Introduction
Ameloblastic carcinoma is a relatively rare type of
tumor. According to the World Health Organization (WHO), the
carcinoma can be classified as metastasizing (malignant)
ameloblastoma or ameloblastic carcinoma (1). Metastasizing ameloblastoma is defined
as an ameloblastoma, which metastasizes and the primary and
metastatic tissues demonstrate benign histological features
(2). Whereas ameloblastic carcinoma
exhibit malignant features, such as cellular atypia and mitosis
(3). Ameloblastic carcinoma
consists of two subtypes; primary and secondary. The primary type
demonstrates malignancy in the primary tumor with characteristics
of ameloblastoma and cytologic atypia. The secondary type consists
of malignant changes, which originate in a previously existing
ameloblastoma, regardless of the presence or absence of metastasis.
The secondary type of ameloblastic carcinoma can be divided into
two further subtypes. The intraosseous type arises within a
pre-existing benign intraosseous ameloblastoma and the peripheral
type arises within a pre-existing benign peripheral ameloblastoma
(4). Angiero et al (5) reported that ameloblastic carcinoma
possess unique histopathological features. At the early stages of
malignancy or dedifferentiation, epithelial tumor nests and islands
surrounded by a layer of stellate basaloid cells are observed in
the mesenchymal tissue. Mubeen et al (6) recognized that these cells exhibit
malignant features, such as cellular pleomorphism, mitoses, focal
necrosis, perineural invasion and nuclear hyperchromatism.
Furthermore, ameloblastic carcinoma exhibit histological features
of ameloblastoma and carcinoma.
Ameloblastic carcinoma is an uncommon tumor type,
and therefore, the clinical characteristics, appropriate treatment
and response rates have not been well characterized. Benlyazid
et al (7) retrospectively
reviewed 66 patients with ameloblastic carcinoma that were reported
between 1927 and 2006, and the majority exhibited lung metastasis,
which indicated the requirement for systemic therapy. As a
relatively rare malignant tumor, further detailed and systematic
studies regarding ameloblastic carcinoma are required.
Patients and methods
Patients
In total, 12 patients with ameloblastic carcinoma
who were treated at the West China Hospital of Stomatology, Sichuan
University (Chengdu, China) between 2000 and 2008 (Table I), and 20 more cases reported
between 2005 and 2010 identified by searching PubMed (http://www.ncbi.nlm.nih.gov/pubmed; Table II) were retrospectively
reviewed.
 | Table IReview of 12 cases of ameloblastic
carcinoma with a follow-up of >36 months from the West China
Hospital of Stomatology between 2000 and 2008. |
Table I
Review of 12 cases of ameloblastic
carcinoma with a follow-up of >36 months from the West China
Hospital of Stomatology between 2000 and 2008.
| Case, n | Gender/age,
years | Type | Location | Therapy | Met. | Re. | Follow-up,
months | Prognosis |
|---|
| 1 | M/36 | S | Mandible | Partial
resection | - | Y | 120 | Re. |
| 2 | F/40 | S | Mandible | Expand resection | - | - | 120 | DF |
| 3 | M/47 | S | Maxillary | Partial
resection | - | Y | 108 | Re. |
| 4 | M/61 | P | Mandible | Partial
resection | - | - | 108 | DF |
| 5 | M/40 | P | Mandible | Expand resection | - | - | 96 | DF |
| 6 | F/39 | P | Mandible | Partial
resection | - | - | 84 | DF |
| 7 | M/42 | P | Mandible | Expand resection | - | - | 72 | DF |
| 8 | M/46 | P | Mandible | Partial
resection | - | - | 60 | DF |
| 9 | M/32 | P | Mandible | Partial
resection | - | - | 60 | DF |
| 10 | M/30 | P | Mandible | Marginal
ostectomy | - | - | 48 | DF |
| 11 | M/35 | P | Mandible | Partial
resection | - | - | 36 | DF |
| 12 | M/75 | S | Mandible | Expand resection | Lung | - | 36 | Met. |
 | Table IIReview of 20 cases of ameloblastic
carcinoma from an evidence-based literature review between 2005 and
2010. |
Table II
Review of 20 cases of ameloblastic
carcinoma from an evidence-based literature review between 2005 and
2010.
