Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide. Chemotherapy is the
predominant treatment for lung cancer, which may improve patient
survival and quality of life, particularly in advanced cases
(1). Cisplatin is one of the
cytotoxic agents used in clinical chemotherapy. However, the
therapeutic effects of cisplatin are limited due to intrinsic or
acquired drug resistance. Anticancer drugs used in chemotherapy may
increase the acquired resistance of tumor cells. This increased
resistance enhances tumor metastasis, which further increases their
drug resistance (2,3). At present, none of the available
treatment regimens are capable of preventing the metastasis of
drug-resistant tumor cells. Epidermal growth factor receptor (EGFR)
has been found to correlate with key characteristics of cancer,
including cell proliferation, apoptosis and tumor metastasis
(4,5), and the dysregulation of EGFR has been
associated with chemoresistance in lung cancer (6,7).
Gefitinib and erlotinib are EGFR-tyrosine kinase inhibitors (TKIs)
that have been approved for lung cancer treatment (8). Clinical studies have shown that these
EGFR-TKIs were effective in patients who had been treated
previously with multiple cytotoxic agents, however, no significant
effects were identified in patients who had not received
chemotherapy (9–12). AG1478 is a quinazoline with a
similar chemical structure and mechanism of action as erlotinib and
gefitinib (13,14). To determine whether AG1478 inhibits
A549/DDP cell growth, migration and invasion in vitro, the
antitumor mechanism of AG1478 in the A549/DDP and A549 cell lines
was investigated.
Materials and methods
Reagents
AG1478 was purchased from Merck KGaA (Darmstadt,
Germany). The rabbit polyclonal antibody against matrix
metalloproteinase (MMP)-9, the retinoblastoma (Rb) antibody sampler
kit, including the phosphor-Rb antibodies Ser780, Ser795 and
Ser807, as well as the total Rb mouse monoclonal antibody and the
rabbit monoclonal antibody against GAPDH (14C10) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). The
horseradish peroxidase-conjugated affinipure goat anti-rabbit IgG
(H+L) and goat anti-mouse IgG (H+L) secondary antibodies were
purchased from ZSGB-BIO (Beijing, China) and the reverse
transcription and quantitative polymerase chain reaction (qPCR)
kits were purchased from Takara Biotechnology (Dalian) Co., Ltd.,
(Dalian, China). The rabbit polyclonal antibody against E2F1 was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The E2F1 short-interfering RNA (siRNA) and HiPerFect
transfection reagent were purchased from Qiagen (Hilden, Germany),
and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) was purchased from Invitrogen Life Technologies (Carlsbad,
CA, USA).
Cell lines
The cisplatin-resistant A549/DDP and
cisplatin-sensitive A549 cell lines were provided by the Tianjin
Lung Cancer Institute (Tianjin, China). Cells were cultured and
maintained in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS) and 2 mmol/l glutamine (both Gibco-BRL, Grand Island,
NY, USA) at 37°C in a humidified atmosphere of 5%
CO2.
Cell proliferation assay
Cells were cultured in 96-well plates (8,000
cells/well) overnight and treated with dimethyl sulfoxide (DMSO) as
the control or AG1478 for 48 h. The effects of AG1478 on the
proliferation of the A549/DDP and A549 cell lines were measured
using the MTT assay, as previously described (15). The MTT solution (5 mg/ml) was added
to the cell cultures and incubated for 4 h at 37°C. The cell
suspensions were treated with DMSO and subjected to colorimetric
measurement at a wavelength of 570 nm using the TriStar LB 941
apparatus (Berthold Technologies U.K. Ltd., Harpenden, UK). DMSO
was used for the blank absorbance readings. The rate of cell growth
inhibition and IC50 were calculated using the GraphPad
Prism 4 software (GraphPad Software, Inc., La Jolla, CA, USA).
Wound healing assay
The cells were seeded in six-well plates to 100%
confluence. A wound was induced by scratching the cell cultures
with a pipette tip. Following rinsing with phosphate-buffered
saline (PBS) to remove the detached cells, AG1478 (at near
IC50 concentration) was added to culture in a 5%
CO2 incubator for 48 h at 37°C. The cells were incubated
and allowed to migrate in the medium. Images were immediately
captured from each well, and again after 48 h using a TE2000
inverted fluorescence microscope (Nikon Corporation, Tokyo, Japan)
in four random fields at ×40 magnification. The width of the wound
at these specific locations was visualized on each plate to
quantify the rate of cell migration.
Cell invasion assay
Cell invasion assays were performed using 24-well
Transwell plates (8 mm pore size; Corning Inc., Acton, MA, USA)
coated with 1 mg/ml Matrigel (BD Biociences, Franklin Lakes, NJ,
USA). A total of 1.0×105 cells/well were suspended in
300 μl of serum-free media and added to the upper compartment of
the Transwell plates. Next, 500 μl complete media containing 10%
FBS was added to the bottom wells of each plate. The cells were
then incubated in a 5% CO2 incubator, with or without
AG1478 (at near IC50 concentration), for 48 h at 37°C.
