Introduction
Non-small cell lung cancer (NSCLC) is a common cause
of cancer-related mortality in China. Although several novel
targeted anticancer agents are available, platinum-based
chemotherapy remains the first-line therapy, achieving better
progression free survival (PFS) rates than non-platinum-based
regimens (1).
Epidermal growth factor receptor (EGFR) tyrosine
kinase inhibitors (TKIs), such as gefitinib and erlotinib, have
been shown to play a significant role in the treatment of untreated
advanced NSCLC, particularly in NSCLC patients with EGFR mutations.
Two phase III studies (NEJ002 and WJTOG3405) showed an improved PFS
rate in NSCLC patients harboring sensitizing EGFR mutations
(2,3). Therefore, gefitinib and erlotinib can
be used as the first-line treatment of patients with advanced or
metastatic NSCLC with activating EGFR mutations.
The present study describes the case of a patient
with an EGFR mutation in NSCLC treated with gefitinib, achieving a
marked efficacy. Patient provided written informed consent.
Case report
A 58-year-old male, with no significant medical
history developed a dry cough in November 2011. The patient had
previously smoked 10 cigarettes per day for 30 years, but stopped
smoking in January 2012. A chest computed tomography (CT) scan
revealed a mass in the left inferior lobe, resulting in the patient
being admitted to The Fourth Affiliated Hospital of Soochow
University (Wuxi, Jiangsu, China). A brain CT scan showed no
evidence of any distant metastasis. A bone emission CT (ECT) scan
showed multiple bone metastases. Positron emission tomography-CT
scan showed a large soft-tissue mass in the left inferior lobe of
the lung, and multiple masses in the right lung, right adrenal
glands and bones. Fiber bronchoscopy found cancer cells in the
section analyzed, and histopathology revealed an adenocarcinoma.
The patient was diagnosed with adenocarcinoma by a CT-guided
percutaneous core needle biopsy. The clinical stage was stage IV.
EGFR mutations were detected using the peptide nucleic acid-locked
nucleic acid polymerase chain reaction clamp method. An EGFR
mutation was found with deletions in E746-A750 of exon 19.
Due to a metastasis in the eleventh and twelfth
thoracic vertebrae that caused spinal cord compression, the patient
initially received 30 Gy radiation of 3 Gy per fraction. Following
this, 1.6 g gemcitabine (GEM 1.0 g/m2) was administered
on days one and eight, and 30 mg cisplatin (DDP) was administered
on days one to four. Six cycles were administered every three weeks
(February-July, 2012). No adverse events (AEs) were reported.
A chest CT scan carried out in August 2012 showed
residual disease in the left inferior lobe of the lung (2 cm in
diameter), and metastatic lesions of the right lung, the right
