Introduction
Due to germline and epigenetic gene inactivation,
tumors with microsatellite instability (MSI) account for ~20% of
all types of colorectal cancer (CRC) (1,2).
Compared with microsatellite stable (MSS) tumors, sporadic MSI
tumors exhibit recognizable clinicopathological features, including
the absence of necrosis, the presence of a Crohn’s-like reactions,
a right-sided location, a lower pathological stage and an improved
prognosis (2–4). Genetic instability in sporadic MSI
tumors primarily reflects variation in microsatellite tracts as a
result of defective surveillance mechanisms controlled by the DNA
mismatch repair system. At present, 30 target genes have been
identified to be involved in MSI carcinogenesis, including genes of
the homologous recombination repair (HRR) pathway for double-strand
breaks (DSBs), such as MRE11, RAD50 and Ku80 (5,6). Thus,
other HRR genes, for example, X-ray repair complementing defective
repair in Chinese hamster cells 2 (XRCC2) may also be associated
with MSI.
Located on chromosome 7q36.1, XRCC2 encodes a member
of the Rad51 family of related proteins that maintains chromosome
stability by participating in homologous recombination and repairs
DNA damage. XRCC2 has roles in the HRR pathway of double-stranded
DNA, which repairs chromosomal fragmentation, deletions and
translocations (7–9). In total, ~622 single nucleotide
polymorphisms (SNPs) have been reported in XRCC2, including
rs3218536 (Arg188His), rs718282, rs3218384, rs3218550, rs3218408,
rs2040639 and rs3218499. Studies have been performed on the
association between SNPs and the cancer incidence risk in ~250,000
individuals from a number of countries, including the United States
of America, China, Japan, Poland and Iran, however, the findings
have been inconsistent (10–16).
However, certain studies have shown that XRCC2 SNPs increase the
risk of CRC (16,17).
DSBs are one of the most significant threats to
genomic integrity and induce the activation of repair proteins that
are involved in the HRR and non-homologous end-joining (NHEJ)
pathways; for example, D-NHEJ, which uses DNA-dependent protein
kinase, and B-NHEJ, which uses DNA ligase III and poly(ADP-ribose)
polymerase (PARP) 1. PARP1 has a key role in HRR and NHEJ.
Inhibitors of PARP1 increase the levels of persistent single-strand
breaks that lead to DNA DSBs upon replication (18,19).
According to the theory of synthetic lethality, cells may survive
when either a HRR gene, such as XRCC2, is mutated, or when PARP1 is
inhibited, but not when both occur simultaneously (20). The present study aimed to
investigate the activity of the PARP1 inhibitor olaparib (AZD2281)
in CRC cell lines with XRCC2 SNP mutations.
Materials and methods
Cell lines
CRC cell lines (LoVo, LS174T, HT29, SW480 and SW620)
were purchased from the American Type Culture Collection (Manassas,
VA, USA) and maintained in Dulbecco’s modified Eagle’s medium
(Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (HyClone Laboratories, Inc., Logan, UT,
USA). The cells were maintained in a humidified atmosphere at 37°C
with 5% CO2.
SNaPshot® analysis of SNP
genotypes
SNaPshot analysis of SNP genotypes was performed
using a SNaPshot Multiplex kit system (Applied Biosystems, Foster
City, CA, USA). Products were treated with 1 unit of shrimp
alkaline phosphatase at 37°C for 60 min and 75°C for 15 min,
followed by a denaturation step at 95°C for 5 min. Detection was
performed using 0.5 μl SNaPshot products mixed with 9 μl HiDi™
formamide and 0.5 μl GeneScan-120LIZ size standard (Applied
Biosystems). Data were generated following capillary
electrophoresis on an automated sequencer (ABI 3130 Genetic
Analyzer; Applied Biosystems) with a 36-cm length capillary and
POP-7™ polymer and analyzed using GeneMapper® software
version 3.7 (Applied Biosystems).
MTT assay
MTT assays were performed to quantify the viability
of the CRC cells following treatment with the PARP1 inhibitor,
AZD2281, and cisplatin (DDP). MTT stock solution (0.5 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) was added to the incubation
medium in the wells at a final concentration of 25 μmol/l AZD2281
(Selleckchem, Houston, TX, USA) and 0.75 μg/ml DDP (Nuoxin; Jiangsu
Hansoh Pharmaceutical Co., Ltd., Group, Lianyungang, China). The
cells were incubated for 36 h at 37°C in a humidified 5%
CO2 atmosphere. The culture medium was then removed and
the formazan crystals in the cells were solubilized using dimethyl
sulfoxide (Sigma-Aldrich), with plate agitation for 30 min. The
absorbance was measured at 570 nm, with a reference wavelength of
655 nm.