Invasive and non-invasive cells on the upper and lower surface of
the membrane were stained according to the manufacturer’s
instructions. Non-invasive cells retained in the upper chamber were
removed with a cotton swab and the invasive cells were examined
using a TE2000 inverted fluorescence microscope in ph1 mode (Nikon
Corporation).
Cell cycle analysis
Cell lines were treated with AG1478 (at near
IC50 concentration) for 48 h. Cells were then collected
by trypsinization and fixed with 70% ethanol by incubating them
overnight at 4°C in the dark. The cell pellets were then
resuspended in PBS and stained with propidium iodide/RNase staining
buffer (BD Biociences) for 30 min at 37°C. Analysis was performed
using a FACS Aria flow cytometer (Becton Dickenson, Franklin Lakes,
NJ, USA), and the cell cycle data were processed using the ModFit
LT cell cycle analysis software (Verity Software House, Topsham,
ME, USA).
qPCR analysis
The total RNA was extracted from the cells using
TRIzol (Invitrogen Life Technologies). Reverse transcription was
performed using a DNA Engine Peltier Thermal Cycler (Bio-Rad,
Hercules, CA, USA) using a reverse transcription kit (Takara
Biotechnology (Dalian) Co., Ltd.) according to the manufacturer’s
instructions, as previously described (16). Standard qPCR was performed using the
following primers: Forward, 5′-CATCCCAGGAGGTCACTTCTG-3′ and
reverse, 5′-GACAACAGCGGTTCTTGCTC-3′ for E2F1; forward,
5′-GGGACGCAGACATCGTCATC-3′ and reverse, 5′-TCGTCATCGTCGAAATGGGC-3′
for MMP-9; and forward, 5′-GGAGTCAACGGATTTGGTCG-3′ and reverse,
5′-CTTGATTTTGGAGGGATCTCG-3′ for GAPDH. All primers were synthesized
by the Bejing Genomics Institute (Shenzhen, China). mRNA levels
were detected by qPCR using SYBR Green stain. The PCR reaction
conditions used were as previously described (16).
Western blot analysis
Following treatment with or without AG1478 (at near
IC50 concentration) for 24 and 48 h, the A549/DDP and
A549 cells were lysed in pre-warmed Laemmli buffer (Bio-Rad).
Western blot analysis was performed as previously described
(17). Briefly, the same amount of
total protein from each sample was resolved by SDS-PAGE on a well
of 10% polyacrylamide gel (Amersham Pharmacia Biotech, Piscataway,
NJ, USA) and resolved by SDS-PAGE. The phosphor-Rb (Ser780, Ser795
and Ser807) and total Rb antibodies were used at 1:500 dilutions,
whereas all the other primary antibodies (E2F1, MMP-9 and GAPDH)
were used at 1:750 dilutions. Protein expression was quantified by
densitometry using the Transparency Adapter for PowerLook 2100XL
(UTA-2100XL; UMAX, Mountain View, CA, USA).
E2F1 siRNA transfection
The E2F1 siRNA-1 (5′-CAGGACCTTCGTAGCATTGCA-3′) and
siRNA-2 (5′-ACGCTATGAGACCTCACTGAA-3′) were transfected using the
HiPerFect transfection reagent (Qiagen), according to the
manufacturer’s instructions. Cells were transfected for 24 or 48 h
and washed twice with cold PBS. The cell pellets were subsequently
collected to determine their E2F1 and MMP-9 expression levels using
qPCR and western blot analysis, as described above.
Statistical analysis
Student’s t-test was used to determine the
significance of the differences between the control and
experimental groups. Error bars were used to indicate the standard
deviation of the data and P<0.05 was considered to indicate a
statistically significant difference.