| Case, n | First author (year)
[ref] | Gender/ age,
years | Type | Location | Therapy | Met. | Re. | Follow-up,
months | Outcome |
|---|
| 1 | Lucca et al
(2010) [9] | M/69 | P | Maxillary | Jaw extended
resection | - | - | 11 | DF |
| 2 | Karakida et al
(2010) [10] | M/43 | S | Mandible | Jaw extended
resection, neck dissection | - | - | 46 | DF |
| 3 | Jindal et al
(2010) [11] | M/60 | S | Mandible | Jaw extended
resection | - | - | 19 | DF |
| 4 | Jeremic et al
(2010) [12] | M/58 | P | Mandible | Jaw extended
resection, neck dissection, radiation and chemotherapy following 9
months | Lung | - | 21 | DF |
| 5 | Devenney-Cakir et
al (2010) [13] | M/16 | P | Mandible | Partial resection,
lymph node dissection | - | - | 48 | Met. (lung) |
| 6 | Yoon et al
(2009) [14] | M/63 | P | Maxillary | Jaw extended
resection, radiation | - | Y | 13 | DF |
| | F/73 | P | Maxillary | Jaw extended
resection | - | - | 31 | DF |
| | M/61 | P | Maxillary | Jaw extended
resection | - | - | 13 | DF |
| | M/46 | P | Mandible | Jaw extended
resection, neck dissection, radiation | LN | Y | 18 | DF |
| | M/58 | P | Maxillary | Jaw extended
resection, neck dissection | - | - | 12 | DF |
| | M/65 | P | Mandible | Jaw extended
resection, neck dissection, radiation | LN | - | 13 | DF |
| 7 | Ismail et al
(2009) [15] | F/21 | P | Mandible | Jaw extended
resection, neck dissection | - | - | 36 | DF |
| 8 | Yazici et al
(2008) [16] | M/10 | P | Maxillary | Jaw extended
resection, radiation | - | - | 6 | DF |
| 9 | Angiero et
al (2008) [5] | M/68 | P | Maxillary | Jaw extended
resection | - | - | 6 | DF |
| 10 | Ward et al
(2007) [4] | M/64 | P | Maxillary | Jaw extended
resection | - | - | 42 | DF |
| 11 | Naik and Kale
(2007) [17] | M/70 | P | Maxillary | Partial
resection | - | - | 12 | DF |
| 12 | Benlyazid et
al (2007) [7] | M/90 | P | Maxillary | Partial
resection | - | - | 25 | STD |
| 13 | Akrish et al
(2007) [18] | M/80 | S | Mandible | Partial resection,
neck dissection | - | - | 12 | DF |
| 14 | Suomalainen et
al (2006) [2] | F/21 | P | Mandible | Partial resection,
neck dissection | - | - | 30 | DF |
| 15 | Miyake et al
(2006) [19] | F/91 | P | Mandible | Jaw extended
resection | - | - | 6 | DF |
Classification of lesions
The ameloblastic carcinomas were classified as
primary or secondary by a reviewing pathologist using the 2005 WHO
criteria (8). The gender, age,
primary site, surgical procedures, pathology and outcome were
identified. Primary and metastatic lesions were confirmed by X-ray,
computed tomography (CT) and pathological examination. Written
informed consent was obtained from all patients and approval of the
study was obtained from the Ethics Committee of Sichuan University.
The study was also approved by the West China Hospital of
Stomatology Review and Ethics Board.
Procedures
An extended jaw resection was performed if the X-ray
and CT scan demonstrated destruction of the cortical bone,
involvement of the periosteum, or invasion of soft tissue. In cases
where the tumor invaded the cortical bone and exhibited no invasion
of the soft tissue, a partial jaw resection was performed. A
marginal ostectomy was performed for tumors that were limited to
the alveolar bone without cortical invasion.
Results
Patients from the West China Hospital of
Stomatology
A total of 12 patients with ameloblastic carcinoma
were treated at the West China Hospital of Stomatology between 2000
and 2008. The incidence of ameloblastic carcinoma was relatively
uncommon compared with that of ameloblastoma (12/538; 2.23% of
overall cases). The male:female ratio was 5:1 and the mean age was
44 years (range, 30–75 years). Tumors occurred more frequently in
the mandible than in the maxillary (11:1) and eight tumors were
primary and four were secondary.
