Introduction
Lung cancer is the second most frequently occurring
cancer in the world, and is the leading cause of cancer-related
mortality (1). Non-small cell lung
cancer (NSCLC) has a poor prognosis, with a five-year survival rate
of 40–50% in patients with pathological stage I or II disease who
undergo a primary resection (2–4).
Gender, age, type of surgery, tumor diameter and post-operative
N-stage are significant contributing factors to the recurrence
risk, with pathological stage and histological type significantly
affecting recurrence-free survival (5,6)
Increased levels of inflammatory cytokines have been
reported as risk factors for recurrence in a number of cancer types
(7). Macrophage inflammatory
protein-3 (MIP-3α), also known as CCL20, has been linked to the
propagation of several malignancies, including prostate, hepatic
and pancreatic carcinomas, raising the possibility that MIP-3α
plays a role in lung carcinogenesis (8), thereby affecting the prognosis of lung
cancer. However, there have been no studies regarding the
association between the serum level of MIP-3α and the prognosis of
NSCLC patients following primary resection. The present study
sought to characterize the role of MIP-3α in NSCLC patients with
early recurrence or metastasis.
Materials and methods
Study population
A total of 20 healthy subjects were enrolled,
together with 239 NSCLC patients whose diagnosis (pathological
Stage I or II, pre-operative assessments) was confirmed by
histological examination, between February 2006 and July 2010, at
the Hebei Province General Hospital (Shijiazhuang, Hebei, China).
From all the patients with lung cancer who underwent pulmonary
resections by video-assisted thoracoscopic surgery (VATS), the
following patients were excluded: Four patients that were converted
to a thoracotomy for bleeding and dense fibrous adhesions; three
with pathologically-positive cancer of the bronchial stump; four
that presented with surgically unresectable cancer with metastatic
lymph nodes; four with pleural disseminations; five with chest wall
cancer stumps; eight with serious complications or who succumbed;
and eight with inadequate follow-up data. The remaining 203
patients with post-operative histopathological stage I or II NSCLC
were included in this study. Staging prior to and following the
surgery was based on histopathological analysis according to the
International Union Against Cancer tumor-node-metastasis staging
system (3). The study was approved
by the Medical Ethics Committee of Hebei Province General Hospital
on human research (code, 2005-123). Informed consent was obtained
from each patient. Patients were followed up for two years
post-operatively.
Surgical procedures
General anesthesia with double-lumen endotracheal
intubation and one-lung ventilation was used in all patients. With
the VATS procedure, three working ports were made for the insertion
of thoracoscopic instruments, located at the 7th or 8th intercostal
space in the mid-axillary line, the 4th or the 5th intercostal
space in the anterior axillary line, and the 8th or the 9th
intercostal space in the scapular line. Specimen bags were used for
removing the samples. Neither muscles (latissimus dorsi or serratus
anterior muscles) nor ribs were cut. During the surgery, the
quantity of hemorrhage (QOH) and duration of surgery (DOS) were
recorded.
Enzyme-linked immunosorbent assay (ELISA)
for serum MIP-3α level
Blood samples were collected in the morning prior to
the surgery and on post-operative days (PODs) 30, 90 and 180. Blood
samples were also collected from healthy subjects. The serum was
obtained by centrifugation at 3,000 × g for 10 min and stored at
−80°C until analysis. Serum MIP-3α level was measured using a
commercially available ELISA, following the manufacturer’s
instrctions (RayBiotech, Inc., Norcross, GA, USA). Briefly, 96-well
plates coated with the monoclonal mouse anti-human IgG1 MIP3a
antibody were incubated with standards at different concentrations,
and serum samples at room temperature for 2.5 h. Subsequent to
three washes, the wells were incubated with a biotinylated
polyclonal goat anti-human MIP3a antibody, at room temperature for
1 h, prepared HRP-conjugated streptavidin for 45 min at room
temperature and TMB One-step Substrate Reagent (RayBiotech, Inc.)
at room temperature for 30 min. Enzymatic reactions were developed
and the absorbance was measured at 450 nm in a Multiskan FC
microplate (Thermo Fisher Scientifitic, Waltham, MA, USA). Protein
levels were calculated according to standard curves.