Results
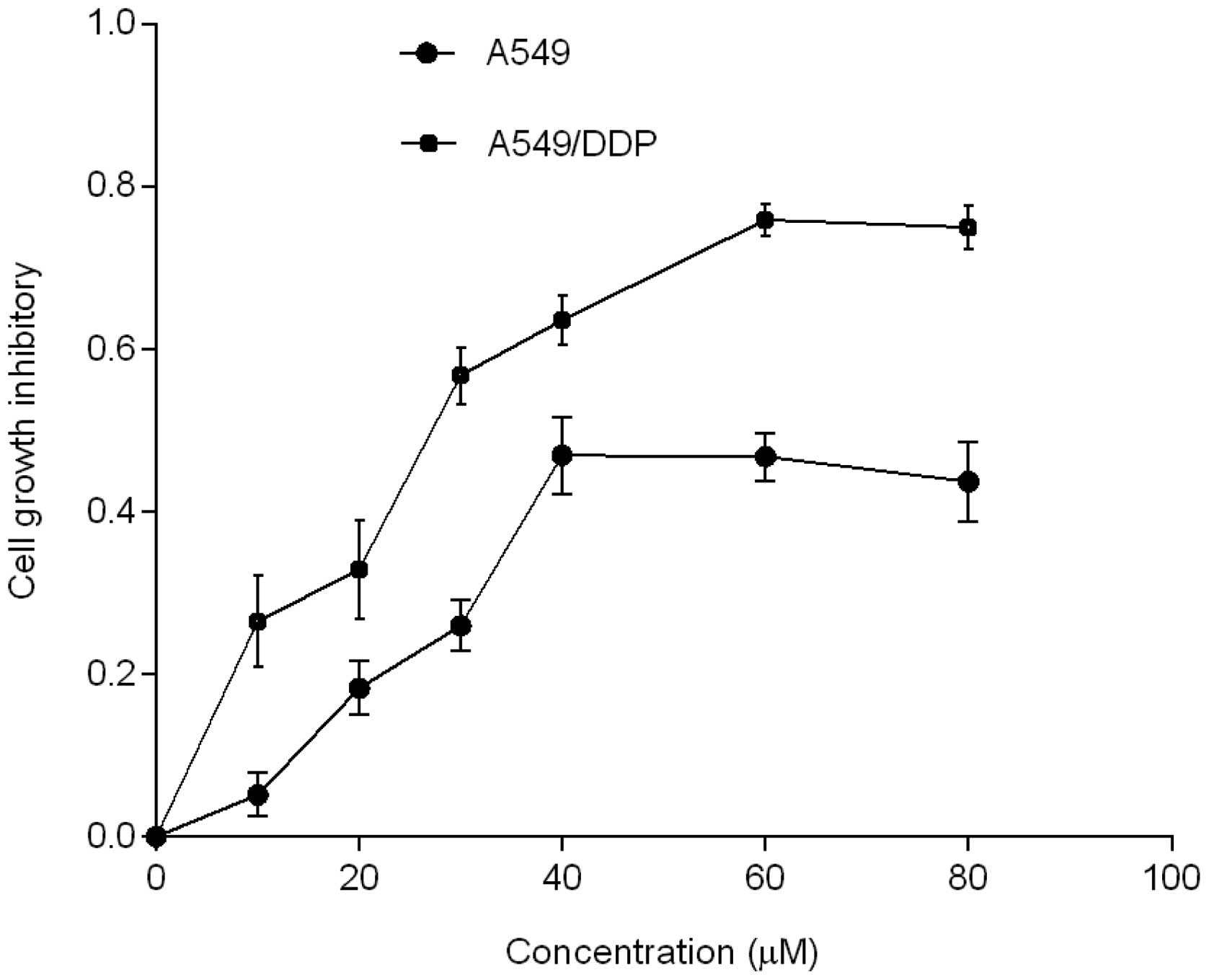
AG1478 inhibits cell proliferation
The results indicated that AG1478 inhibited the
growth of the two cell lines with varying potency. The
IC50 values of AG1478 in the cisplatin-resistant
A549/DDP cell line (33.6±3.45 μM) were lower than those of the
corresponding parental A549 cell line (65.6±5.92 μM), as shown in
Fig. 1.
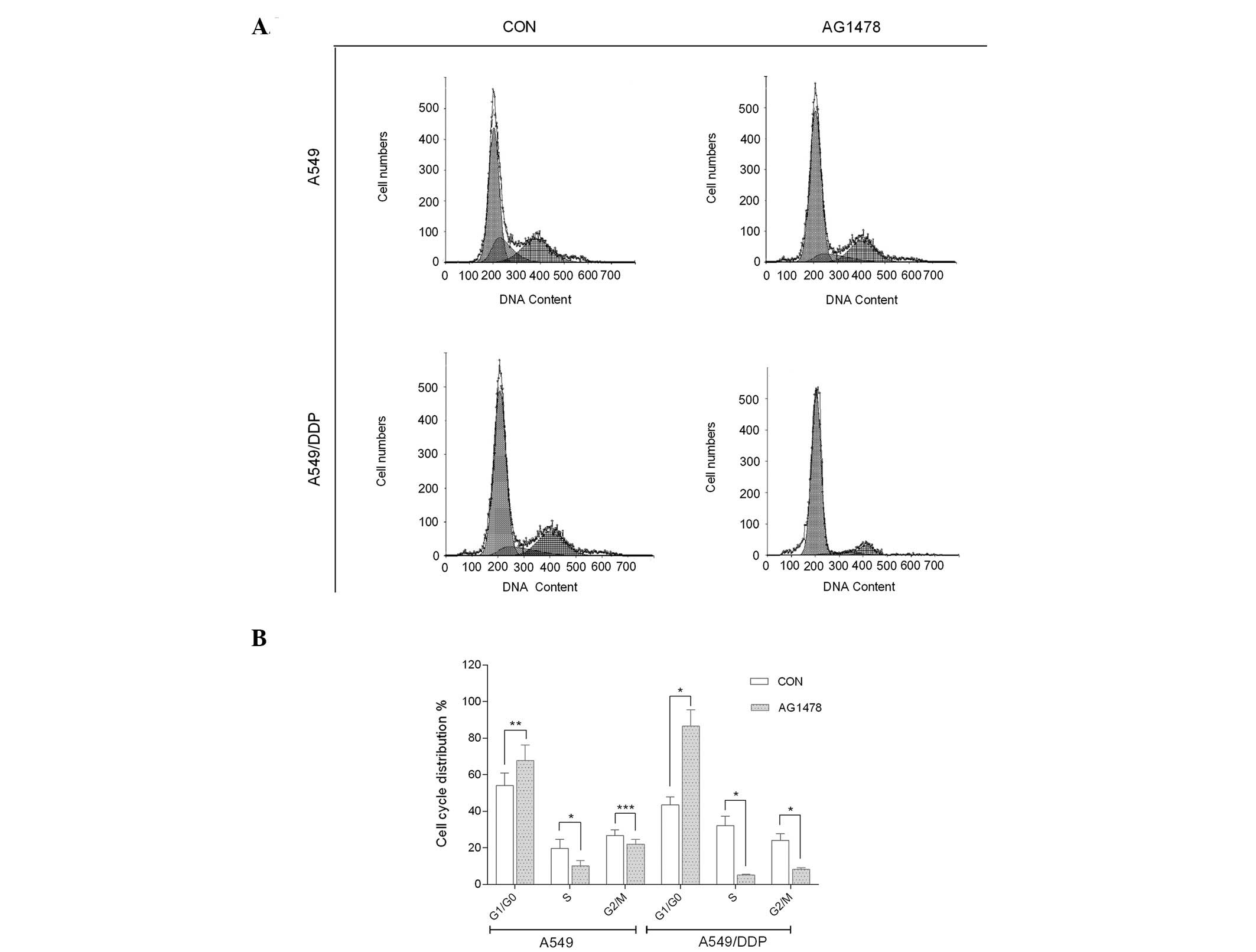
AG1478 arrests cells at G1
phase
To further examine whether AG1478 inhibits cell
proliferation, the percentage cell distribution at various stages
of the cell cycle was determined in the two cell lines based on
their DNA content by flow cytometry. The results showed that AG1478
significantly inhibited DNA synthesis in the treated cells, when
compared with the untreated cells (P<0.001). In addition, FACS
analysis revealed that the untreated proliferating A549/DDP cells
exhibited the following cell cycle distributions: 43.5±4.50% in
G1/G0 phase; 32.2±5.21% in S phase; and
24.3±3.53% in G2/M phase. However, the parental A549
cells were composed of the following cell cycle distributions:
54.0±6.91% in G1/G0 phase; 18.9±5.01% in S
phase; and 26.7±3.22% in G2/M phase. By contrast, the
treated proliferative A549/DDP cells exhibited the following cell
cycle distribution: 86.6±8.91% in G1/G0
phase; 5.19±0.52% in S phase; and 8.22±0.92% in G2/M
phase. However, the treated A549 cells exhibited the following
distributions: 67.8±8.42% in G1/G0 phase;
10.1±3.03% in S phase; and 22.1±2.52% in G2/M phase.
