Introduction
The correlation between malignancy and sarcoidosis
remains controversial (1). To the
best of our knowledge, the first attempt to quantify the incidence
of this phenomenon occurred in a retrospective study, which
included 2,544 subjects presenting with sarcoidosis (2). Additional evidence that was obtained
from retrospective clinical studies (3,4) and
genetic linkage-analyses (5,6)
focused on the association of sarcoidosis and malignancies and
indicated a potential etiological correlation between the two
clinical entities. These data have enabled, in certain cases, the
diagnosis of a rare pathological condition defined as
sarcoidosis-lymphoma syndrome (SLS) (1). Typically, SLS refers to patients with
chronic, active sarcoidosis onset at a median age that is greater
than that of the non-oncologic population, who develop a lymphoma
[most frequently Hodgkin’s lymphoma (HL)] after 1–2 years. A small
number of patients with SLS and a clinical history of lymphoma or
leukemia present with late-onset sarcoidosis (7,8,9).
In conventional SLS, the sarcoidotic pathway with
the subsequent chronic inflammatory immune status, may determine a
dysregulation of the cell immunological pattern, permitting the
onset of a lymphomatous/leukemic disorder. By contrast, the
physiopathology of the non-conventional presentation of SLS remains
unclear (10).
This report presents a case of sarcoidosis with
prevalent hepatic and cutaneous localizations, which developed 10
years after the diagnosis of Helicobacter pylori (H.
pylori)-positive gastric mucosa-associated lymphoid tissue
(MALT) lymphoma. The patient was treated with H.
pylori-eradicating therapy only.
Case report
This report presents an 83-year-old male, who in
2001, in the absence of any significant pre-existing pathology and
presenting with gastritis-like symptoms, was diagnosed with H.
pylori-positive gastric MALT lymphoma. The complete disease
staging was negative due to the pathological presence of
lymphadenomegaly in the neck, chest, abdomen and pelvis, as well as
secondary involvment in the liver, spleen and bone marrow. The
patient was treated only with a specific H.
