Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common type of cancer and the third most common cause of
cancer-associated mortality worldwide (1,2). It
has been estimated that 82% of HCC cases occur in developing
countries and 55% occur in China (3). Several causes of HCC have been
identified, including hepatitis virus infection, aflatoxin B1 and
alcoholic liver cirrhosis, among others. One of most significant
risk factors is chronic hepatitis B virus (HBV) infection (4).
Increasing evidence suggests that aberrant
expression of the Wnt signaling pathway is involved in the
development of HCC (5). By binding
to their receptors, termed frizzled (Fzd) proteins, on the cell
membrane (6), the Wnt proteins
exhibit an important function during animal embryogenesis, growth
and development (7,8). Previous studies have shown that the
Wnt/β-catenin pathway is involved in the tumorigenesis of several
cell types; its involvement has been identified in breast,
colorectal, lung and bladder cancer, as well as HCC (9–13).
The secreted Fzd-related proteins (SFRPs) family
comprises of five members (SFRP1-SFRP5), which share 30–50%
sequence similarity with Fzd proteins. These molecules have been
identified as possible negative modulators of the Wnt signaling
pathway, which bind directly to Wnt proteins or block their
receptors (14).
Recently, it has become clear that aberrant
epigenetic modifications of tumor-associated genes are involved in
liver tumorigenesis. Of these candidate genes, the sfrp
family members are increasingly being investigated, with regard to
their roles and mechanisms during HCC development. One of the most
important observations is that the hypermethylation of sfrps
has been frequently reported in several types of cancer (15,16).
Notably, sfrp1, sfrp2 and sfrp5 methylation
has also been frequently detected in HBV-associated chronic
hepatitis and liver cirrhosis (17), which are considered to be
pre-neoplastic lesions. Downregulated SFRP5 expression at the mRNA
and protein levels was also detected in the tissue of other tumor
types (18). However, thus far, few
studies have investigated the serum levels of SFRP5 in patients
with HBV-associated infections and HCC. In the present study,
patient characteristics and serum SPFR5 levels were investigated in
patients with different HBV-associated liver disease statuses.
Materials and methods
Patients
A total of 147 patients with HBV-associated chronic
infection or HCC were enrolled at the Second Affiliated Hospital of
Chongqing Medical University (Chongqing, China) between March 2013
and July 2013. The patients were divided into two groups based on
clinical diagnosis, an HBV group (75 patients with HBV-positive
chronic hepatitis or liver cirrhosis) and an HBV-C group (72
patients with HBV-associated HCC). A comparison group (CT group) of
38 subjects without any history of chronic hepatitis, liver
cirrhosis or HCC was enrolled at the same hospital. The study was
approved by the Ethical Committee of Chongqing Medical University
(Chongqing, China) and written informed consent was obtained from
all subjects.
Clinical characteristics of patients
The clinical patient information included age,
gender, liver cirrhosis status and serum HBV DNA quantitative
analysis results, including serum levels of alanine
aminotransferase (ALT), aspartate aminotransferase (AST), albumin,
total bilirubin (TB), combining bilirubin (CB) and unconjugated
bilirubin (UCB); prothrombin time (PT) and prothrombin activity
(PTA). In addition, serum samples from the CT group were collected
as normal controls for SFRP5 level comparison.
Serum SFRP5 level determination
Blood samples were collected from the subjects
following overnight fasting. Serum samples were obtained by
centrifugation at 4°C and stored at −80°C for future use. Serum
SFRP5 levels were determined using the ELISA method (Human ELISA
kit; Uscn Life Science Inc., Wuhan, China).
Statistical analysis
The data are presented as the mean ± standard
deviation. Data with a skewed distribution were logarithmically
transformed for further statistical analysis. Student’s t-test and
analysis of variance were used for serum SFRP5 level comparison.
Correlational analysis of serum SFRP5 levels with liver disease
status (control, HBV-associated chronic hepatitis, liver cirrhosis
and HCC) was performed using Spearman’s rank correlation. All tests
were two-tailed and P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS, version 17.0 (SPSS, Inc., Chicago, IL,
USA).
Results
Patient characteristics
The clinical characteristics of the patients are
shown in Table I. No significant
differences were identified in albumin (35.13±8.51 g/l vs.