AG1478 significantly arrested A549/DDP cells in G1 phase
(P<0.001), with a corresponding reduction in the S and
G2/M phases (P<0.001). AG1478 similarly blocked the
A549 cells from progressing beyond the G1 phase
(P<0.05), with a simultaneous reduction in the S phase
(P<0.001). However, a significant reduction in the
G2/M phase was not observed in the A549 cells
(P>0.05), as shown in Fig.
2.
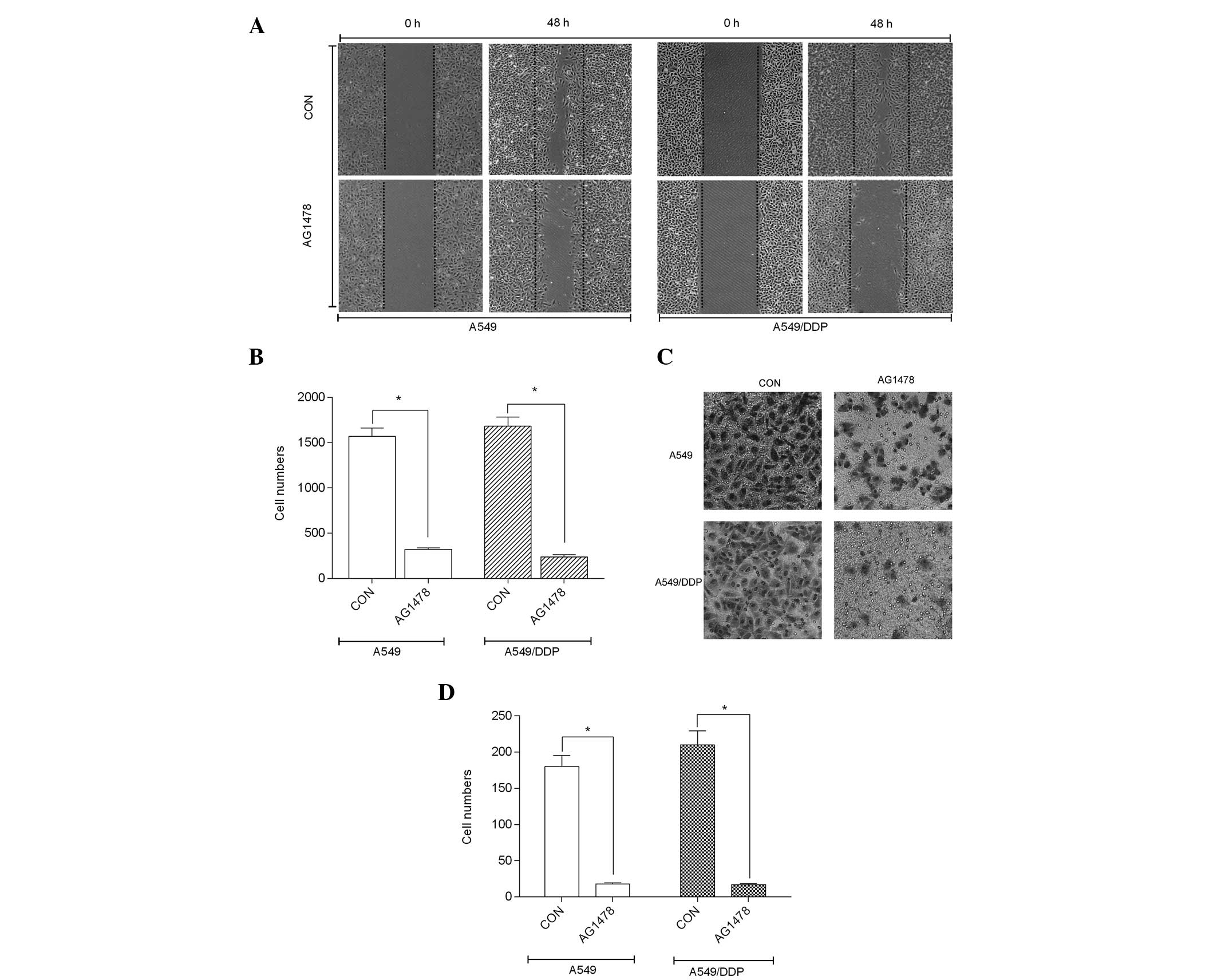
AG1478 inhibits cell migration and
invasion
To investigate the effect of AG1478 on migration and
invasion, the two cell lines were treated with AG1478 (almost
IC50, respectively) for 48 h, whereas the controls were
left untreated. Cell migration was analyzed using the wound-healing
assay, as described above. Wounds generated on the AG1478-treated
cells did not heal for 48 h, whereas wounds in the untreated cell
lines had almost completely healed, as shown in Fig. 3A. Furthermore, the migration levels
of the A549/DDP and A549 cells were reduced to 15.4±1.21 and
13.6±1.68%, respectively, after 48 h (P 0.001), as shown in
Fig. 3B. AG1478 inhibited the
migration of the two cell lines, as confirmed by quantitative
analysis using a Transwell system. The mean invasive proportion of
the AG1478-treated cell lines was reduced to 10.1±1.31% in the
A549/DDP cells (P<0.001) and 8.7±0.63% in the A549 cells
(P<0.001), as compared with the control, as shown in Fig. 3C and D.
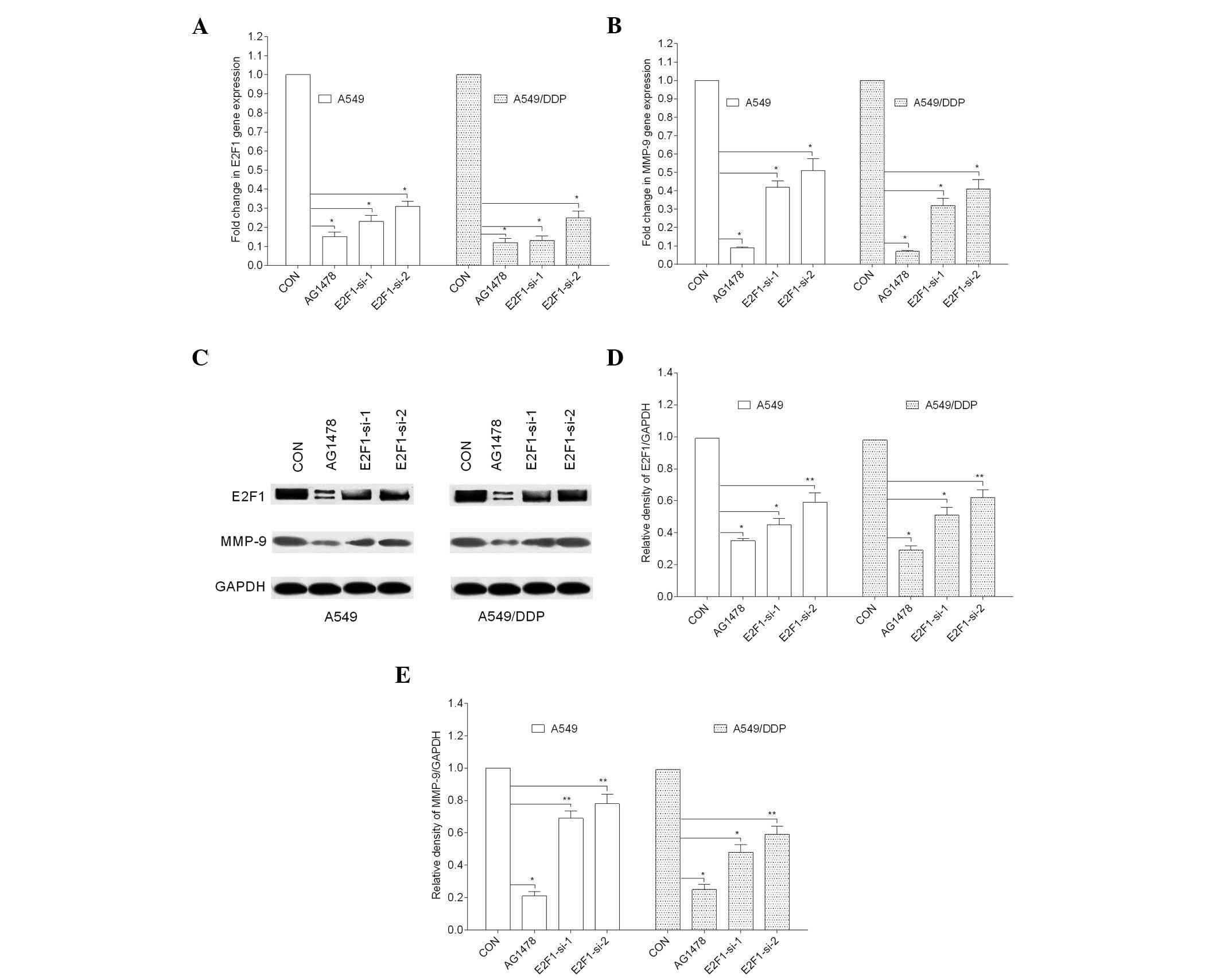
AG1478 regulates MMP-9 expression via