All of the patients with primary tumors (8/8) were
cured following extended resection (2), partial resection (5), or marginal ostectomy (1), while only one patient with a secondary
tumor was cured (1/4; 25%). Patients exhibiting secondary tumors
were treated by extended (2) or
partial resection (2); two of the
four (50%) patients developed a local recurrence and one (1/4; 25%)
exhibited metastases. No patients underwent cervical lymph node
dissection, chemotherapy or radiotherapy. The cure rate of males
and females was 80% (8/10) and 100% (2/2), respectively. The cure
rate was 75% (3/4) following extended jaw resection, 71% (5/7)
following partial jaw resection and 100% (1/1) following marginal
ostectomy.
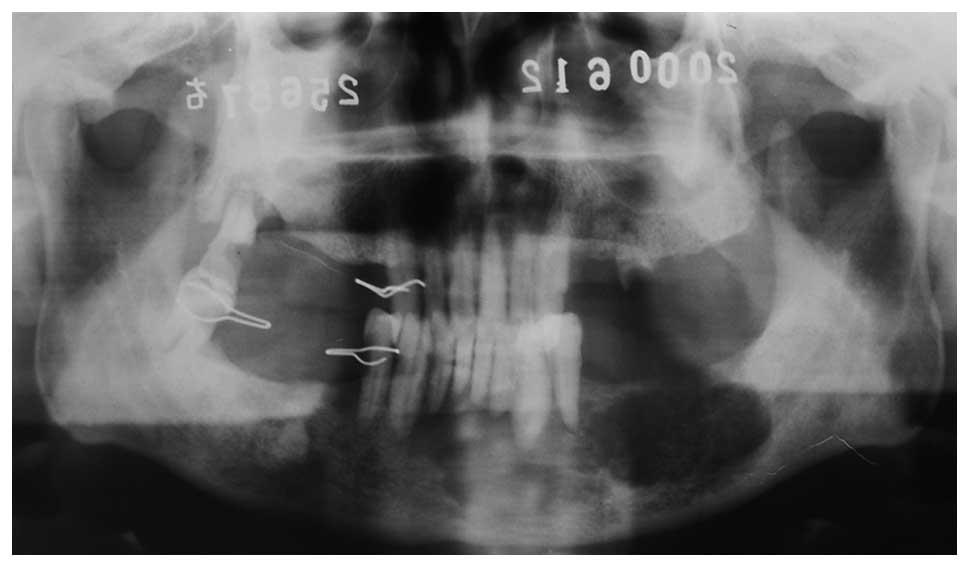
A 75-year-old male was diagnosed with left jaw
ameloblastoma. Following curettage in 1978 at the West China
Hospital of Stomatology, the chest radiograph was normal. However,