Expression of XRCC2 wild-type and mutant
transcripts and PARP1
Total RNA was extracted from the cultured cells
using TRIzol® reagent (Invitrogen Life Technologies)
according to the manufacturer’s instructions. The cDNA was
amplified and quantified using an ABI Prism 7500 Sequence Detection
System (Applied Biosystems) with SYBR Green I dye (Invitrogen Life
Technologies). The following primers were used: XRCC2 forward,
TCACCTGTGCATGGTGATATT and reverse, TTCCAGGCCACCTTCTGATT; PARP1
forward, ACAGTGTGCAGGCCAAGGTG and reverse,
CTCGGCTTCTTCAGAATCTCTGTC; β-actin forward, TGGCACCCAGCACAATGAA and
reverse, CTAAGTCATAGTCCGCCTAGAAGCA. Expression data were normalized
to the expression of β-actin and calculated as 2− [(Ct of
gene) − (Ct of β-Actin)], where Ct represents the threshold
cycle for each transcript.
Western blot analysis
The cells were harvested in sampling buffer [62.5
mmol/l Tris-HCl (pH 6.8), 10% glycerol and 2% SDS] and heated for 5
min at 100°C. The protein concentration was determined using the
Bradford assay using a commercial kit purchased from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA). Equal quantities of protein
were separated electrophoretically on 12% SDS polyacrylamide gels
and transferred onto polyvinylidene difluoride membranes (Roche,
Basel, Switzerland). The membranes were probed for 3 h with mouse
anti-XRCC2 (dilution, 1:1,000; ab20253; Abcam Plc, Cambridge, UK)
and mouse anti-PARP1 (dilution, 1:1,000; ab96476; Abcam Plc)
antibodies. The expression of the target proteins was determined
using horseradish peroxidase-conjugated anti-rabbit/anti-mouse
immunoglobulin G (dilution, 1:3,000) and enhanced chemiluminescence
(Pierce Chemical Company, Rockford, IL, USA) according to the
manufacturer’s instructions. The membranes were stripped and
reprobed with an anti-β-actin mouse monoclonal antibody (dilution,
1:1,000; Sigma-Aldrich) as a loading control.
Statistical analysis
Differences among the cell lines were analyzed
following treatment with AZD2281 and DDP using repeated measures
analysis of variance (ANOVA), covariance analysis and pairwise
comparison methods. The two-tailed Student’s t-test was used to
assess the significance of the differences between two groups of
data. P<0.05 was considered to indicate a statistically
significant difference.
Results