pylori-eradicating therapy, which resulted in a complete
clinical remission with the eradication of the bacteria and
histologically documented normal gastric mucosa. Written informed
consent was obtained from the son of the patient for publication of
this case report.
The patient underwent regular clinical and
instrumental follow-up examinations that revealed no disease
relapse. In December 2010, the patient presented with a recent
onset of asthenia, nausea, dyspepsia and moderate weight loss.
Based on the suspected disease relapse, an endoscopic examination
was performed, which was negative for non-specific lesions and
lymphoid infiltration, and revealed a mild-grade chronic gastritis
pattern. An ultrasound liver examination identified multiple solid
heterogeneous lesions that were confirmed by computed tomography
(CT) scanning, which also revealed multiple abnormal mediastinal
and retroperitoneal lymph nodes. Routine blood tests, including
tumor marker assays, lactate dehydrogenase and β2 microglobulin
were considered to be in the normal range. The circulating
leukocyte pattern showed a non-significant increment in the
CD8+ cell subset, diffuse presence of activated
CD3+/HLA-DR T-cells and rare B-cells. Positron emission
tomography was negative for ipercaptant lesions/lymph nodes and for
suspected lymphomatous localizations. A bone biopsy excluded
lymphoma relapse in the bone marrow. An ultrasound-guided biopsy
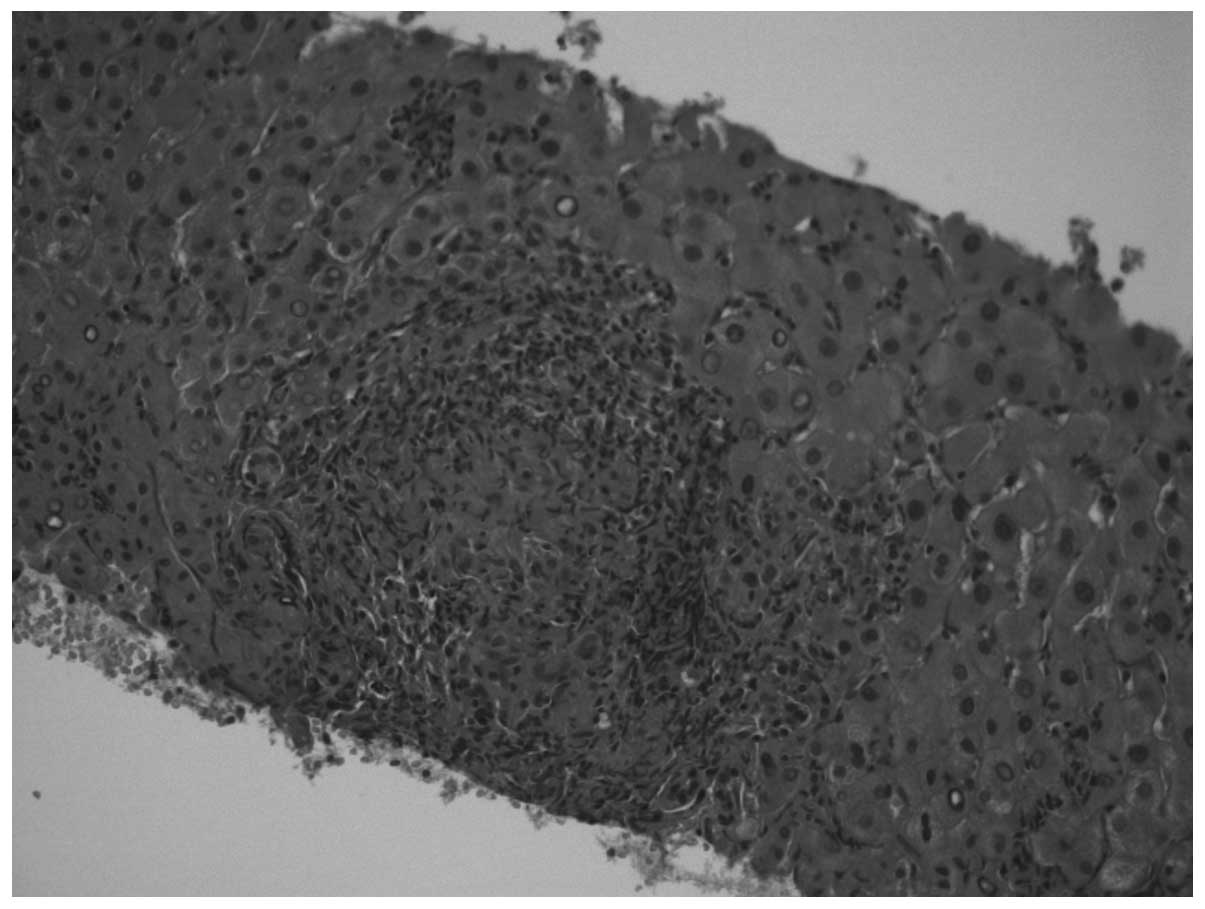
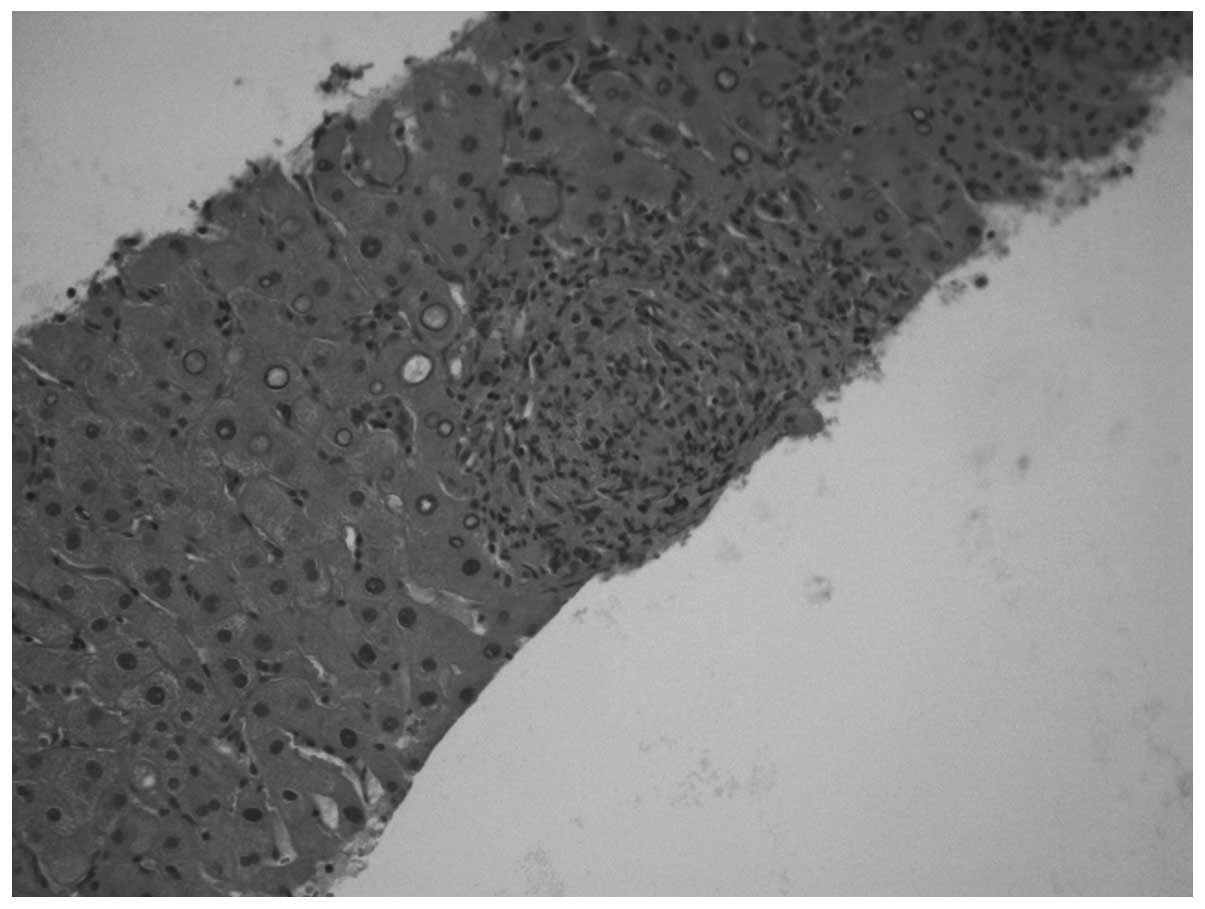
was performed on one of the major hepatic nodules and non-specific
granulomatous epithelial-like hepatitis was diagnosed (Figs. 1 and 2). Staining for acid-fast bacilli and
fungi was performed and excluded these organisms as causative
agents. Autoimmunity tests, the viral hepatitis screening panel,
virus and bacteria-associated infection tests, and the Mantoux test
were all negative.
The clinical condition of the patient as well as the
routine blood analyses remained stable until the end of April 2011.
The patient then presented with a mild-grade fever, and rapid and
significant weight loss associated with nodular lesions (with an