22 years later, panoramic radiographs demonstrated bone destruction
(Fig. 1) and a chest X-ray
demonstrated a solitary nodule. A needle biopsy of the lung nodule
revealed ameloblastoma and the patient underwent a partial
mandibulectomy; the pathological examination demonstrated
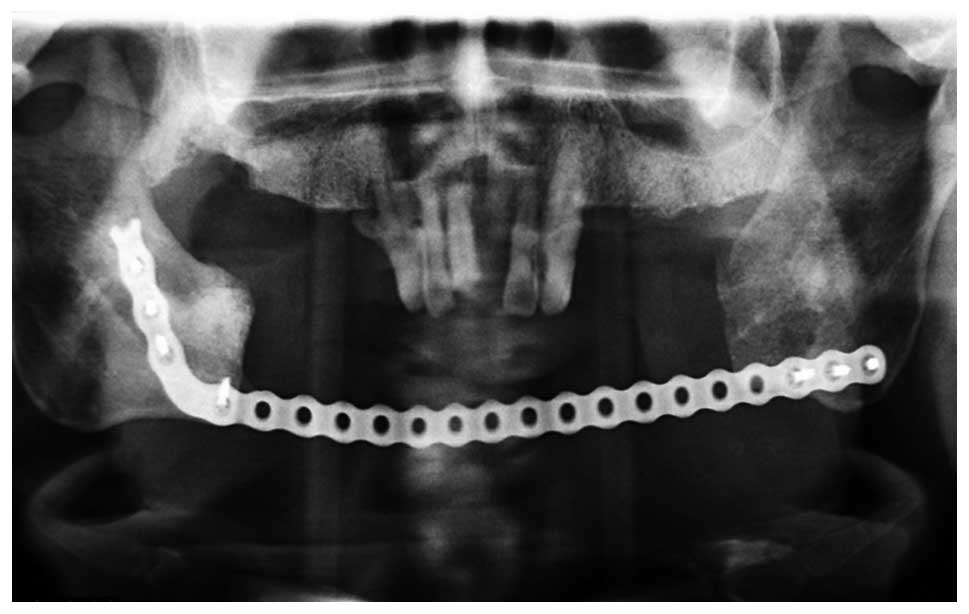
metastatic ameloblastoma. Eight years later, the tumor recurred
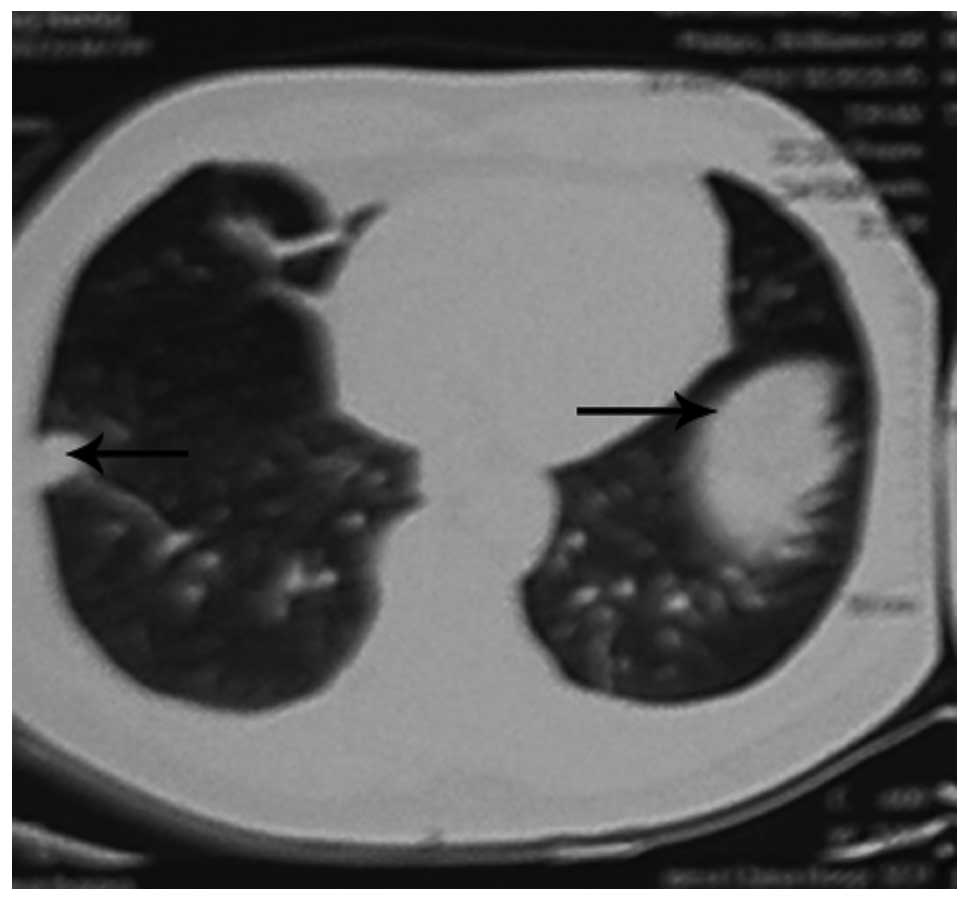
(Fig. 2) and a chest CT showed
bilateral lung nodules (Fig. 3).
Pathological review of the needle biopsy demonstrated ameloblastic
carcinoma. Subsequently, the patient underwent an extended mandible
resection of the ameloblastic carcinoma (secondary type; Figs. 4 and 5). The patient experienced various stages
of disease development: a malignant transformation from
ameloblastoma, metastatic ameloblastoma, to ameloblastic carcinoma,
and survival with a tumor for 30-years, which is particularly
rare.