XRCC2 rs3218536 polymorphism mutation is
associated with MSI CRC cell lines
LoVo and LS174T are MSI cell lines, while SW480,
SW620 and HT29 are MSS cell lines (21). In the present study, mutations in
the coding microsatellite tracts of XRCC2 were assessed in a panel
of five CRC cell lines. All the MSI cell lines were found to have a
mutation in XRCC2, while none of the MSS cell lines were found to
have a mutation (P=0.025). The two MSI cell lines (LoVo and LS174T)
were observed to have mutations in the poly-(A) tract located on
exon 3 of XRCC2; the rs3218536 mutation was homozygous, while the
rs3218550 mutation was heterozygous. The rs3218550 mutation was not
located within a coding DNA sequence (CDS); therefore, the present
study focused on the rs3218536 mutation. None of the MSS cell lines
had mutations in XRCC2 (Fig. 1;
Table I).
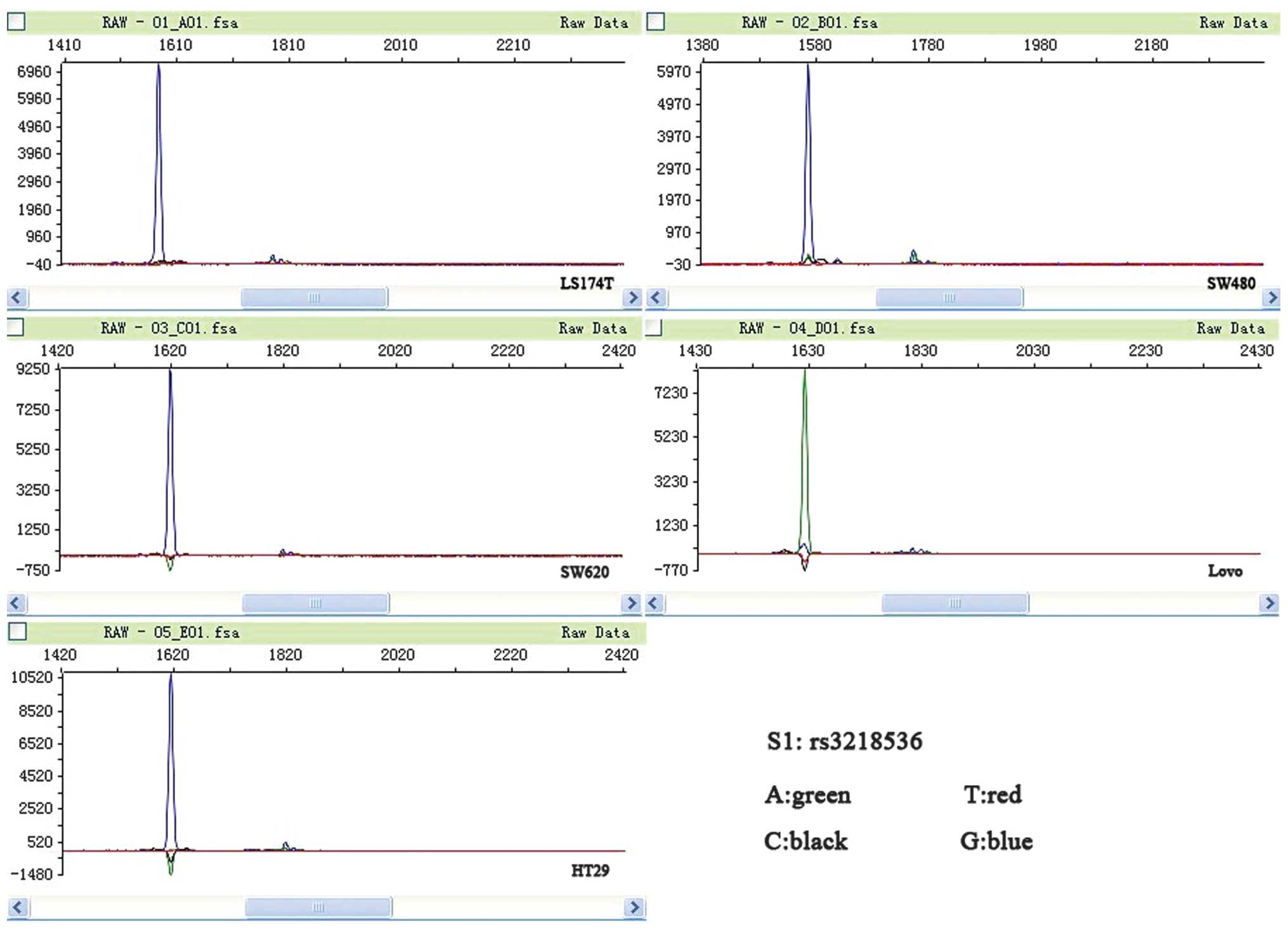 | Figure 1Peak figure of XRCC2 rs3218536. A
green peak, representing A, is apparent in the microsatellite
instability (MSI) LoVo cell line, and blue peaks, representing G,
are apparent in the other cell lines. A, adenine; T, thymine; C,
cytosine; G, guanine; XRCC2, X-ray repair complementing defective
repair in Chinese hamster cells 2. |
 | Table IPrimer sequences used for multiplex
polymerase chain reaction amplification panels. |
Table I
Primer sequences used for multiplex
polymerase chain reaction amplification panels.
| Gene | SNP | Forward (5′-3′) | Reverse (5′-3′) | Amplicon size,
bp |
|---|
| XRCC2 | rs3218536 |
TGTAGTCACCCATCTCTCTGC |
CACAGTCGTCGAGAGGCATGA | 240 |
| rs718282 |
GATACTTGGGAGATTGAGGCA |
GCCTGCTGTTATGAGTGTGAA | 205 |
| rs3218384 |
ACACCCTATTGCGCATGCTCC |
CCCATCTCCCTCACTCCCAAC | 233 |
| rs3218550 |
CATTCAACCCAGCAATCTCAT |
CACGCCCAGTCAGTCTTGTT | 190 |
| rs2040639 |
ATGCCTACCAGCAGTTTGTGA |
CAGTCTCCACACTGTTCCTAATG | 183 |
| rs3218499 |
ATATCTTCAAGTGCCAAACCT |
GATCCTCAAGATCAAAACCTG | 186 |
| rs3218408 |
TAGGCGATATACTGATGCCCT |
CAAGGCATGCACAGGCAGAAC | 190 |
Low AZD2281-sensitivity of MSI LoVo
cells
The five cell lines were divided into three groups:
Group 1, LoVo cells that harbored a mutation in the poly-(A) tract
of XRCC2; group 2, LS174T cells that harbored another mutation not
located within a CDS region; and group 3 (control), SW480, SW620
and HT29 cells that did not harbor mutations in the poly-(A) tract
of XRCC2.