36.43±8.26 g/l), ALT (156.46±183.20 U/l vs. 95.60±220.02 U/l) or
AST (129.04±146.69 U/l vs. 104.15±213.76 U/l) levels between the
HBV and HBV-C groups. The mean age of the HBV-C group (53.31±11.93
years) was higher than that of the HBV group (42.37±15.28 years)
(P<0.001; Table I). In addition,
the mean PT was higher in the HBV group (16.34±3.73 sec) than that
of the HBV-C group (14.95±2.19 sec) (P<0.01; Table I). By contrast, the PTA of the HBV
group (73.51±23.24) was lower when compared with the HBV-C group
(80.89±19.27%) (P<0.05; Table
I). In the HBV group, the levels of TB (75.58±109.28 μmol/l vs.
27.87±28.70 μmol/l), CB (50.15±77.26 μmol/l vs. 14.58±17.77
μmol/l), UCB (22.17±34.89 μmol/l vs. 13.29±12.73 μmol/l) and CB/TB
(0.59±0.16 vs. 0.50±0.09) were significantly increased (P<0.01;
Table I).
 | Table IClinical characteristics of
patients. |
Table I
Clinical characteristics of
patients.
| Parameter | HBV-associated
chronic hepatitis group | HBV-associated HCC
group |
|---|
| Total no. | 75 | 72 |
| Males, n | 58 | 64 |
| Females, n | 17 | 8 |
| Age (years ± SD) | 42.37±15.28 | 53.31±11.93b |
| PT (sec ± SD) | 16.34±3.73 | 14.95±2.19b |
| PTA (% ± SD) | 73.51±23.24 | 80.89±19.27a |
| Albumin (g/l ±
SD) | 35.13±8.51 | 36.43±8.26 |
| ALT (U/l ± SD) | 156.46±183.20 | 95.60±220.02 |
| AST (U/l ± SD) | 129.04±146.69 | 104.15±213.76 |
| TB (μmol/l ± SD) | 75.58±109.28 | 27.87±28.70b |
| CB (μmol/l ± SD) | 50.15±77.26 | 14.58±17.77b |
| UCB (μmol/l ±
SD) | 22.17±34.89 | 13.29±12.73a |
| CB/TB (±SD) | 0.59±0.16 | 0.50±0.09b |
Serum SFRP5 levels decrease in
HBV-associated liver diseases