Statistical analysis
Statistical analysis was performed using SPSS 13
(SPSS, Inc., Chicago, IL, USA). Serum MIP-3α levels and clinical
and operative continuous variables are presented as the mean ±
standard deviation. ANOVA and χ2 tests for independence
were used for the comparison of clinical data and serum MIP-3α
levels at each time-point. Independent sample t-tests were employed
to investigate the differences in serum MIP-3α levels between
patients with and without adjuvant chemotherapy. The χ2
test was used to examine the recurrence rate between high and low
serum MIP-3α level groups. The Kaplan-Meier method was used to
compare recurrence-free survival rates using a log-rank test to
examine differences in recurrence free times for high and low serum
MIP-3α level groups. Cox’s proportional hazards regression was
further employed to estimate the hazard risk ratio of early
recurrence for levels of serum MIP-3α and other clinical and
operative predictors. ANOVA was used to examine differences in the
serum MIP-3α levels among different metastatic or recurrent
position groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Association between tumor recurrence or
metastasis and clinicopathological parameters
A total of 203 patients were reviewed for up to two
years post-operatively. In total, 63 patients experienced
recurrence of NSCLC, with a two-year recurrence rate of 31.0%. The
serum MIP-3α levels on POD 180 were significantly higher in the
recurrence group than those in the non-recurrence group and healthy
subjects (P=0.001). The adjuvant chemotherapy rate was low in the
non-recurrence group (P=0.035), while there were no significant
differences in age, gender, QOH, DOS and histological type between
the recurrence and non-recurrence groups (P>0.05) (Table I).
 | Table IClinical data and serum MIP-3α level
in healthy subjects, recurrence and non-recurrence groups. |
Table I
Clinical data and serum MIP-3α level
in healthy subjects, recurrence and non-recurrence groups.
| Patient
characteristics | Healthy | Rec (−) | Rec (+) | P-value |
|---|
| Total, n | 20 | 140 | 63 | |
| Age, years | 62.75±9.76 | 62.37±8.94 | 62.05±9.88 | 0.818 |
| Gender (male/female),
n | 10/10 | 81/59 | 39/24 | 0.587 |
| Histological type
(Ad/Sq/LC/other), n | | 75/61/2/2 | 33/27/2/1 | 0.930 |
| Adjuvant/non-adjuvant
chemotherapy, n | | 45/95 | 30/33 | 0.035 |
| QOH, ml | | 99.66±28.59 | 94.46±26.24 | 0.204 |
| DOS, min | | 165.89±35.19 | 162.77±32.16 | 0.537 |
| MIP-3α, pg/ml | 51.06±8.19 | | | |
| Pre-operatively | | 48.10±12.35 | 51.25±10.69 | 0.157 |
| POD 30 | | 49.79±10.54 | 47.66±11.63 | 0.315 |
| POD 90 | | 52.83±13.66 | 49.74±11.55 | 0.268 |
| POD 180 | | 53.96±10.38 | 71.70±15.41 | 0.001 |
Adjuvant chemotherapy has no significant
effect on serum MIP-3α level
Adjuvant chemotherapy was carried out in certain
patients after POD 30. To evaluate whether adjuvant chemotherapy
contributes to the value of serum MIP-3α, the serum MIP-3α levels
were compared at PODs 90 and 180 in the recurrence and
non-recurrence groups, respectively, considering adjuvant
chemotherapy as a main factor for the recurrence and non-recurrence
groups. It was found that there was no significant difference in
the serum MIP-3α level at PODs 90 and 180 in all groups (P>0.05)
(Tables II and III).
 | Table IISerum MIP-3α level (pg/ml) in the
recurrence group. |
Table II
Serum MIP-3α level (pg/ml) in the
recurrence group.
| Day | Adjuvant chemotherapy
(n=30) | Non-adjuvant
chemotherapy (n=33) | P-value |
|---|
| POD 90 | 50.75±10.13 | 48.51±9.15 | 0.359 |
| POD 180 | 68.94±12.11 | 72.49±10.23 | 0.192 |
 | Table IIISerum MIP-3α level (pg/ml) in the
non-recurrence group. |
Table III
Serum MIP-3α level (pg/ml) in the
non-recurrence group.