adrenal glands and bone were stable. Chemotherapy was subsequently
continued. The patient received four cycles of chemotherapy
consisting of 1.6 g GEM on days one an eight, and 30 mg DDP on days
one to four. The last chemotherapy treatment was on November 24,
2012. No AEs were reported. A CT scan of the thorax was assessed as
stable. In May 2013, another ECT scan revealed one new lesion in
the right femur, indicating progression of the disease. The patient
received another 30 Gy radiation, 3 Gy/FX.
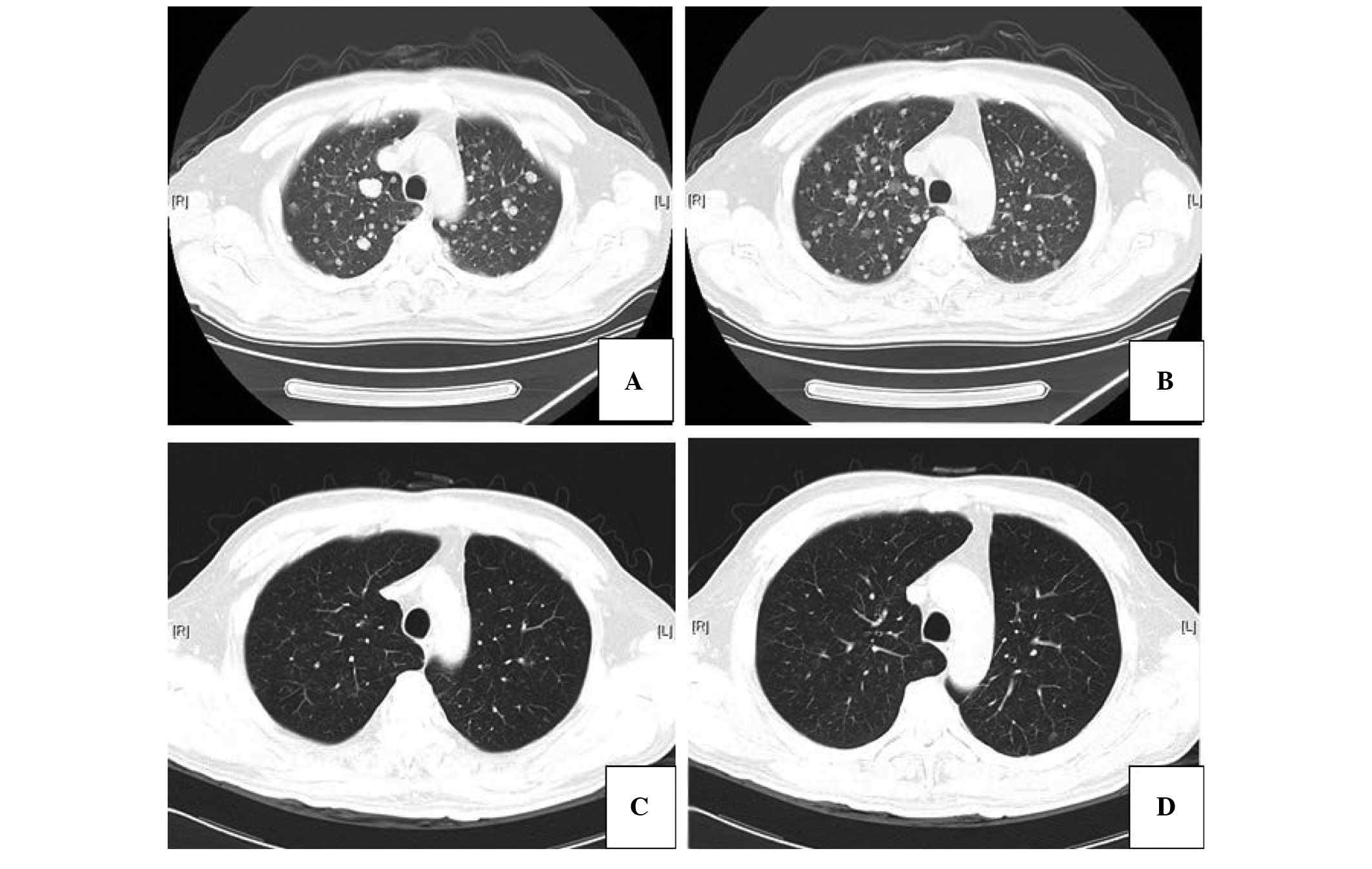
Another Chest CT scan showed widespread metastases
in the right and left lung (Fig.
1). The patient was consequently administered 250 mg oral
gefitinib once daily in June 2013. A grade 1 acne-like rash
developed on the face and back, which was treated with 4.5 g
piperacillin-tazobactam twice daily for 5 days. The rash lasted the
clinical course of the treatment. Chest CT scans showed that the
metastatic tumors were improved following gefitinib treatment
(Fig. 1). To date, the disease
remains stable and the patient continues to receive gefitinib
orally.
Discussion
The current study presents the case of an NSCLC
patient with an EGFR mutation treated with gefitinib. For an
unknown EGFR status, platinum-based chemotherapy remains in use as
the first-line management of NSCLC (4,5).
However, gefitinib is the first targeted agent to be approved for
the treatment of EGFR mutation-positive lung adenocarcinoma, which
has showed evident clinical efficacy, particularly among
non-smokers, East Asian females and patients with adenocarcinoma
(6,7). EGFR mutation analyses have
demonstrated that patients with activating EGFR gene mutations
obtained more benefit than those of non-EGFR gene mutations
(8). A recent meta-analysis also
showed that gefitinib yielded a statistically significant benefit
in progression-free survival compared with gefitinib-free therapy
(9).
Drug-related side-effects are significantly more
common in patients receiving gefitinib therapy (9). EGFR-TKI therapy causes the development
of a rash in numerous patients (10). In the patient of the present study,
a grade 1 acne-like rash developed on the face and back following
the start of gefitinib treatment. The rash persisted throughout the
treatment period. Rash development has been associated with
EGFR-TKI efficacy in NSCLC, and a meta-analysis previously showed
that a skin rash was an independent predictive factor for
progression and survival in EGFR TKI-treated NSCLC patients
(10). Other adverse events, such
as diarrhea, dry skin, pruritus and paronychia, have also been
notable in the current literature. Therefore, the status of the
patient should be considered prior to gefitinib treatment to ensure
that the best therapy is being selected.
EGFR-TKI therapy may prolong life expectancy.