Serum levels of SFRP5 decreased in the HBV
(10.69±6.44 ng/ml) and HBV-C (8.65±5.33 ng/ml) groups when compared
with the CT group (12.33±4.32 ng/ml)(P<0.05). Notably, the serum
SFRP5 levels were decreased in the HBV-C group when compared with
the HBV group (P<0.05; Fig.
1A).
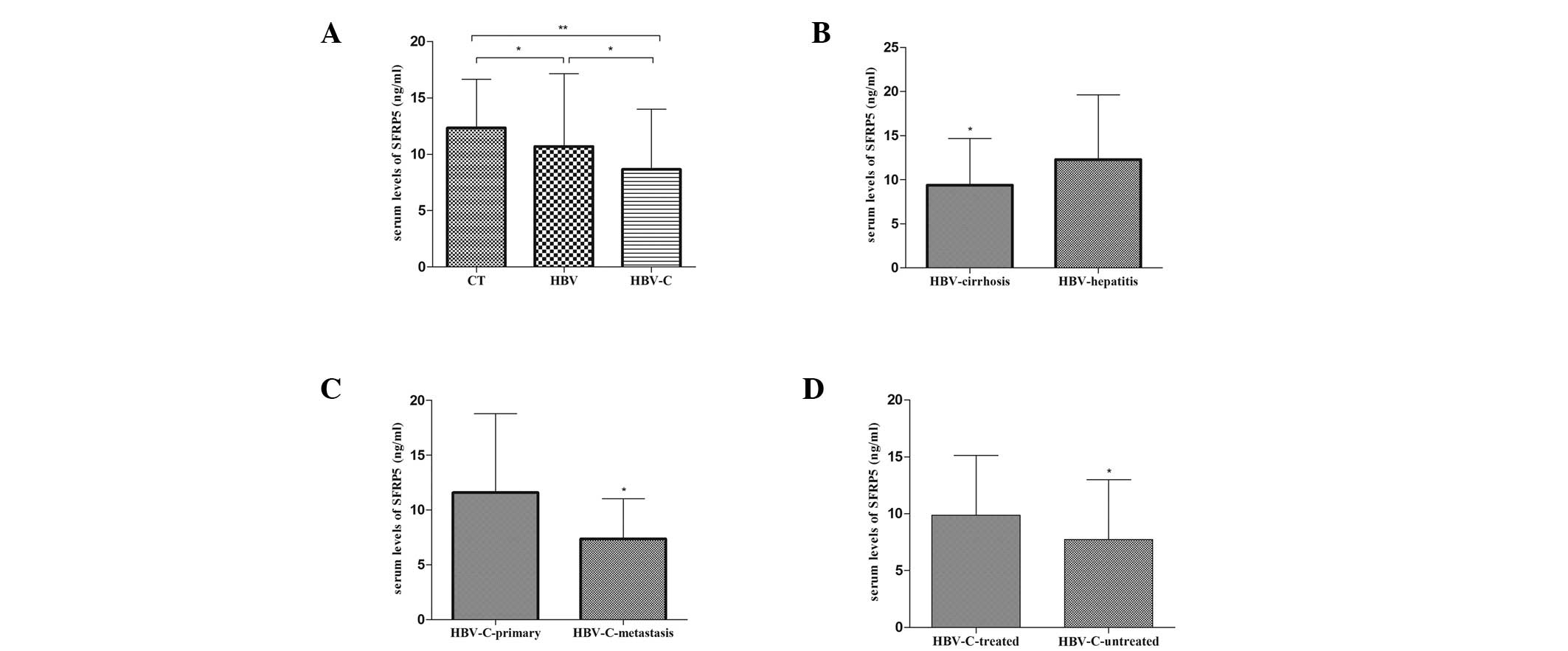 | Figure 1Serum SFRP5 levels in the different
subgroups. (A) Comparison of SFRP5 levels in CT, HBV and HBV-C
groups (**P<0.01, vs. CT group;
*P<0.05, vs. HBV group). (B) Comparison of SFRP5
levels in HBV-cirrhosis and HBV-hepatitis subgroups
(*P<0.05). (C) Comparison of SFRP5 levels in
HBV-C-primary and HBV-C-metastasis subgroups
(*P<0.05). (D) Comparison of SFRP5 levels in
HBV-C-treated and HBV-C-untreated subgroups
(*P<0.05). Error bars represent SD. SFRP5, secreted
frizzled-protein 5; CT, control group; HBV, hepatitis B virus; HCC,
hepatocellular carcinoma; HBV-C, HBV-associated HCC; HBV-cirrhosis,
subgroup of HBV-associated liver cirrhosis; HBV-hepatitis, subgroup
of HBV-associated chronic hepatitis without liver cirrhosis;
HBV-C-primary, subgroup of HCC patients without metastasis;
HBV-C-metastasis, subgroup of HCC patients with metastasis;
HBV-C-treated, subgroup of HCC patients receiving medical
treatment; HBV-C-untreated, subgroup of HCC patients without
medical treatment. |
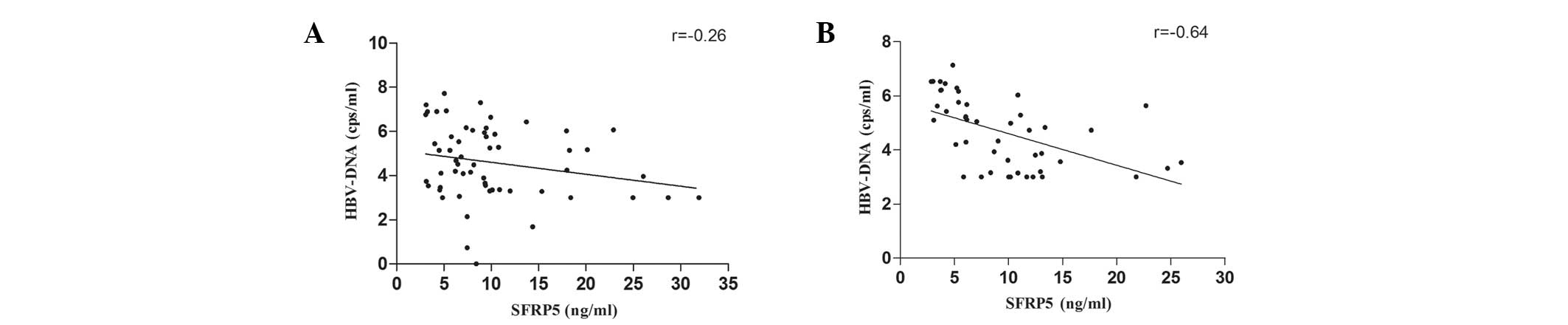
Inverse correlation between serum SFRP5
levels and HBV DNA level
Spearman’s rank correlational analysis identified a
significant inverse correlation between serum SFRP5 levels and HBV
DNA levels in the HBV (r=−0.26; P<0.05) and HBV-C (r=−0.64;
P<0.01) groups (Fig. 2A and B,
respectively).