| Day | Adjuvant chemotherapy
(n=45) | Non-adjuvant
chemotherapy (n=95) | P-value |
|---|
| POD 90 | 53.31±11.12 | 49.59±11.75 | 0.077 |
| POD 180 | 55.83±9.81 | 52.59±10.75 | 0.089 |
High serum MIP-3α level contributes to a
high recurrence risk
The patients were divided into high and low serum
MIP-3α level groups by the median value of the serum MIP-3α level
(57 pg/ml) at POD 180. The recurrence rate in the high serum MIP-3α
level group was significantly higher than that in the low serum
MIP-3α level group (P=0.006) (Table
IV).
 | Table IVRecurrence rate in the high and low
serum MIP-3α level groups. |
Table IV
Recurrence rate in the high and low
serum MIP-3α level groups.
| serum MIP-3α level at
POD 180, pg/ml | Number | Rec (−) | Rec (+) | Rate, % |
|---|
| Low (<57), n | 119 | 91 | 28 | 23.53 |
| High (>57), n | 84 | 49 | 35 | 41.67 |
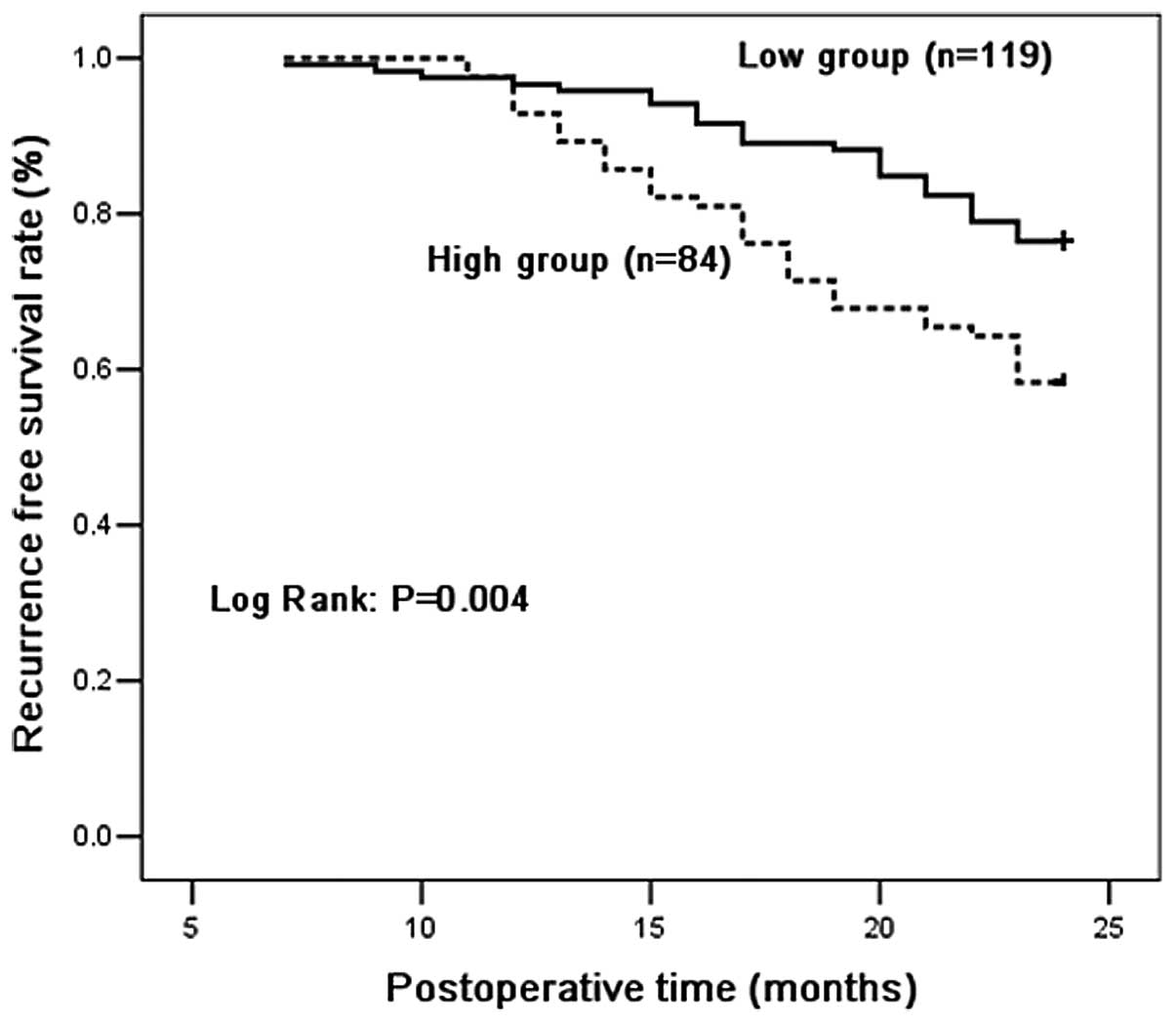
Recurrence-free survival analysis
Recurrence-free survival curves for the high and low
MIP-3α groups are shown in Fig. 1.
