-institution
registries will also provide useful clues to deciding on the
clinical management in such rare cancers.
References
|
1
|
Eng TY, Fuller CD, Jagirdar J, Bains Y and
Thomas CR Jr: Thymic carcinoma: state of the art review. Int J
Radiat Oncol Biol Phys. 59:654–664. 2004.
|
|
2
|
Shimosato Y, Kameya T, Nagai K and Suemasu
K: Squamous cell carcinoma of the thymus. An analysis of eight
cases. Am J Surg Pathol. 1:109–121. 1977.
|
|
3
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: World health organization classification of
tumors. Pathology and Genetics of Tumors of the Lung, Pleura,
Thymus and Heart. Chapter 3. 3rd edition. IARC Press; Lyon: pp.
145–197. 2004
|
|
4
|
Okumura M, Ohta M, Tateyama H, et al: The
world health organization histologic classification system reflects
the oncologic behavior of thymoma: a clinical study of 273
patients. Cancer. 94:624–632. 2002.
|
|
5
|
Quintanilla-Martinez L, Wilkins EW Jr,
Choi N, Efird J, Hug E and Harris NL: Thymoma. Histologic
subclassification is an independent prognostic factor. Cancer.
74:606–617. 1994.
|
|
6
|
Müller-Hermelink HK, Marino M and Palestro
G: Pathology of thymic epithelial tumors. Curr Top Pathol.
75:207–268. 1986.
|
|
7
|
Petrini I, Zucali PA, Lee HS, et al:
Expression and mutational status of c-kit in thymic epithelial
tumors. J Thorac Oncol. 5:1447–1453. 2010.
|
|
8
|
Mimae T, Tsuta K, Kondo T, et al: Protein
expression and gene copy number changes of receptor tyrosine kinase
in thymomas and thymic carcinomas. Ann Oncol. 23:3129–3137.
2012.
|
|
9
|
Fornasiero A, Daniele O, Ghiotto C, et al:
Chemotherapy for invasive thymoma. A 13-year experience. Cancer.
68:30–33. 1991.
|
|
10
|
Loehrer PJ Sr, Kim K, Aisner SC, et al:
Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or
recurrent thymoma: final results of an intergroup trial. The
Eastern Cooperative Oncology Group, Southwest Oncology Group, and
Southeastern Cancer Study Group. J Clin Oncol. 12:1164–1168.
1994.
|
|
11
|
Lemma GL, Lee JW, Aisner SC, et al: Phase
II study of carboplatin and paclitaxel in advanced thymoma and
thymic carcinoma. J Clin Oncol. 29:2060–2065. 2011.
|
|
12
|
National Comprehensive Cancer Network.
Thymomas and thymic carcinomas (version 1. 2014). http://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf.
Accessed April 1, 2014
|
|
13
|
Girard N: Thymic epithelial tumours: From
basic principles to individualised treatment strategies. Eur Respir
Rev. 22:75–87. 2013.
|
|
14
|
Okuma Y, Hosomi Y, Takagi Y, et al:
Clinical outcomes with chemotherapy for advanced thymic carcinoma.
Lung Cancer. 80:75–80. 2013.
|