Differential decrease in serum SFRP5
levels in HBV-associated liver diseases
SFRP5 levels in patients with different liver
disease statuses were investigated. Compared with HBV-associated
chronic hepatitis patients (12.28±7.34 ng/ml), patients with liver
cirrhosis exhibited lower serum SFRP5 levels (9.38±5.31 ng/ml)
(P<0.05; Fig. 1B). In the HBV-C
group, patients with metastasis exhibited significantly lower SFRP5
levels (7.36±3.67 ng/ml) when compared with those without
metastasis (11.58±7.20 ng/ml) (P<0.05; Fig. 1C). Similarly, the SFRP5 levels were
significantly lower in patients prior to receiving medical
treatment (7.72±5.27 and 9.88±5.25 ng/ml, respectively) (P<0.05;
Fig. 1D).
Discussion
HBV infection is a major public health challenge
worldwide. It is estimated that ~30% of the global population
exhibit serological evidence of current or past HBV infection
(19). HBV infection may cause
liver diseases, including acute and chronic hepatitis and liver
cirrhosis. HBV infection is also considered to be a major risk
factor for the development of HCC. With regard to the process of
HBV inducing HCC, numerous observations from previous studies have
revealed that abnormal activation of the Wnt pathway is important
(20,21). The Wnt proteins are a large family
of palmitoylated secreted glycoproteins, which has 19 known members
in humans. These secreted proteins bind to the Fzd proteins and low
density lipoprotein receptor-associated proteins on the cell
membrane (6). Wnt proteins activate
at least three different signaling pathways: The Wnt/β-catenin,
Wnt/non-canonical and Wnt/Ca2+ pathways (22). There are several known types of Wnt
pathway antagonists, including the Cerberus, Wnt inhibitory factor
1, SFRP and Dickopf families. The SFRP family comprises five
glycoproteins, identified as putative inhibitors of the Wnt
signaling pathway. Numerous studies have identified frequent
epigenetic inactivation of sfrp genes in numerous types of
cancer (23,24). However, few studies have
investigated the characteristics of SFRP5 levels in serum. In the
present study, decreased serum SFRP5 levels were observed in HBV
chronic infection and HCC patients when compared with the control
group. These results further support the theory that inactivation
of the sfrp5 gene is frequently found in HBV-associated
liver diseases. Two different mechanisms are involved in the
downregulation of SFRP expression in cancer cells: Allelic loss and
epigenetic silencing. SFRP5, together with SFRP1 and SFRP2, has
dense CpG islands which flank the first exon. The frequent
occurrence of the downregulation of SFRP expression has been
observed in several types of cancer due to promoter
hypermethylation of these genes (25–27).
SFRP1 promoter hypermethylation is a common event occurring in
HBV-induced chronic infections and HCC (28). The results of the present study
revealed that serum SFRP5 levels were found to negatively correlate
with HBV DNA levels. This observation suggested that HBV-associated
infection was possibly responsible for decreased SFRP5 serum
levels. Furthermore, HBV infection-associated oxidative stress and
the HBV encoded X antigen are considered to cause SFRP promoter
hypermethylation (29–31).
Another important observation was that serum SFRP5
levels decreased differentially in HBV chronic infection and
HBV-associated HCC. SFRP5 levels were found to be lower in HCC
patients than in patients with only chronic hepatitis or
HBV-associated liver cirrhosis. These results are consistent with
previous studies, which revealed that methylation was detected to
be weaker in hepatitis and cirrhosis patients when compared with
liver cancer cell lines or in primary HCC (17). Therefore, it is possible that low
levels of sfrp gene promoter methylation or the relatively
low serum SFRP5 levels observed in HBV-associated chronic hepatitis
and liver cirrhosis may serve as biomarker for the risk of
developing HCC.