erythema nodosum-like pattern) and a pruritus-causing
cutaneous-rash predominantly localized to the legs. The test
results for bacterial or viral infections were consistently
negative. Hepatic lesions and lymph node characteristics were
stable following the CT examination, however, blood analyses
revealed that the angiotensin-converting enzyme (ACE) serum level
was higher (156 U/l) compared with that of the normal level (8–52
U/l).
The patient was admitted to the Department of
Internal Medicine at the Civic Hospital Vigevano (Pavia, Italy) at
the beginning of June. Blood analyses revealed mild anemia (a
haemogloblin concentration of <9.0 g/dl) with negative fecal
hemoccult test findings, high β2 microglobulin values (11.2 mg/l)
and the ACE serum level was 235 U/ml. The upper gastrointestinal
endoscopy was negative for ulcerative lesions and gastric bleeding,
and only showed a chronic erythematous gastritis pattern. The CT
findings were comparable to the results of CT scans performed
during the previous six months as follows: Liver lesions appeared
stable, lung parenchyma remained negative for disease secondary
localizations and the abnormal lymph nodes were unchanged. The
results of the skin biopsy indicated granulomatous non-caseating
lesions with a psoriasis-like pattern.
Oral corticosteroid therapy (37.5 mg prednisone per
day) was administered and after one week a progressive reduction of
symptoms was noted. Complete resolution of the skin lesions and the
disappearance of the fever were subsequently achieved. The patient
was discharged from hospital, however, continued with the steroid
therapy for two weeks, which was followed by a progressive dosage
reduction. Immediately following the first tapering of prednisone
(25 mg prednisone per day), the fever reappeared with the same
characteristics. All of the blood tests, including the repeated
searches for bacterial or viral agents, were negative and the CT
scan was stable. The results from fresh hepatic and bone marrow
biopsies were negative for lymphomatous lesions and cell
infiltraion. The fever disappeared when the previous steroid dosage
was resumed.