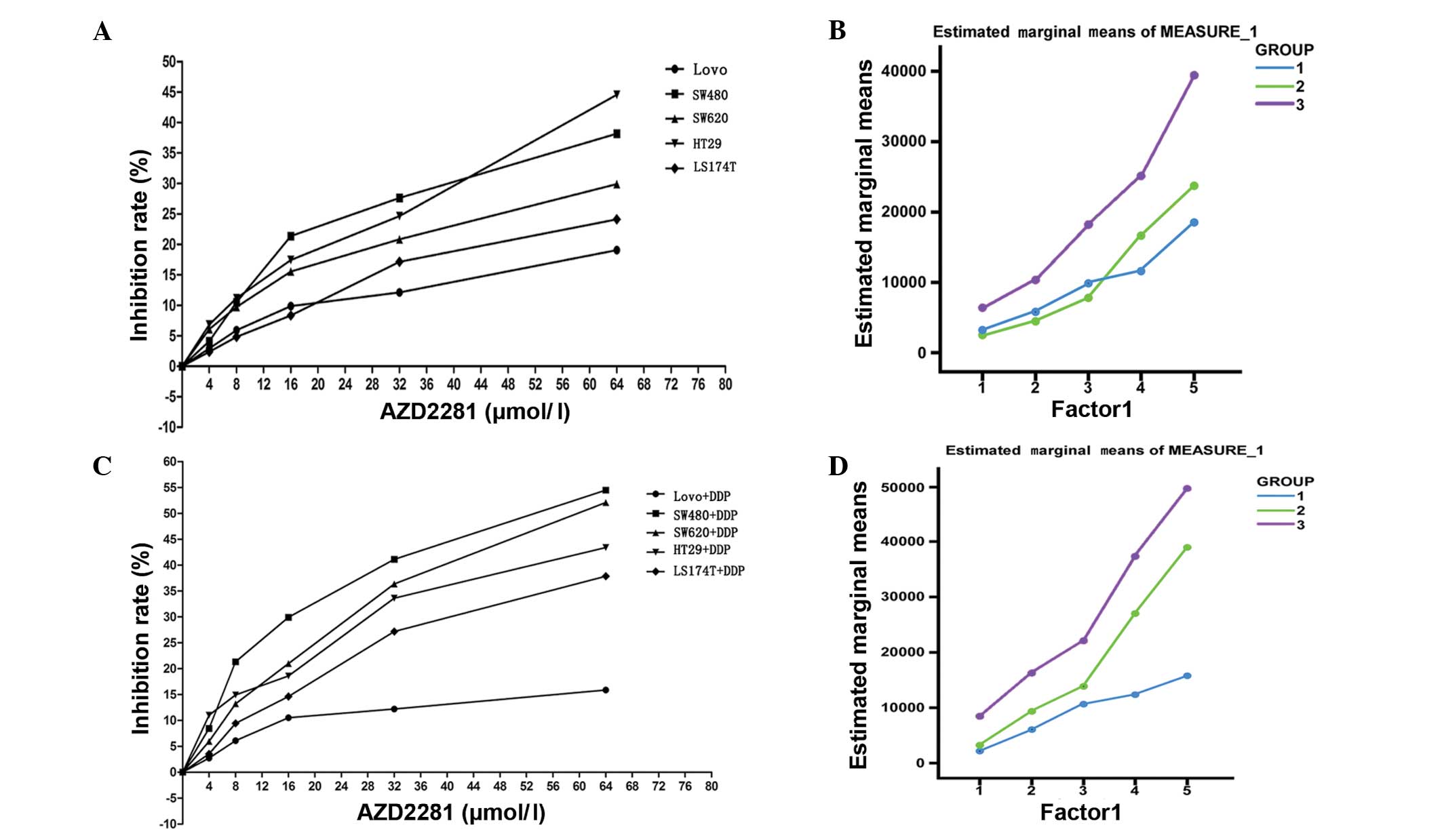
The relative growth inhibition rate of the cell
lines mediated by different concentrations of the novel PARP1
inhibitor, AZD2281, was analyzed. The half maximal inhibitory
concentration (IC50) in the cells in group 1 (1797.2
μmol/l) was approximately three-fold higher than that in the cells
in the other two groups (group 2, 631.7 μmol/l; group 3, 579.58
μmol/l). The relative inhibition rate of the cells was observed to
be affected by the AZD2281 concentration (0–64 μmol/l; P<0.001).
Furthermore, the relative inhibition rate of the LoVo cells in
response to AZD2281 was found to be significantly lower than that
of the other cell lines (P=0.002) (Fig.
2A; Table II). There was no
significant difference in the estimated marginal means of the cells
in group 1 and group 2 (P=0.415), while the estimated marginal mean
of the cells in group 1 was significantly lower than that of group
3 (P<0.001; Fig. 2B).
 | Table IIRepeated measures analysis of variance
of cells following treatment with AZD2281. |
Table II
Repeated measures analysis of variance
of cells following treatment with AZD2281.
| | | | 95% confidence
interval |
|---|
| | | |
|
|---|
| Group | Mean | Std | Pairwise comparisons
P-value | Lower bound | Upper bound |
|---|
| 1 | 9.967 | 1.157 | | 7.447 | 12.487 |
| 2 | 11.349 | 1.157 | 0.415 | 8.828 | 13.869 |
| 3 | 19.221 | 0.668 | <0.001 | 17.766 | 20.676 |
According to repeated measures ANOVA, the relative
inhibition rate of the cells was affected by the concentration of
AZD2281 and DDP (P<0.001) and the defining group factors
(P<0.001). Furthermore, there were cross-effects between the
different concentrations of AZD2281 and DDP and the defining group
factors (P<0.001; Fig. 2C;
Table III). The estimated
marginal mean of group 1 was found to be lower than that of group 2
(P=0.004) and group 3 (P<0.001; Fig.