Despite the fact that the treatment time is short, we believe that
if used long-term, maintenance therapy would benefit those patients
with EGFR mutations. Furthermore, a retrospective study analyzed
301 long-term NSCLC survivors to confirm which patient may benefit
most from erlotinib treatment (11). The long-term gefitinib benefits were
not only relative to good prognostic factors (e.g., good
performance status, never-smoker status and the female gender), but
also included negative factors. A study of 124 advanced NSCLC
patients treated with chemotherapy [64 (51.6%) patients treated
with gefitinib] as the initial therapy revealed that 8% of the
patients survived for >5 years (12). In addition, another study reported
that three refractory NSCLC patients survived for >3 years with
gefitinib treatment (13). It was
concluded that patients with EGFR mutations gained the greater
benefit, particularly those who were older or had a poor
performance status, from first-line EGFR-TKI therapy (12).
In conclusion, this study suggests that gefitinib
therapy has a significant role in advanced lung adenocarcinoma. A
limitation of the current study was the small sample size. Future
studies with larger sample size are required to investigate EGFR
mutations.
References
|
1
|
Yu Y, Xu X, Du Z and Shi M: Non-platinum
regimens of gemcitabine plus docetaxel versus platinum-based
regimens in first-line treatment of advanced non-small cell lung
cancer: a meta-analysis on 9 randomized controlled trials. Cancer
Chemother Pharmacol. 69:1265–1275. 2012.
|
|
2
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, et al; West Japan Oncology Group. Gefitinib
versus cisplatin plus docetaxel in patients with non-small-cell
lung cancer harbouring mutations of the epidermal growth factor
receptor (WJTOG3405): an open label, randomised phase 3 trial.
Lancet Oncol. 11:121–128. 2010.
|
|
3
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, et al; North-East Japan Study Group.
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Eng J Med. 362:2380–2388. 2010.
|
|
4
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, et al: Gefitinib or carboplatin-paclitaxel in
pulmonary adenocarcinoma. N Eng J Med. 361:947–957. 2009.
|
|
5
|
Gridelli C, Butts C, Ciardiello F, Feld R,
Gallo C and Perrone F: An international, multicenter, randomized
phase III study of first-line erlotinib followed by second-line
cisplatin/gemcitabine versus first-line cisplatin/gemcitabine
followed by second-line erlotinib in advanced non-small-cell lung
cancer: treatment rationale and protocol dynamics of the TORCH
trial. Clin Lung Cancer. 9:235–238. 2008.
|
|
6
|
Thatcher N, Chang A, Parikh P, Rodrigues
Pereira J, Ciuleanu T, von Pawel J, et al: Gefitinib plus best
supportive care in previously treated patients with refractory
advanced non-small-cell lung cancer: results from a randomised,
placebo-controlled, multicentre study (Iressa Survival Evaluation
in Lung Cancer). Lancet. 366:1527–1537. 2005.
|
|
7
|
Maruyama R, Nishiwaki Y, Tamura T,
Yamamoto N, Tsuboi M, Nakagawa K, et al: Phase III study, V-15-32,
of gefitinib versus docetaxel in previously treated Japanese
patients with non-small-cell lung cancer. J Clin Oncol.
26:4244–4252. 2008.
|
|
8
|
Ebi N, Semba H, Tokunaga SJ, Takayama K,
Wataya H, Kuraki T, et al; Lung Oncology Group in Kyushu, Japan. A
phase II trial of gefitinib monotherapy in chemotherapy naïve
patients of 75 years or older with advanced non-small cell lung
cancer. J Thorac Oncol. 3:1166–1171. 2008.
|
|
9
|
Zhou H, Zeng C, Wang LY, Xie H, Zhou J,
Diao P, et al: Chemotherapy with or without gefitinib in patients
with advanced non-small-cell lung cancer: a meta-analysis of 6,844
patients. Chin Med J (Engl). 126:3348–3355. 2013.
|
|
10
|
Petrelli F, Borgonovo K, Cabiddu M, Lonati
V and Barni S: Relationship between skin rash and outcome in
non-small-cell lung cancer patients treated with anti-EGFR tyrosine
kinase inhibitors: a literature-based meta-analysis of 24 trials.
Lung Cancer. 78:8–15. 2012.
|
|
11
|
Schumann C, Heigener D, Dittrich I, et al:
Long term benefit from erlotinib treatment is independent of
prognostic factors and therapeutic response (abstract 9146). Eur J
Cancer. 7:12009.
|
|
12
|
Kaira K, Takahashi T, Murakami H, Tsuya A,
Nakamura Y, Naito T, et al: Long-term survivors of more than 5
years in advanced non-small cell lung cancer. Lung Cancer.
67:120–123. 2010.
|
|
13
|
Nakatomi K, Soda H, Kitazaki T, Nakano H,
Uchida K, Urabe S, et al: Long-term survival in three patients with
metastatic non-small cell lung cancer treated with gefitinib. Lung
Cancer. 52:253–255. 2006.
|