Review of cases from the literature
In total, 20 patients with ameloblastic carcinoma,
which were reported between 2005 and 2010 were identified by
searching PubMed (Table II). The
postoperative follow-up ranged between six and 48 months. The
male:female ratio was 4:1 and the mean patient age was 56.3 years
(range, 10–91 years). A total of 10 patients exhibited maxillary
tumors and 10 had mandibular tumors. Furthermore, 17 patients had
primary tumors and three exhibited secondary tumors. Among the
patients with primary tumors, 2/17 exhibited cervical lymph node
metastases and 1/17 exhibited lung metastases. Among the patients
with secondary tumors, 2/3 exhibited lymph node metastases.
The cure rate of males and females was 87.5% (14/16)
and 100% (4/4), respectively. The cure rate was 88.2% (15/17) in
patients with primary tumors and 100% (3/3) in patients with
secondary tumors (3/3). In addition, the cure rate was 100% (15/15)
following extended jaw resection and 60% (3/5) following partial
jaw resection. Two patients with lymph node metastasis were also
treated with radical neck dissection and seven patients with lymph
node metastasis underwent prophylactic lymph node dissection. The
cure rate was 88.9% (8/9) following lymph node dissection and 90.9%
(10/11) without lymph node dissection. Chemotherapy and
radiotherapy were administered for lung metastases, and
radiotherapy alone was administered for lymph node metastasis. The
response rates were 100% (1/1) for chemotherapy and radiotherapy,
100% (4/4) for radiotherapy, and 86.7% (13/15) without chemotherapy
or radiotherapy (Table III). For
the 17 patients with primary tumors, the cure rate was 100% (13/13)
following extended resection and 50% (2/4) following partial
resection (2/4); one patient succumbed to the disease and one
developed metastases. The cure rate was 85.7% (6/7) following lymph
node dissection and 90% (9/10) without lymph node dissection. Only
primary tumors were treated with radiotherapy and chemotherapy. The
cure rate was 100% (5/5) for patients treated with chemotherapy and
radiotherapy, and 83.3% (10/12) for patients treated with surgery
alone. The cure rate for secondary tumors was 100% (3/3); two
patients were treated with an extended resection and one was
treated with a partial resection. The cure rate was 100% (2/2) for
patients with secondary tumors following lymph node dissection, and
100% without lymph node dissection (1/1).
 | Table IIIAmeloblastic carcinoma cure rates at
the West China Hospital of Stomatology and from the literature. |
Table III
Ameloblastic carcinoma cure rates at
the West China Hospital of Stomatology and from the literature.
| Cure rate, n
(%) |
|---|
|
|
|---|
| Variable | West China Hospital
(n=12) | PubMed literature
(n=20) |
|---|
| Gender |
| Male | 10 (80.0) | 16 (87.5) |
| Female | 2 (100.0) | 4 (100.0) |
| Type |
| Primary | 8 (100.0) | 17 (88.2) |
| Secondary | 4 (25.0) | 3 (100.0) |
| Therapy |
| Extended
resection | 4 (75.0) | 15 (100.0) |
| Partial
resection | 7 (71.4) | 5 (60.0) |
| Marginal
ostectomy | 1 (100.0) | N/A |
| Neck
dissection | N/A | 9 (88.9) |
| Primary type | N/A | 7 (85.7) |
| Secondary
type | N/A | 2 (100.0) |
| No neck
dissection | N/A | 11 (90.9) |
| Primary type | N/A | 10 (90.0) |
| Secondary
type | N/A | 1 (100.0) |
| Radiation and
chemotherapy | N/A | 5 (100.0) |
| Primary type | N/A | 5 (100.0) |
| Secondary
type | N/A | N/A |
| No radiation and
chemotherapy | N/A | 15 (86.7) |
| Primary type | N/A | 3 (83.3) |
| Secondary
type | N/A | 3 (100.0) |
Discussion
The evolution of ameloblastoma to ameloblastic
carcinoma is controversial (14).