2D).
 | Table IIIRepeated measures analysis of variance
of cells following treatment with AZD2281and DDP. |
Table III
Repeated measures analysis of variance
of cells following treatment with AZD2281and DDP.
| | | | 95% confidence
interval |
|---|
| | | |
|
|---|
| Group | Mean | Std | Pairwise comparisons
P-value | Lower bound | Upper bound |
|---|
| 1 | 9.482 | 1.795 | | 5.571 | 13.393 |
| 2 | 18.505 | 1.795 | 0.004 | 14.594 | 22.416 |
| 3 | 26.986 | 1.036 | <0.001 | 24.728 | 29.244 |
PARP1 expression is not associated with
the XRCC2 rs3218536 mutation
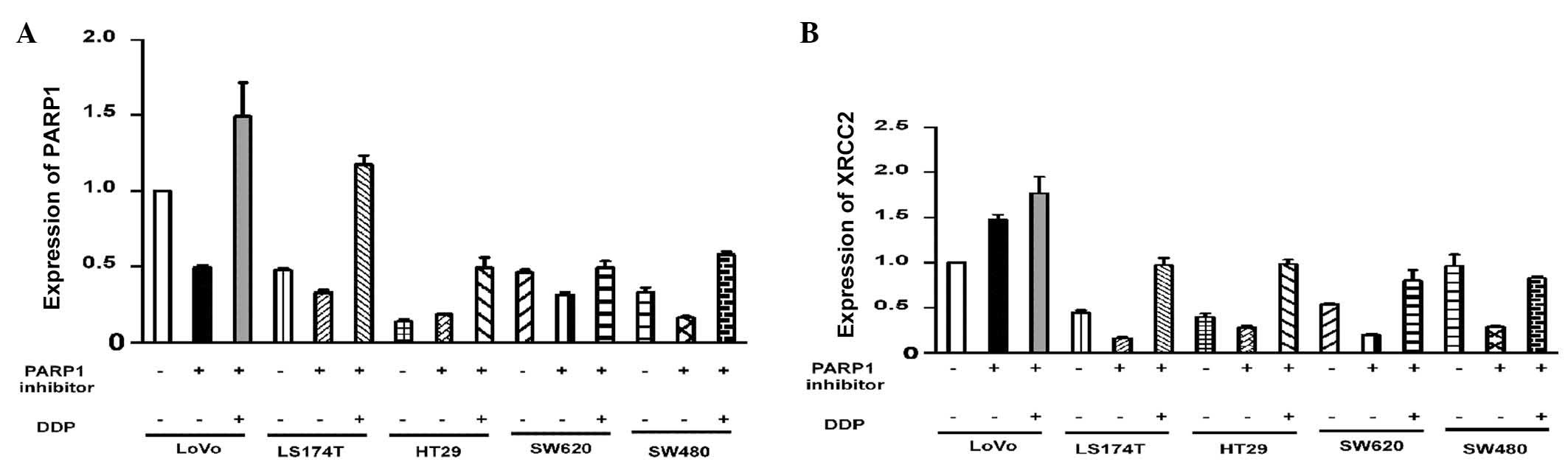
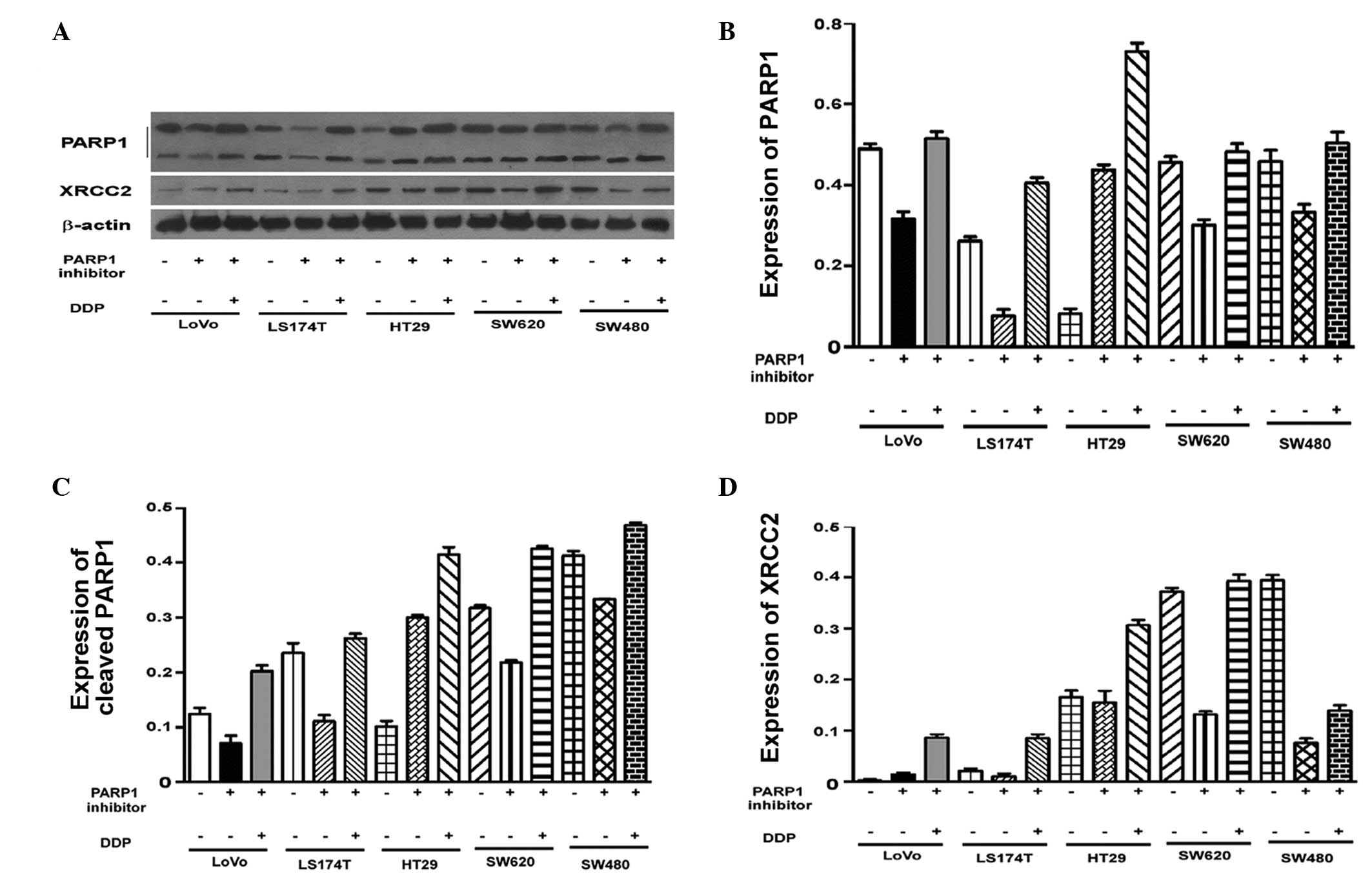
Varying levels of PARP1 expression were observed in
the untreated cell lines (P<0.001; Fig. 3A). To assess the effect of AZD2281
and DDP on the expression of PARP1, the mRNA levels in each cell
line were adjusted to the same level. Pairwise comparisons of the
PARP1 mRNA levels in the cells revealed that there were no
significant differences in PARP1 mRNA expression in the LoVo cells
compared with the other four cell lines following treatment with
AZD2281 and DDP (P>0.05; Table
IV). Western blot analysis demonstrated that there were no
significant differences in PARP1 protein expression between the
cell lines (Fig. 4). Thus, the
effect of AZD2281 and DDP on PARP1 mRNA levels was unaffected by
the original PARP1 mRNA levels (P=0.835; P>0.05).
 | Table IVPairwise comparisons of PARP1 mRNA
expression between different cell lines. |
Table IV
Pairwise comparisons of PARP1 mRNA
expression between different cell lines.
| | | | 95% confidence
interval |
|---|
| | | |
|
|---|
| Group | Mean | Standard error | Pairwise comparisons
P-value | Lower bound | Upper bound |
|---|
| LoVo | 0.429 | 0.152 | | 0.086 | 0.773 |
| LS174T | 0.329 | 0.009 | 0.527 | 0.308 | 0.349 |