E2F1
E2F1 is a transcriptional activator of MMP-9 that
regulates lung cancer cell invasion and metastasis (18). The present study showed that AG1478
almost completely eliminated MMP-9 and E2F1 gene expression
(P<0.001). To further determine whether AG1478 modulates the
expression of MMP-9 in A549/DDP and A549 cells via the E2F1
transcription factor, the two cell lines were transfected with 10
nM siRNA against E2F1 or with a non-targeting control siRNA. MMP-9
gene and protein expression following transfection with
E2F1-targeting siRNA were significantly reduced in the two cell
lines (P<0.001 and P<0.05), as shown in Fig. 4.
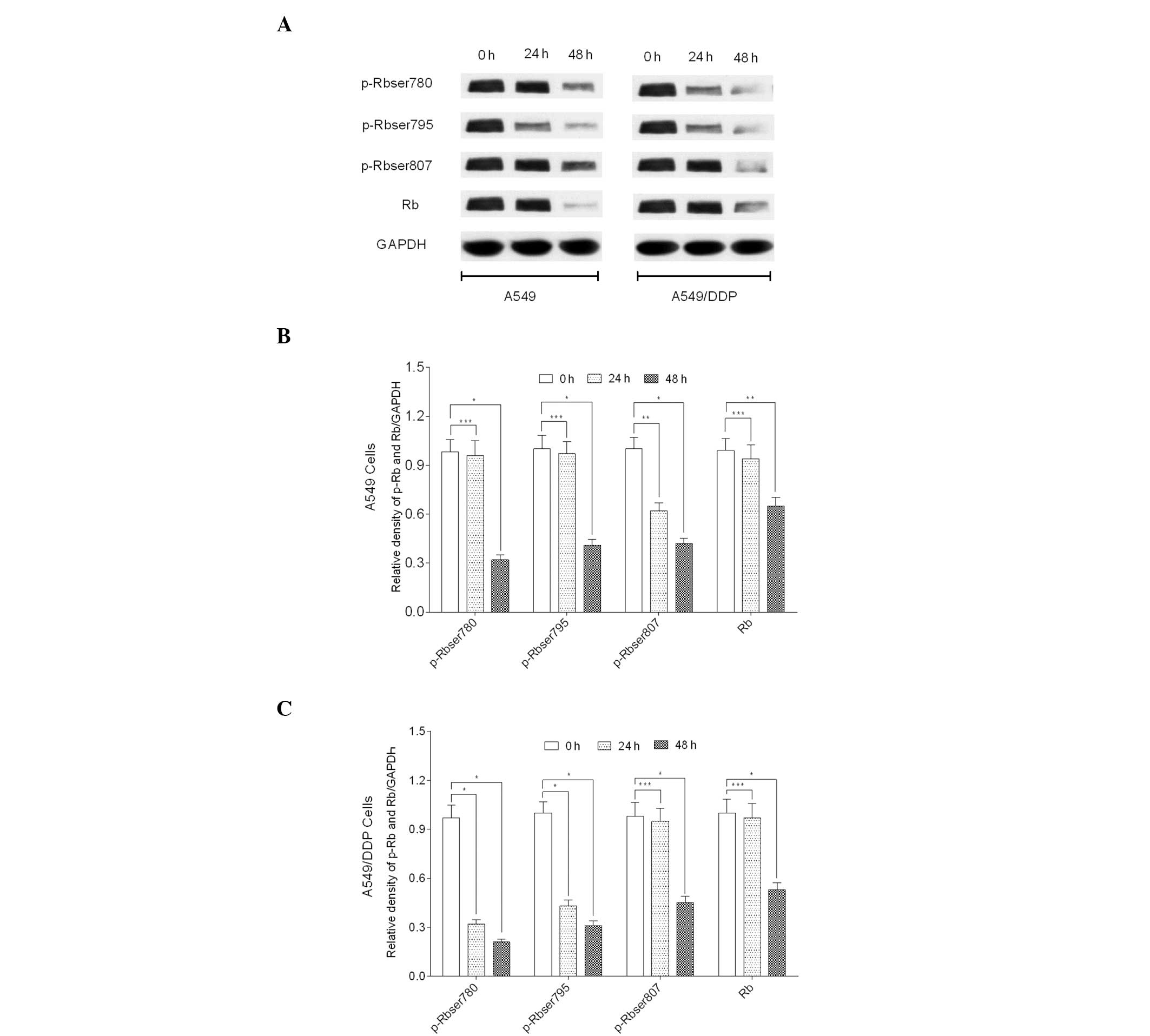
AG1478 modulates Rb protein status
To further determine whether AG1478 modulates the
key cell cycle protein, Rb, the phosphorylation status of Rb
protein was determined. The results revealed that AG1478
significantly inhibited the phosphorylation of Rb at Ser780 and
Ser795 sites following the exposure of A549/DDP cells to AG1478 for
24 h (P<0.001). However, no significant reduction in the
phosphorylation at the Ser807 site was identified (P>0.05). By
contrast, AG4178 significantly inhibited the phosphorylation of Rb
at Ser807 sites in A549 cells following 24 h exposure (P<0.05);
however, the Ser780 and Ser795 sites were not affected (P>0.05).
Furthermore, a significant reduction in Rb phosphorylation was
identified at Ser780, Ser795 and Ser807 sites of A549/DDP
(P<0.001) and A549 (P<0.05) cell lines at 48 h (Fig. 5).