Patients in the high and low groups had two year recurrence-free
rates of 76.5 and 58.3%, respectively. There were significant
differences in overall recurrence-free survival between patients
with high and low serum MIP-3α level. Patients with high serum
levels of MIP-3α had a significantly shorter overall
recurrence-free time compared with those with low levels
(P=0.004).
Multivariate Cox’s regression
analysis
The study further investigated the independent
effects of MIP-3α on early recurrence or metastasis with respect to
age, gender, QOH, DOS, histological type and adjuvant chemotherapy
rate using Cox’s regression models. Only the serum MIP-3α level on
POD 180 was a significant predictor for early recurrence or
metastasis. The MIP-3α level on POD 180 had a hazard ratio of
1.061, with a 95% confidence interval of 1.044–1.078 and a P-value
of 0.001. In the multivariate Cox’s regression analyses, only the
serum MIP-3α level was significant. Other factors were not
independent predisposing factors for post-operative early
recurrence or metastasis (Table
V).
 | Table VRecurrence factors in Cox’s
proportional hazards model. |
Table V
Recurrence factors in Cox’s
proportional hazards model.
| Patient
characteristics | HR (95% CI) | P-value |
|---|
| Age, years | 0.989
(0.962–1.017) | 0.443 |
| Gender
(male/female) | 0.990
(0.544–1.803) | 0.974 |
| Histological type
(Ad/Sq/LC/Other) | 0.508
(0.989–2.298) | 0.076 |
| Adjuvant/non-adjuvant
chemotherapy | 1.405
(0.976–1.985) | 0.069 |
| QOH, ml | 0.996
(0.987–1.135) | 0.407 |
| DOS, min | 1.002
(0.994–1.010) | 0.624 |
| MIP-3α, pg/ml |
| Pre-operatively | 1.003
(0.985–1.021) | 0.730 |
| POD 30 | 1.012
(0.986–1.038) | 0.366 |
| POD 90 | 1.002
(0.984–1.020) | 0.821 |
| POD 180 | 1.061
(1.044–1.078) | 0.001 |
Serum MIP-3α level of varying recurrence
or metastasis sites
In the two years of follow-up, the earliest and
average recurrence times were 7 and 17.25 months after surgery,
respectively. The serum MIP-3α level was also investigated at POD
180 in the patients with varying recurrence or metastasis sites, as
shown in Table VI. The serum
MIP-3α levels in the patients with liver and bone metastases were
significantly higher than those in the patients with other sites of
recurrence (P<0.05). The patients with pleural dissemination had
to be excluded from the analysis due to the small sample size.
 | Table VIPost-operative tumor recurrence or
metastasis site and serum MIP-3α level. |
Table VI
Post-operative tumor recurrence or
metastasis site and serum MIP-3α level.
| Recurrence site | Number (n=63) | Serum MIP-3α level at
POD 180, pg/ml |
|---|
| Lung | 10 | 65.92±9.15 |
| Lymph node | 11 | 64.45±8.49 |
| Brain | 7 | 67.65±8.24 |
| Bone | 7 | 85.10±9.95a |
| Liver | 6 | 87.31±11.12a |
| Pleural
dissemination | 2 | 68.85±2.33 |
| Lung+mediastinal
lymph node | 7 | 67.95±8.40 |
| Lung+brain | 7 | 66.67±9.74 |
| Lung+bone | 6 | 88.34±12.46a |
Discussion
MIP-3α is the only cytokine known to interact with
CC chemokine receptor 6 (CCR6), a property shared with the
antimicrobial β-defensins. The ligand-receptor pair MIP-3α-CCR6 is
responsible for the chemoattraction of immature dendritic cells,
effector/memory T cells and B cells, and plays a critical role in
cancer and rheumatoid arthritis (9). MIP-3α has been linked to the
development of malignant tumors. The high expression of MIP-3α has
been reported in cancers of the colon (10), pancreas (11), prostate (12), nasopharynx (13) and liver (14). There is evidence that MIP-3α
enhances tumor growth in numerous types of cancer (15,16).