| HT29 | 0.227 | 0.101 | 0.445 | −0.002 | 0.457 |
| SW480 | 0.183 | 0.045 | 0.240 | 0.082 | 0.284 |
| SW620 | 0.317 | 0.010 | 0.493 | 0.293 | 0.341 |
High XRCC2 expression in LoVo cells
Following AZD2281 treatment, XRCC2 expression was
found to be associated with the cell group (P<0.001). The LoVo
cells had significantly higher XRCC2 mRNA levels compared with the
other four cell lines (P<0.001). Notably, XRCC2 expression
following AZD2281 and DDP treatment, was also closely associated
with the cell group (P<0.001). The level of XRCC2 mRNA
expression in the LoVo cells was significantly higher than that in
the other four cell lines (P<0.001; Fig. 3B; Table
V). The results of the western blot analysis were consistent
with the results of the quantitative polymerase chain reaction
analysis (Fig. 4A and D).
 | Table VPairwise comparisons of XRCC2 mRNA
expression of cells. |
Table V
Pairwise comparisons of XRCC2 mRNA
expression of cells.
| | | | 95% confidence
interval |
|---|
| | | |
|
|---|
| Group | Mean | Standard error | Pairwise comparisons
P-value | Lower bound | Upper bound |
|---|
| LoVo | 1.470 | 0.055 | | 1.346 | 1.594 |
| LS174T | 0.168 | 0.040 | <0.001 | 0.078 | 0.257 |
| HT29 | 0.286 | 0.046 | <0.001 | 0.182 | 0.390 |
| SW480 | 0.285 | 0.050 | <0.001 | 0.172 | 0.397 |
| SW620 | 0.202 | 0.027 | <0.001 | 0.142 | 0.263 |
Discussion
Simultaneous deficiencies in two genes have been
proposed to introduce lethality in biologic systems that would
otherwise tolerate the loss of one of the genes (22). According to this theory, the
inhibition of PARP is a potential therapeutic strategy for the
treatment of types of cancer that have specific DNA repair defects,
including those that arise in individuals who are carriers of
mutations in BRCA1 or BRCA2 (23).