Discussion
The chemoresistance of cancer cells is a major
obstacle in the treatment of malignant cancers. The enhanced
sensitivity of cancer cells to chemotherapy is highly desirable.
The present study demonstrated that the A549/DDP cell line was more
sensitive to AG1478 than the A549 cell line. Similar results have
been observed in two other cisplatin resistant oral squamous
carcinoma cell lines, with an increased sensitivity to the novel
EGFR inhibitor AG1478 (19). As a
cytotoxic chemotherapeutic agent, cisplatin causes DNA damage and
may arrest cells in the G2 phase (20,21).
The current study revealed that AG1478 blocked the two cell lines
in the G1 phase of the cell cycle, with a concomitant
decrease in the proportion of cells in S phase, which caused cell
cycle redistribution. Rb is a key cell cycle protein, which
inhibits entry into S phase during the cell cycle. Rb functions
together with the E2F-family of transcription factors to activate
or inhibit cell proliferation (22). As one of the activating
transcription factors, E2F1 promotes cell cycle progression into S
phase when Rb is inactivated by phosphorylation (23), and E2F1 functions as an activator of
MMPs to modulate MMP-9 expression (18). The present study found that AG1478
inhibited E2F1 and MMP-9 expression, and reduced the levels of E2F1
via RNA interference, which consequently decreased the expression
of MMP-9. Cell cycle progression usually occurs when Rb is
inactivated by phosphorylation, leading to the release of free E2F1