The exact mechanism of the malignant transformation is currently
unknown due to the limited number of reports. One study has shown
that malignant transformation requires a relatively long duration,
and multistage carcinogenesis is a reasonable suggestion as to the
underlying mechanism of malignant transformation (20). Makiguchi et al (21) demonstrated that the malignant and
benign regions are distinguishable by preoperative 18F-α-methyl
tyrosine positron emission tomography and magnetic resonance
imaging to avoid excessive resection, severe functional loss and a
poor facial appearance. However, the exact mechanism requires
further investigation. The present study presents the progression
from benign ameloblastoma to metastatic ameloblastoma and
eventually to ameloblastic carcinoma in a 75-year-old male.
Surgical treatment alone was effective for this patient. Rare
diseases, including the ameloblastoma to ameloblastic carcinoma
spectrum, require randomized multicenter studies in order to define
novel and improved treatments.
The most common clinical manifestation of
ameloblastoma is a swollen, occasionally painful, jaw. However,
certain tumors grow rapidly and limit the opening of the mouth. In
addition, rapid tumor growth may perforate the cortical bone and
extend into the soft tissue, causing pain and paresthesia. Akrish
et al (18) retrospectively
analyzed 37 patients with ameloblastic carcinoma, which were
reported between 1984 and 2007. In these patients, the male:female
ratio was 2:1, the mean age was 52 years (range, 15–84 years), and
the maxillary:mandible tumor ratio was 13:25. In the current study
PubMed was used and 20 cases of ameloblastic carcinoma (reported
between 2005 and 2010) and staged according to the 2005 WHO
classification were identified. The male:female ratio was 4:1,
maxillary:mandible tumor ratio was 10:10, and mean age was 56.3
years (range, 10–91 years). A total of 17 patients had primary
tumors and three exhibited secondary tumors. By contrast, the
majority of the patients treated at the West China Hospital of
Stomatology were male and aged between 30 and 45 years. In
addition, the majority of tumors were in the mandible (92%),
including eight primary and four secondary tumors.
Ameloblastic carcinoma occurs in a wide range of age
groups with no apparent gender predilection. The most commonly
involved area is the mandible and the most common pathology is the
primary type. All the secondary tumors presented in the present
study and in the identified literature were of the intraosseous
type. The peripheral type of ameloblastic carcinoma, arising within
a pre-existing benign peripheral ameloblastoma, was relatively
rare.
The optimal treatment for ameloblastic carcinoma
remains unknown. In the present study, the cure rate of the primary
tumors was higher than that observed in the literature (100 vs.
88.2%), however, the recurrence rates of the secondary tumors were
higher than those presented in the literature (16.7 vs. 0%). The
incidence of lymph node metastases in the present study was less
frequent than in the literature (0 vs. 10%). Two patients with lung
metastases were identified in the present study group (primary
type) and the literature (secondary type). Ameloblastic carcinoma
exhibits the histological features and behavior of malignancy, and
therefore, definitive surgical treatment is required (20). In the literature the cure rate
following extended jaw resection was 100% for primary and secondary
tumors. In the present group of patients, the recurrence rate
following partial resection was 50%. Therefore, these results
support the use of extended jaw resection to prevent local
recurrence. Yoon et al (14)
reported a recurrence rate of 92.3% following curettage alone, and
28.3% following partial resection. Extended jaw resection involves
a margin of resection of 2–3 cm, including the normal bone,
periosteum and soft tissue. This approach has been found to reduce
the local recurrence rate by 15% when compared with partial
resection (22). Naik and Kale
(17) also reported that surgical
stimulation and incomplete resection may induce the degeneration of
early lesions, and explain the higher recurrence rate following
partial resection, which was identified in patients with secondary
ameloblastic carcinoma compared with primary.
At present, the use of cervical lymph node
dissection with ameloblastic carcinoma is under review. Jeremic
et al (12) proposed the use
of parotid gland resection and regional lymph node dissection to
achieve adequate margins. Angiero et al (5) argued that since metastases is able to
occur via the blood stream, cervical lymph node dissection should
not be routinely performed. In the present study, patients with
primary tumors and no prophylactic lymph node dissection exhibited
higher cure rates. In addition, the cure rate of secondary tumors
was higher compared with the cases in the literature. However, due
to the small number of patients, elective neck dissection is not
recommended for this type of lesion. Yoon et al (14) also revealed that neck dissections
should not be recommended for primary or secondary lesions without
evidence of cervical lymph node involvement.