The results of the current study also indicate that
different serum SFRP5 levels may be exhibited among HCC patients
according to disease status. Firstly, it was found that patients
usually exhibited lower SFRP5 levels prior to receiving any
therapy. This observation suggests that therapeutic measurement,
including tumorectomy, chemotherapy or antiviral therapy, may
contribute to the alleviation of HBV-associated oxidative stress
and increase serum SFRP5 levels. However, the association between
serum SFRP5 levels and anticancer therapy remains unclear and
further investigation is required. Secondly, significantly lower
levels of serum SFRP5 were observed in HCC patients who exhibited
metastasis. The reason for this is not clear; however, it may
involve the expression of other Wnt target genes. Furthermore, Zhao
et al (32) identified an
inverse correlation between SFRP5 expression and matrix
metalloproteinase 7 (MMP-7) and membrane type 1-MMP expression,
which are known as regulators of invasion and metastasis.
Therefore, it is possible that the downregulation of SFRP5 leads to
the activation of Wnt pathways and subsequently increases the
transcription of Wnt target genes, including c-myc,
cyclin D1 and certain MMPs. It would be of interest
to investigate the association between SFRP5 and these members of
the downstream Wnt pathway. This may lead to the development of
novel approaches to prevent HBV-associated HCC, as well as novel
therapeutic strategies.
Recent studies have investigated the role of
sfrp gene methylation in the initiation and progression of
HCC. However, the majority of these studies have not investigated
the characteristics of serum SFRP5 levels. The most significant
result obtained from this study is that serum SFRP5 levels
differentially decreased in HBV-associated chronic infection and
HCC patients. This difference is dependent on the disease
condition. These results suggested that serum SFRP5 levels may
present a possible biomarker for the severity of HBV-associated
chronic infection and the risk of HCC initiation and progression.
However, prior to the use of serum SFRP5 as a reliable biomarker in
clinical practice, large-scale studies are required to clarify the
relevant factors. In addition, exploration of the manner in which
the HBV infection triggers epigenetic modulation and subsequently
regulates the serum SFRP5 level may aid in understanding the
function of SFRP5 in the Wnt signaling pathways.
Acknowledgments
This study was supported in part by the National
Natural Science Foundation of China (grant no. 81270947), the
National Key Basic Research Development Program (grant no.
2012CB517505), the Doctoral Fund of Ministry of Education of China
(grant no. 20115503110008) and a Grant for Natural Science Research
(grant no. cstc2012jjA10015).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108.
2005.
|
|
3
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: a global and regional perspective. Oncologist. 15(Suppl
4): S5–S13. 2010.
|
|
4
|
Zhu AX, Chabner BA and Tanabe KK: New
trends and novel treatment for hepatocellular carcinoma: a global
perspective. Oncologist. 15(Suppl 4): S1–S4. 2010.
|
|
5
|
Bengochea A, de Souza MM, Lefrançois L, et
al: Common dysregulation of Wnt/Frizzled receptor elements in human
hepatocellular carcinoma. Br J Cancer. 99:143–150. 2008.
|
|
6
|
Camilli TC and Weeraratna AT: Striking the
target in Wnt-y conditions: intervening in Wnt signaling during
cancer progression. Biochem Pharmacol. 80:702–711. 2010.
|
|
7
|
Liu H, Fergusson MM, Wu JJ, et al: Wnt
signaling regulates hepatic metabolism. Sci Signal. 4:ra62011.
|
|
8
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009.
|
|
9
|
Uematsu K, He B, You L, Xu Z, McCormick F
and Jablons DM: Activation of the Wnt pathway in non small cell
lung cancer: evidence of dishevelled overexpression. Oncogene.
22:7218–7221. 2003.
|
|
10
|
Segditsas S and Tomlinson I: Colorectal
cancer and genetic alterations in the Wnt pathway. Oncogene.
25:7531–7537. 2006.
|
|
11
|
Urakami S, Shiina H, Enokida H, et al:
Combination analysis of hypermethylated Wnt-antagonist family genes
as a novel epigenetic biomarker panel for bladder cancer detection.
Clin Cancer Res. 12:2109–2116. 2006.
|
|
12
|
Thompson MD and Monga SP: WNT/beta-catenin
signaling in liver health and disease. Hepatology. 45:1298–1305.