(24). This phenomenon facilitates
the expression of E2F1 target genes and promotes cell
proliferation. The current study revealed that AG1478 selectively
inhibited the phosphorylation of Rb, thereby facilitating its
activation in various sites at different time points in the two
cell lines, and consequently eliminated the expression of E2F1.
These results suggest that Rb may be activated by AG1478 via
dephosphorylation, thereby preventing the release of free
activating E2F1. This may consequently inhibit the expression of
target genes, such as MMP-9, preventing the progression of the cell
cycle and subsequently leading to the suppressed tumor cell
migration and invasion observed. This study may provide a promising
therapeutic approach for a particular type of cisplatin-resistant
lung cancer.
Acknowledgements
This study was supported by the Wu Jieping Medical
Foundation of China (grant nos. 320.6750.11003, 320.6799.1112 and
3206720.10021), the Key Project from the National Natural Science
Foundation of China (grant no. 30430300), the China-Sweden
Cooperative Foundation (grant no. 09ZCZDSF04100), the Major State
Basic Research Development Program of China (grant no.
2010CB529405), the Tianjin Scientific Innovation System Program
(grant nos. 07SYSYSF05000 and 07SYSYJC27900) and the Major Project
of Tianjin Sci-Tech Support Program (grant no. 06YFSZSF05300). The
authors would also like to thank Dr Jao Feng for assistance with
cell cycle detection.