The literature review identified four patients that
were treated with radiotherapy and one, which was treated with a
combination of chemotherapy and radiotherapy. All five patients had
primary tumors. The cure rate was 100% for the combination of
chemotherapy and radiotherapy, and 100% for radiotherapy alone.
Chemotherapy has not been indicated as a primary treatment
(16). Patients with secondary
tumors exhibit a higher rate of recurrence and metastasis and to
date, chemotherapy has shown no favorable results for local control
(23). In addition, few
chemotherapy reports are available and its role is yet to be
confirmed (24). Furthermore, the
combination of chemotherapy and radiotherapy to treat patients with
secondary tumors requires further evaluation.
It is generally acknowledged that ameloblastic
carcinoma is considered to be a radioresistant tumor. However, pre-
or postoperative radiotherapy may reduce the size of ameloblastic
carcinomas (17). Dhir et al
(25) reported that 50% of
postoperative patients developed local recurrence or metastasis,
and could be treated with radiotherapy. Radiotherapy alone is
appropriate for patients who are not surgical candidates, or
exhibit advanced local or metastatic disease.
Certain novel treatment methods have gradually been
put forward. Ion beam therapy, which delivers high doses to the
target tumor while sparing the normal surrounding tissues, is a
novel therapy for cancer. Recently, Jensen et al (26) reported the use of carbon ion therapy
for recurrent ameloblastic carcinoma over four weeks, which showed
no recurrence following three months. In addition, compared with
the conventional radiotherapy, carbon ion therapy exhibits less
severe complications. Perera et al (23) considered Gamma Knife stereotactic
radiosurgery as a promising option for instances where tumors
present in surgically complex regions.
In conclusion, ameloblastic carcinoma is a
relatively rare type of tumor, occurring in only 2.23% (12/538) of
patients presenting with ameloblastoma at the West China Hospital
of Stomatology. Ameloblastic carcinoma exhibit an aggressive
clinical behavior, including rapid tumor growth, painful swelling
and perforation of the cortex. The proposed mechanisms underlying
the transformation of a classic benign ameloblastoma into a
malignant tumor remain controversial. It has been indicated that
wide local excision with postoperative radiation therapy should be
employed. However, novel therapeutic regimens must be considered,
including carbon ion therapy and Gamma Knife stereotactic
radiosurgery. Controlled studies with larger groups of patients are
required to increase the accuracy of results.
Acknowledgements
This study was supported by grants from the Chinese
National Natural Science Foundation (grant no. 81170940), the
National Key Technology R&D Program (grant nos. 2012BAH07F01
and 2009BAI8IB03) and the National 863 Program (grant nos.
2013AA013803 and 2013AA040804).
References
|
1
|
Abiko Y, Nagayasu H, Takeshima M, et al:
Ameloblastic carcinoma ex ameloblastoma: report of a case-possible
involvement of CpG island hypermethylation of the p16 gene in
malignant transformation. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 103:72–76. 2007.
|
|
2
|
Suomalainen A, Hietanen J, Robinson S and
Peltola JS: Ameloblastic carcinoma of the mandible resembling
odontogenic cyst in a panoramic radiograph. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 101:638–642. 2006.
|
|
3
|
Kamath KP, Vidya M, Shetty N, et al:
Nucleolar organizing regions and alpha-smooth muscle actin
expression in a case of ameloblastic carcinoma. Head Neck Pathol.
4:157–162. 2010.
|
|
4
|
Ward BB, Edlund S, Sciubba J and Helman
JI: Ameloblastic carcinoma (primary type) isolated to the anterior
maxilla: case report with review of the literature. J Oral
Maxillofac Surg. 65:1800–1803. 2007.
|
|
5
|
Angiero F, Borloni R, Macchi M and Stefani
M: Ameloblastic carcinoma of the maxillary sinus. Anticancer Res.