Moreover, MIP-3α is considered to be involved in carcinogenesis,
angiogenesis and invasion (17,18).
Surgical procedures are an important primary
approach for the treatment of early-stage lung cancer patients.
However, metastasis and recurrence are the most common risks for
treatment failure following surgery, with a reported 50% recurrence
rate of lung cancer within one year in the treatment of NSCLC
(19).
In the present study, it was found that there was a
higher post-operative level of MIP-3α (POD 180) and a relatively
higher adjuvant chemotherapy rate NSCLC patients (post-operative
histopathological stage I or II) with recurrence or metastasis.
Consistent with this finding, Kirshberg et al (20) reported that the MIP-3α/CCR6 axis
promoted NSCLC disease progression. However, in the multivariate
Cox’s regression analyses of the present study, only serum MIP-3α
level (POD 180) was significant for post-operative early recurrence
or metastasis, specifically for bone or liver metastasis. The study
indicated that a high post-operative level of MIP-3α (POD 180) was
significantly associated with the early recurrence or metastasis of
NSCLC. This is the first study to identify the serum MIP-3α immune
response marker as a predictor for the early post-operative
recurrence of NSCLC. The study highlighted the fact that the
post-operative MIP-3α level may therefore be a useful marker to
determine the requirement for adjunctive anti-cancer therapy, and
the fact that the MIP-3α/CCR6/IL-17 axis should be investigated
further as a potential novel therapeutic target.
To the best of our knowledge, no previous study has
examined the post-operative value of serum MIP-3α in NSCLC patients
with adjuvant chemotherapy. In the present study, adjuvant
chemotherapy was applied in certain patients after POD 30. It was
found that there was no significant difference in the serum MIP-3α
level at PODs 90 and 180 in all patients. This indicated that
MIP-3α was independent of adjuvant chemotherapy, which was
consistent with the result of the multivariate Cox’s regression
analyses. Iwata et al (21)
also showed that the serum MIP-3α status was an independent
prognostic factor for overall CRC patients regardless of
therapeutic interventions.
In statistical analyses, the patients of the present
study were divided into low and high groups according the serum
MIP-3α level following primary pulmonary resection. The patients
with a high serum MIP-3α level had a higher recurrence rate than
the patients with a low level. Recurrence-free survival curves
showed that there were significant differences in recurrence-free
survival between the two groups. This indicated that post-operative
patients with high serum MIP-3α levels have a high risk of tumor
recurrence or metastasis.
In further experiments, the serum MIP-3α levels were
studied in patients with recurrence and different recurrence or
metastasis sites. The results showed the the serum MIP-3α levels
were significantly higher in the patients with liver and bone
metastases. Iwata et al (21) also reported that the serum MIP-3α
status was significantly associated with synchronous liver
metastasis and was as independent predictive factor for liver
metastasis.
However, several limitations, including the
relatively small sample and the requirement for validation studies
in independent samples, exist in this study. More studies are
required to further clarify the association between MIP-3α and
recurrence or metastasis in NSCLC patients.
In conclusion, in the present study, high
post-operative serum MIP-3α levels were associated with an
increased risk of post-operative early recurrence or metastasis in
NSCLC patients (post-operative histopathological stage I or II),
specifically in those with bone or liver metastases.
Acknowledgements
The authors would like to thank their colleagues in
the Department of Thoracic Surgery (Hebei Province General
Hospital) for collecting the samples and Hanying Xing and Zhijuan
Hu for providing technical assistance.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108.
2005.
|
|
2
|
Goya T, Asamura H, Yoshimura H, et al;
Japanese Joint Committee of Lung Cancer Registry. Prognosis of 6644
resected non-small cell lung cancers in Japan: A Japanese lung
cancer registry study. Lung Cancer. 50:227–234. 2005.
|
|
3
|
Mountain CF: Revisions in the
International System for Staging Lung Cancer. Chest. 111:1710–1717.