The clinical conditions of the patient remained
stable until the beginning of September 2011, when, despite steroid
therapy, they rapidly worsened with a reappearance of a fever and
the multiple organ dysfunction/failure syndrome, which resulted in
the patient succumbing to acute renal failure in November 2011.
Discussion
Bichel and Brincker (1,11–13)
were the first to examine cases of sarcoidosis that were
co-presenting with malignant tumors identified in the Danish Cancer
and Sarcoidosis Registries (14).
Bichel and Brincker noted a higher incidence of lymphoma in the
sarcoidosis population compared with that in the general
population. Thus, SLS was used to describe a pathological entity
that was characterized by the onset of a lymphoma (most commonly
HL) following a previous diagnosis of sarcoidosis, whose
development was noted ~10 years later, in comparison to a diagnosis
of sarcoidosis that was made in the general population.
Two previous studies failed to confirm the
association between sarcoidosis and lymphoma (15,16).
Furthermore, in an attempt to quantify the association between
non-HLs (NHLs) and various autoimmune and chronic inflammatory
disorders, Mellemkjaer et al (17) conducted an analysis on >25,000
patients with sarcoidosis that were obtained from the Swedish and
Danish Cancer Registries (14). A
notable increase in the risk of NHL was observed in patients with a
previous history of sarcoidosis (odds ratio, 1.9). A previous
linkage analysis supports this association and indicated that ≥25%
of patients with sarcoidosis may develop a malignancy (5,6).
Based on the abovementioned observations, certain
investigators aimed to clarify, from a pathogenetic perspective,
the significant association between sarcoidosis and malignancies,
and proposed an immunopathogenetic model. Noor and Knox (10) observed that sarcoidosis is
invariably accompanied by significant alterations in the immune
system, predominantly hyperstimulation and the increased
mitogenesis of B and T lymphocytes. This may predispose the subject
to the development of lymphoid malignancies.
In a small percentage of SLS cases the diagnosis of
lymphoma was given prior to the onset of sarcoidosis. In these rare
cases, it was indicated that anticancer chemotherapy contributed to
the worsening of the clinical manifestations of the underlying
sarcoidosis (2).
In this context, the patient in the current study
presented certain noteworthy characteristics as follows: i) The
unusual presentation of NHL, which preceded sarcoidosis and was not
treated with chemotherapy; ii) the long interval between the two
diagnoses; iii) the particular type of NHL, a gastric MALT lymphoma
that, to the best of our knowledge, was previously reported in
<10 patients (2,18,19);
and iv) the unusual presentation of sarcoidotic lesions, in
particular, the hepatic and cutaneous manifestations (a
psoriasis-like sarcoidosis pattern). These findings were not
classically pathognomonic of sarcoidosis and complicated the
differential diagnosis between other possible causes of
granulomatous disorders (20,21).
Skin manifestations in sarcoidosis occur in ~20–35% of patients and
are typically present at the onset of sarcoidosis (22).
In conclusion, due to the long interval that elapsed
between the two pathological onsets and the abovementioned unusual
localizations, the differential diagnosis between a lymphoma
relapse and a de novo sarcoidosis was challenging.
Acknowledgements
The authors would like to thank Dr. Vittorio
Perfetti of the Medical Oncology Division IRCCS Foundation
Policlinico San Matteo (Pavia, Italy) for useful suggestions and
careful revision.
References
|
1
|
Brincker H: The
sarcoidosis-lymphoma-syndrome. Br J Cancer. 54:467–473. 1986.
|
|
2
|
Goswami T, Siddique S, Cohen S and Cheson
BD: The sarcoid-lymphoma syndrome. Clin Lymphoma Myeloma Leuk.
10:241–247. 2010.
|
|
3
|
Karakantza M, Matutes E, MacLennan K,
O’Connor NT, Srivastava PC and Catovsky T: Association between
sarcoidosis and lymphoma revisited. J Clin Pathol. 49:208–212.