28:3847–3854. 2008.
|
|
6
|
Mubeen K, Shakya HK and Jigna VR:
Ameloblastic carcinoma of mandible. A rare case report with review
of literature. J Clin Exp Dent. 2:e83–e87. 2010.
|
|
7
|
Benlyazid A, Lacroix-Triki M, Aziza R, et
al: Ameloblastic carcinoma of the maxilla: case report and review
of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 104:e17–e24. 2007.
|
|
8
|
Barnes L, Eveson J, Reichat P, et al;
World Health Organization. Classification of Tumours; Pathology and
Genetics of Head and Neck Tumours. IARC Press; Lyon: 2005
|
|
9
|
Lucca M, D‘Innocenzo R, Kraus JA, et al:
Ameloblastic carcinoma of the maxilla: a report of 2 cases. J Oral
Maxillofac Surg. 68:2564–2569. 2010.
|
|
10
|
Karakida K, Aoki T, Sakamoto H, et al:
Ameloblastic carcinoma, secondary type: a case report. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 110:e33–e37. 2010.
|
|
11
|
Jindal C, Palaskar S, Kaur H and Shankari
M: Low-grade spindle-cell ameloblastic carcinoma: report of an
unusual case with immunohistochemical findings and review of the
literature. Curr Oncol. 17:52–57. 2010.
|
|
12
|
Jeremic JV, Nikolic ZS, Boricic IV, et al:
Total mandibular reconstruction after resection of rare
‘honeycomb-like’ ameloblastic carcinoma - a case report. J
Craniomaxillofac Surg. 38:465–468. 2010.
|
|
13
|
Devenney-Cakir B, Dunfee B, Subramaniam R,
et al: Ameloblastic carcinoma of the mandible with metastasis to
the skull and lung: advanced imaging appearance including computed
tomography, magnetic resonance imaging and positron emission
tomography computed tomography. Dentomaxillofac Radiol. 39:449–453.
2010.
|
|
14
|
Yoon HJ, Hong SP, Lee JI, et al:
Ameloblastic carcinoma: an analysis of 6 cases with review of the
literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
108:904–913. 2009.
|
|
15
|
Ismail SB, Zain RB, Yaacob HB and Abraham
MT: Ameloblastic carcinoma (spindle cell variant). Pathology.
41:292–295. 2009.
|
|
16
|
Yazici N, Karagöz B, Varan A, et al:
Maxillary ameloblastic carcinoma in a child. Pediatr Blood Cancer.
50:175–176. 2008.
|
|
17
|
Naik V and Kale AD: Ameloblastic
carcinoma: a case report. Quintessence Int. 38:873–879. 2007.
|
|
18
|
Akrish S, Buchner A, Shoshani Y, et al:
Ameloblastic carcinoma: report of a new case, literature review,
and comparison to ameloblastoma. J Oral Maxillofac Surg.
65:777–783. 2007.
|
|
19
|
Miyake T, Tanaka Y, Kato K, et al: Gene
mutation analysis and immunohistochemical study of beta-catenin in
odontogenic tumors. Pathol Int. 56:732–737. 2006.
|
|
20
|
Avon SL, McComb J and Clokie C:
Ameloblastic carcinoma: case report and literature review. J Can D
Assoc. 69:573–576. 2003.
|
|
21
|
Makiguchi T, Yokoo S, Miyazaki H, et al:
Treatment strategy of a huge ameloblastic carcinoma. J Craniofac
Surg. 24:287–290. 2013.
|
|
22
|
Phaik IKS and Ong ST: Ameloblastic
carcinoma: a case with cervical node and pulmonary metastases.
Annals Dent Univ Malaya. 5:49–52. 1998.
|
|
23
|
Perera E, Lindquist C, Hughes C and Thomas
S: The use of Gamma Knife stereotactic radiosurgery in the
treatment of ameloblastic carcinoma. Int J Oral Maxillofac Surg.
42:934–938. 2013.
|
|
24
|
Ozlugedik S, Ozcan M, Basturk O, et al:
Ameloblastic carcinoma arising from anterior skull base. Skull
Base. 15:269–272. 2005.
|
|
25
|
Dhir K, Sciubba J and Tufano RP:
Ameloblastic carcinoma of the maxilla. Oral Oncol. 39:736–741.
2003.
|
|
26
|
Jensen AD, Ecker S, Ellerbrock M, et al:
Carbon ion therapy for ameloblastic carcinoma. Radiat Oncol.
6:132011.
|