Other important genes involved in HRR include MRE11, XRCC2 and
PARP1. In the present study, it was hypothesized that other
components of this pathway may predict an increase in PARP1
inhibitor sensitivity. XRCC2 has roles in the HRR pathway, which
repairs chromosomal fragmentation, deletions and translocations.
Numerous studies have reported an association between XRCC2 SNPs
and the cancer incidence risk; the most common mutation studied
being the XRCC2 rs3218536 SNP (10–17).
The present study aimed to investigate the effect of this SNP on
PARP1 inhibitor sensitivity in CRC cell lines.
Vilar et al (21) reported that LoVo and LS174T are MSI
cell lines that have biallelic mutations in MRE11. However, the
study reported that there were no such mutations in MSS cell lines,
including the SW480, SW620 and HT29 cell lines (21). In the present study, the rs3218536
and rs3218550 SNP mutations of XRCC2 were only found to occur in
two MSI cells. In the MSS cell lines, no SNP mutations were
observed in XRCC2. However, since only five CRC cell lines were
investigated in the present study, whether these SNP mutations
occur only in MSI CRC lines remains to be investigated.
Certain PARP1 inhibitors, including
benzimidazole-4-carboxamides and tricyclic lactam indoles, have
been reported to inhibit cell growth by 50% at concentrations
between 8 and 94 μM (24). Vilar
et al (21) found that
following treatment with the PARP1 inhibitor veliparib (ABT-888) at
10 μM, there was a significant difference in cytotoxicity between
biallelic mutants cell lines, such as LoVo, and wild-type cell
lines, such as SW480 (P=0.028), with a 2.5-fold difference in
IC50 between these two groups (P=0.028). The study
concluded that cell lines, such as LoVo, that harbor biallelic
mutations in MRE11, have a higher sensitivity compared with
wild-type cell lines, such as SW480 (21). In the present study, LoVo cells were
found to harbor the XRCC2 rs3218536 mutation and were considered to
be a SNP mutation-positive cell line compared with the other cell
lines investigated, which were regarded as control cell lines.
According to the theory of synthetic lethality and the findings of
Vilar et al (21), LoVo
cells should have a higher sensitivity to the PARP1 inhibitor
AZD2281, as well as have a correspondingly lower IC50
compared with the other four cell lines, due to the presence of the
XRCC2 rs3218536 mutation. In accordance with this, the relative
growth inhibition rate of the LoVo cells should be higher than that
of the other four cell lines in response to AZD2281. The results of
the present study were contrary to this expectation. In the present
study, the LoVo cells exhibited a lower sensitivity to the PARP1
inhibitor, AZD2281, and a higher IC50 compared with the
other four cell lines. The differences in the results reported in
the present study compared with those reported by Vilar et
al (21) may be due to
differences in the PARP1 inhibitors used.
In the present study, no differences in PARP1 mRNA
levels were detected in the LoVo cells compared with the other four
cell lines. Furthermore, a higher level of XRCC2 mRNA was detected
in the LoVo cells compared with the other four cell lines. These
findings indicate that higher levels of XRCC2 mRNA are associated
with a lower sensitivity to the PARP1 inhibitor, AZD2281. Moreover,
consistent results were found in the analysis of the effect of the
PARP1 inhibitor on the five cell lines in a DNA damage model based
on DDP treatment for 16–32 h. These results are consistent with
those of Vilar et al (21).
In conclusion, the results of the present study show
that there is no association between PARP1 inhibitor sensitivity
and XRCC2 SNP mutations in CRC cells. However, due to the
limitations of the study, this conclusion requires further
investigation to fully elucidate this association. To the best of
our knowledge, there are >600 SNP sites in XRCC2, seven of which
have been reported in previous studies (10–17).
The rs3218536 SNP is located in a CDS region, therefore, it is
possible that this SNP mutation leads to a decreased sensitivity to
AZD2281, while other SNPs may not. Furthermore, a number of types
of PARP1 inhibitors are available, which may yield different
results in similar studies. Moreover, AZD2281 may not have an
effect on the DNA damage induced by DDP. These factors will be
clarified in a future rs3218536 mutation model that will be
developed to facilitate further investigations.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
NSFC-2011-81172339) and the Guangdong Natural Science Foundation
(grant no. 2011B031800118).
References
|
1
|
Hampel H, Frankel WL, Martin E, et al:
Screening for the Lynch syndrome (hereditary nonpolyposis
colorectal cancer). N Engl J Med. 352:1851–1860. 2005.
|
|
2
|
Gryfe R, Kim H, Hsieh ET, et al: Tumor
microsatellite instability and clinical outcome in young patients
with colorectal cancer. N Engl J Med. 342:69–77. 2000.
|
|
3
|
Greenson JK, Bonner JD, Ben-Yzhak O, et
al: Phenotype of microsatellite unstable colorectal carcinomas:
Well-differentiated and focally mucinous tumors and the absence of
dirty necrosis correlate with microsatellite instability. Am J Surg
Pathol. 27:563–570. 2003.
|
|
4
|
Greenson JK, Huang SC, Herron C, et al:
Pathologic predictors of microsatellite instability in colorectal
cancer. Am J Surg Pathol. 33:126–133. 2009.
|
|
5
|
Duval A and Hamelin R: Mutations at coding
repeat sequences in mismatch repair-deficient human cancers: toward
a new concept of target genes for instability. Cancer Res.