1997.
|
|
4
|
Scagliotti GV and Novello S: Adjuvant
therapy in completely resected non-small-cell lung cancer. Curr
Oncol Rep. 5:318–325. 2003.
|
|
5
|
Choi YS, Shim YM, Kim K and Kim J: Pattern
of recurrence after curative resection of local (stage I and II)
non-small cell lung cancer: difference according to the histologic
type. J Korean Med Sci. 19:674–676. 2004.
|
|
6
|
Hjelde H, Sundstrøm S, Ødegård A, et al:
Recurrence and survival after surgical treatment of lung cancer.
Tidsskr Nor Laegeforen. 130:25–28. 2010.(In Norwegian).
|
|
7
|
Jiang J, Goel R, Schmechel S, et al:
Pre-conditioning cryosurgery: Cellular and molecular mechanisms and
dynamics of tnf-α enhanced cryotherapy in an in vivo prostate
cancer model system. Cryobiology. 61:280–288. 2010.
|
|
8
|
Ghadjar P, Rubie C, Aebersold DM and
Keilholz U: The chemokine CCL20 and its receptor CCR6 in human
malignancy with focus on colorectal cancer. Int J Cancer.
125:741–745. 2009.
|
|
9
|
Schutyser E, Struyf S and Van Damme J: The
CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor
Rev. 14:409–426. 2003.
|
|
10
|
Brand S, Olszak T, Beigel F, et al: Cell
differentiation dependent expressed CCR6 mediates ERK-1/2,
SAPK/JNK, and Akt signaling resulting in proliferation and
migration of colorectal cancer cells. J Cell Biochem. 97:709–723.
2006.
|
|
11
|
Rubie C, Frick VO, Ghadjar P, et al:
CCL20/CCR6 expression profile in pancreatic cancer. J Transl Med.
8:452010.
|
|
12
|
Ghadjar P, Loddenkemper C, Coupland SE, et
al: Chemokine receptor CCR6 expression level and aggressiveness of
prostate cancer. J Cancer Res Clin Oncol. 134:1181–1189. 2008.
|
|
13
|
Chang KP, Hao SP, Chang JH, et al:
Macrophage inflammatory protein-3alpha is a novel serum marker for
nasopharyngeal carcinoma detection and prediction of treatment
outcomes. Clin Cancer Res. 14:6979–6987. 2008.
|
|
14
|
Rubie C, Frick VO, Wagner M, et al:
Enhanced expression and clinical significance of CC-chemokine MIP-3
alpha in hepatocellular carcinoma. Scand J Immunol. 63:468–477.
2006.
|
|
15
|
Fujii H, Itoh Y, Yamaguchi K, et al:
Chemokine CCL20 enhances the growth of HuH7 cells via
phosphorylation of p44/42 MAPK in vitro. Biochem Biophys Res
Commun. 322:1052–1058. 2004.
|
|
16
|
Liu J, Zhang N, Li Q, et al:
Tumor-associated macrophages recruit CCR6+ regulatory T
cells and promote the development of colorectal cancer via
enhancing CCL20 production in mice. PLoS One. 6:e194952011.
|
|
17
|
Slettenaar VI and Wilson JL: The chemokine
network: a target in cancer biology? Adv Drug Deliv Rev.
58:962–974. 2006.
|
|
18
|
Tsai ST, Chien IH, Shen WH, et al: ENO1, a
potential prognostic head and neck cancer marker, promotes
transformation partly via chemokine CCL20 induction. Eur J Cancer.
46:1712–1723. 2010.
|
|
19
|
Lee HJ, Jo J, Son DS, et al: Predicting
recurrence using the clinical factors of patients with non-small
cell lung cancer after curative resection. J Korean Med Sci.
24:824–830. 2009.
|
|
20
|
Kirshberg S, Izhar U, Amir G, et al:
Involvement of CCR6/CCL20/IL-17 axis in NSCLC disease progression.
PLoS One. 6:e248562011.
|
|
21
|
Iwata T, Tanaka K, Inoue Y, et al:
Macrophage inflammatory protein-3 alpha (MIP-3α) is a novel serum
prognostic marker in patients with colorectal cancer. J Surg Oncol.
107:160–166. 2013.
|