References
|
1
|
Stinchcombe TE and Socinski MA:
Considerations for second-line therapy of non-small cell lung
cancer. Oncologist. 13(Suppl 1): 28–36. 2008.
|
|
2
|
Chen XL, Hu HJ, Pan YQ, et al: Molecular
mechanism of acquisiton of invasion and metastasis phenotype in
human lung cancer cell line A549/DDP. J Mod Oncol. 21:1670–1674.
2013.
|
|
3
|
Lu LS: Relationship between cancer
metastasis and drug resistance. J Int Oncol. 33:665–667. 2006.
|
|
4
|
Laskin JJ and Sandler AB: Epidermal growth
factor receptor: a promising target in solid tumours. Cancer Treat
Rev. 30:1–17. 2004.
|
|
5
|
Yarden Y: The EGFR family and its ligands
in human cancer. signalling mechanisms and therapeutic
opportunities. Eur J Cancer. 37(Suppl 4): S3–S8. 2001.
|
|
6
|
Veale D, Kerr N, Gibson GJ, Kelly PJ and
Harris AL: The relationship of quantitative epidermal growth factor
receptor expression in non-small cell lung cancer to long term
survival. Br J Cancer. 68:162–165. 1993.
|
|
7
|
Schmidt M and Lichtner RB: EGF receptor
targeting in therapy-resistant human tumors. Drug Resist Updat.
5:11–18. 2002.
|
|
8
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007.
|
|
9
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small-cell lung
cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 21:2237–2246.
2003.
|
|
10
|
Kris MG, Natale RB, Herbst RS, et al:
Efficacy of gefitinib, an inhibitor of the epidermal growth factor
receptor tyrosine kinase, in symptomatic patients with non-small
cell lung cancer: a randomized trial. JAMA. 290:2149–2158.
2003.
|
|
11
|
Giaccone G, Herbst RS, Manegold C, et al:
Gefitinib in combination with gemcitabine and cisplatin in advanced
non-small-cell lung cancer: a phase III trial - INTACT 1. J Clin
Oncol. 22:777–784. 2004.
|
|
12
|
Herbst RS, Giaccone G, Schiller JH, et al:
Gefitinib in combination with paclitaxel and carboplatin in
advanced non-small-cell lung cancer: a phase III trial - INTACT 2.
J Clin Oncol. 22:785–794. 2004.
|
|
13
|
Fry DW, Kraker AJ, McMichael A, et al: A
specific inhibitor of the epidermal growth factor receptor tyrosine
kinase. Science. 265:1093–1095. 1994.
|
|
14
|
Ward WH, Cook PN, Slater AM, Davies DH,
Holdgate GA and Green LR: Epidermal growth factor receptor tyrosine
kinase. Investigation of catalytic mechanism, structure-based
searching and discovery of a potent inhibitor. Biochem Pharmacol.
48:659–666. 1994.
|
|
15
|
Lui VW, Boehm AL, Koppikar P, et al:
Antiproliferative mechanisms of a transcription factor decoy
targeting signal transducer and activator of transcription (STAT)
3: the role of STAT1. Mol Pharmacol. 71:1435–1443. 2007.
|
|
16
|
Wu X, Zhu Y, Yan H, et al: Isothiocyanates
induce oxidative stress and suppress the metastasis potential of
human non-small cell lung cancer cells. BMC Cancer. 10:2692010.
|
|
17
|
Guo L, Li L, Wang W, Pan Z, Zhou Q and Wu
Z: Mitochondrial reactive oxygen species mediates nicotine-induced
hypoxia-inducible factor-1α expression in human non-small cell lung
cancer cells. Biochim Biophys Acta. 1822:852–861. 2012.
|
|
18
|
Johnson JL, Pillai S, Pernazza D, Sebti
SM, Lawrence NJ and Chellappan SP: Regulation of matrix
metalloproteinase genes by E2F transcription factors: Rb-Raf-1
interaction as a novel target for metastatic disease. Cancer Res.
72:516–526. 2012.
|
|
19
|
Hiraishi Y, Wada T, Nakatani K, et al:
EGFR inhibitor enhances cisplatin sensitivity of oral squamous cell
carcinoma cell lines. Pathol Oncol Res. 14:39–43. 2008.
|
|
20
|
Sorenson CM and Eastman A: Mechanism of
cis-diamminedichloroplatinum(II)-induced cytotoxicity: role of G2
arrest and DNA double-strand breaks. Cancer Res. 48:4484–4488.
1988.
|
|
21
|
Sorenson CM, Barry MA and Eastman A:
Analysis of events associated with cell cycle arrest at G2 phase
and cell death induced by cisplatin. J Natl Cancer Inst.
82:749–755. 1990.
|
|
22
|
Knudsen ES and Wang JY: Targeting the
RB-pathway in cancer therapy. Clin Cancer Res. 16:1094–1099.
2010.
|
|
23
|
Li J, Ran C, Li E, et al: Synergistic
function of E2F7 and E2F8 is essential for cell survival and
embryonic development. Dev Cell. 14:62–75. 2008.
|
|
24
|
Cobrinik D: Pocket proteins and cell cycle
control. Oncogene. 24:2796–2809. 2005.
|