2007.
|
|
13
|
Yook JI, Li XY, Ota I, et al: A
Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast
cancer cells. Nat Cell Biol. 8:1398–1406. 2006.
|
|
14
|
Bovolenta P, Esteve P, Ruiz JM, Cisneros E
and Lopez-Rios J: Beyond Wnt inhibition: new functions of secreted
Frizzled-related proteins in development and disease. J Cell Sci.
121:737–746. 2008.
|
|
15
|
Marsit CJ, Karagas MR, Andrew A, et al:
Epigenetic inactivation of SFRP genes and TP53 alteration act
jointly as markers of invasive bladder cancer. Cancer Res.
65:7081–7085. 2005.
|
|
16
|
Nojima M, Suzuki H, Toyota M, et al:
Frequent epigenetic inactivation of SFRP genes and constitutive
activation of Wnt signaling in gastric cancer. Oncogene.
26:4699–4713. 2007.
|
|
17
|
Takagi H, Sasaki S, Suzuki H, et al:
Frequent epigenetic inactivation of SFRP genes in hepatocellular
carcinoma. J Gastroenterol. 43:378–389. 2008.
|
|
18
|
Kawakami K, Yamamura S, Hirata H, et al:
Secreted frizzled-related protein-5 is epigenetically downregulated
and functions as a tumor suppressor in kidney cancer. Int J Cancer.
128:541–550. 2011.
|
|
19
|
Shepard CW, Simard EP, Finelli L, Fiore AE
and Bell BP: Hepatitis B virus infection: epidemiology and
vaccination. Epidemiol Rev. 28:112–125. 2006.
|
|
20
|
Capurro MI, Xiang YY, Lobe C and Filmus J:
Glypican-3 promotes the growth of hepatocellular carcinoma by
stimulating canonical Wnt signaling. Cancer Res. 65:6245–6254.
2005.
|
|
21
|
Lee HC, Kim M and Wands JR: Wnt/Frizzled
signaling in hepatocellular carcinoma. Front Biosci. 11:1901–1915.
2006.
|
|
22
|
Bovolenta P, Rodriguez J and Esteve P:
Frizzled/RYK mediated signalling in axon guidance. Development.
133:4399–4408. 2006.
|
|
23
|
Cheng YY, Yu J, Wong YP, et al: Frequent
epigenetic inactivation of secreted frizzled-related protein 2
(SFRP2) by promoter methylation in human gastric cancer. Br J
Cancer. 97:895–901. 2007.
|
|
24
|
Suzuki H, Gabrielson E, Chen W, et al: A
genomic screen for genes upregulated by demethylation and histone
deacetylase inhibition in human colorectal cancer. Nat Genet.
31:141–149. 2002.
|
|
25
|
Suzuki H, Watkins DN, Jair KW, et al:
Epigenetic inactivation of SFRP genes allows constitutive WNT
signaling in colorectal cancer. Nat Genet. 36:417–422. 2004.
|
|
26
|
Quan H, Zhou F, Nie D, et al: Hepatitis C
virus core protein epigenetically silences SFRP1 and enhances HCC
aggressiveness by inducing epithelial-mesenchymal transition.
Oncogene. 2013.
|
|
27
|
Fukui T, Kondo M, Ito G, et al:
Transcriptional silencing of secreted frizzled related protein 1
(SFRP 1) by promoter hypermethylation in non-small-cell lung
cancer. Oncogene. 24:6323–6327. 2005.
|
|
28
|
Shih YL, Shyu RY, Hsieh CB, et al:
Promoter methylation of the secreted frizzled-related protein 1
gene SFRP1 is frequent in hepatocellular carcinoma. Cancer.
107:579–590. 2006.
|
|
29
|
Park IY, Sohn BH, Yu E, et al: Aberrant
epigenetic modifications in hepatocarcinogenesis induced by
hepatitis B virus X protein. Gastroenterology. 132:1476–1494.
2007.
|
|
30
|
Sasaki Y: Does oxidative stress
participate in the development of hepatocellular carcinoma? J
Gastroenterol. 41:1135–1148. 2006.
|
|
31
|
Tong A, Gou L, Lau QC, et al: Proteomic
profiling identifies aberrant epigenetic modifications induced by
hepatitis B virus X protein. J Proteome Res. 8:1037–1046. 2009.
|
|
32
|
Zhao C, Bu X, Zhang N and Wang W:
Downregulation of SFRP5 expression and its inverse correlation with
those of MMP-7 and MT1-MMP in gastric cancer. BMC Cancer.
9:2242009.
|