62:2447–2454. 2002.
|
|
6
|
Wang HC, Liu CS, Chiu CF, et al:
Significant association of DNA repair gene Ku80 genotypes with
breast cancer susceptibility in Taiwan. Anticancer Res.
29:5251–5254. 2009.
|
|
7
|
Jones NJ, Zhao Y, Siciliano MJ and
Thompson LH: Assignment of the XRCC2 human DNA repair gene to
chromosome 7q36 by complementation analysis. Genomics. 26:619–622.
1995.
|
|
8
|
Thacker J, Tambini CE, Simpson PJ, Tsui LC
and Scherer SW: Localization to chromosome 7q36.1 of the human
XRCC2 gene, determining sensitivity to DNA-damaging agents. Hum Mol
Genet. 4:113–120. 1995.
|
|
9
|
Liu N, Lamerdin JE, Tebbs RS, et al: XRCC2
and XRCC3, new human Rad51-family members, promote chromosome
stability and protect against DNA cross-links and other damages.
Mol Cell. 1:783–793. 1998.
|
|
10
|
Rafii S, O’Regan P, Xinarianos G, et al: A
potential role for the XRCC2 R188H polymorphic site in DNA-damage
repair and breast cancer. Hum Mol Genet. 11:1433–1438. 2002.
|
|
11
|
Wang R, Wang W and Zhang JW: Correlation
between XRCC2 and XRCC5 single nucleotide polymorphisms and
drug-sensitivity of human lung cancer cells. Zhonghua Yi Xue Za
Zhi. 88:3059–3062. 2008.(In Chinese).
|
|
12
|
Romanowicz-Makowska H, Smolarz B, Połać I
and Sporny S: Single nucleotide polymorphisms of RAD51 G135C, XRCC2
Arg188His and XRCC3 Thr241Met homologous recombination repair genes
and the risk of sporadic endometrial cancer in Polish women. J
Obstet Gynaecol Res. 38:918–924. 2012.
|
|
13
|
Han J, Hankinson SE, Hunter DJ and De Vivo
I: Genetic variations in XRCC2 and XRCC3 are not associated with
endometrial cancer risk. Cancer Epidemiol Biomarkers Prev.
13:330–331. 2004.
|
|
14
|
Lin WY, Camp NJ, Cannon-Albright LA, et
al: A role for XRCC2 gene polymorphisms in breast cancer risk and
survival. J Med Genet. 48:477–484. 2011.
|
|
15
|
Yen CY, Liu SY, Chen CH, et al:
Combinational polymorphisms of four DNA repair genes XRCC1, XRCC2,
XRCC3, and XRCC4 and their association with oral cancer in Taiwan.
J Oral Pathol Med. 37:271–277. 2008.
|
|
16
|
Curtin K, Lin WY, George R, et al: Genetic
variants in XRCC2: new insights into colorectal cancer
tumorigenesis. Cancer Epidemiol Biomarkers Prev. 18:2476–2484.
2009.
|
|
17
|
Krupa R, Sliwinski T,
Wisniewska-Jarosinska M, et al: Polymorphisms in RAD51, XRCC2 and
XRCC3 genes of the homologous recombination repair in colorectal
cancer - a case control study. Mol Biol Rep. 38:2849–2854.
2011.
|
|
18
|
Hoeijmakers JH: DNA damage, aging, and
cancer. N Engl J Med. 361:1475–1485. 2009.
|
|
19
|
Farmer H, McCabe N, Lord CJ, et al:
Targeting the DNA repair defect in BRCA mutant cells as a
therapeutic strategy. Nature. 434:917–921. 2005.
|
|
20
|
Whitehurst AW, Bodemann BO, Cardenas J, et
al: Synthetic lethal screen identification of chemosensitizer loci
in cancer cells. Nature. 446:815–819. 2007.
|
|
21
|
Vilar E, Bartnik CM, Stenzel SL, et al:
MRE11 deficiency increases sensitivity to poly(ADP-ribose)
polymerase inhibition in microsatellite unstable colorectal
cancers. Cancer Res. 71:2632–2642. 2011.
|
|
22
|
O’Connor MJ, Martin NM and Smith GC:
Targeted cancer therapies based on the inhibition of DNA strand
break repair. Oncogene. 26:7816–7824. 2007.
|
|
23
|
Fong PC, Boss DS, Yap TA, et al:
Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA
mutation carriers. N Engl J Med. 361:123–134. 2009.
|
|
24
|
Calabrese CR, Batey MA, Thomas HD, et al:
Identification of potent nontoxic poly(ADP-Ribose) polymerase-1
inhibitors: chemopotentiation and pharmacological studies. Clin
Cancer Res. 9:2711–2718. 2003.
|