1996.
|
|
4
|
Cohen PR and Kurzrock R: Sarcoidosis and
malignancy. Clin Dermatol. 25:326–333. 2007.
|
|
5
|
Reich JM, Mullooly JP and Johnson RE:
Linkage analysis of malignancy-associated sarcoidosis. Chest.
107:605–613. 1995.
|
|
6
|
Conde L, Bracci PM, Halperin E and Skibola
CF: A search for overlapping genetic susceptibility loci between
non-Hodgkin lymphoma and autoimmune diseases. Genomics. 98:9–14.
2011.
|
|
7
|
Suen JS, Forse MS, Hyland RH and Chan CK:
The malignancy-sarcoidosis syndrome. Chest. 98:1300–1302. 1990.
|
|
8
|
Merchant TE, Filippa DA and Yahalom J:
Sarcoidosis following chemotherapy for Hodgkin’s disease. Leuk
Lymphoma. 13:339–347. 1994.
|
|
9
|
Haran MZ, Feldberg E and Berrebi A:
Lymphoma masking sarcoidosis. Leuk Lymphoma. 43:1709–1710.
2002.
|
|
10
|
Noor A and Knox KS: Immunopathogenesis of
sarcoidosis. Clin Dermatol. 25:250–258. 2007.
|
|
11
|
Bichel J and Brincker H: Treatment of
pruritus in Hodgkin’s disease and in reticulum cell sarcoma. Scand
J Haematol. 2:85–90. 1965.
|
|
12
|
Brincker H: Sarcoid reactions and
sarcoidosis in Hodgkin’s disease and other malignant lymphomata. Br
J Cancer. 26:120–123. 1972.
|
|
13
|
Brincker H and Wilbek E: The incidence of
malignant tumours in patients with respiratory sarcoidosis. Br J
Cancer. 29:247–251. 1974.
|
|
14
|
Ji J, Shu X, Li X, Sundquist K, Sundquist
J and Hemmiki K: Cancer risk in hospitalized sarcoidosis patients:
a follow-up study in Sweden. Ann Oncol. 20:1121–1126. 2009.
|
|
15
|
Rømer FK, Hommelgaard P and Schou G:
Sarcoidosis and cancer revisited: a long-term follow-up study of
555 Danish sarcoidosis patients. Eur Respir J. 12:906–912.
1998.
|
|
16
|
Ekström Smedby K, Vajdic CM, Falster M,
Engels EA, Martínez-Maza O, Turner J, Hjalgrim H, Vineis P, Seniori
Costantini A, Bracci PM, et al: Autoimmune disorders and risk of
non-Hodgkin lymphoma subtypes: a pooled analysis within the
InterLymph Consortium. Blood. 111:4029–4038. 2008.
|
|
17
|
Mellemkjaier L, Pfeiffer RM, Engels EA,
Gridley G, Wheeler W, Hemminki K, Olsen JH, Dreyer L, Linet MS,
Goldin LR and Landgren O: Autoimmune disease in individuals and
close family members and susceptibility to non-Hodgkin’s lymphoma.
Arthritis Rheum. 58:657–666. 2008.
|
|
18
|
Masuda R, Toyoshima H, Bandou T, Isoyama
T, Matsui Y and Takemura T: Malignant lymphoma of the stomach
associated with systemic sarcoidosis. Cancer. 70:2592–2596.
1992.
|
|
19
|
Fukuda T, Sato K, Tachikawa S, Ohnuki K,
Ohtani H and Suzuki T: Mucosa-associated lymphoid tissue lymphoma
coexisting with epithelioid granulomas in the stomach of a patient
with systemic sarcoidosis. Pathol Int. 47:870–875. 1997.
|
|
20
|
Costabel U, Guzman J and Baughman RP:
Systemic evaluation of a potential cutaneous sarcoidosis patient.
Clin Dermatol. 25:303–311. 2007.
|
|
21
|
Lim EJ, Johnson PD, Crowley P and Gow PJ:
Granulomatous hepatitis: tuberculosis or not? Med J Aust.
188:166–167. 2008.
|
|
22
|
Fernandez-Faith E and McDonnell J:
Cutaneous sarcoidosis: differential diagnosis. Clin Dermatol.
25:276–287. 2007.
|