Introduction
Human PPPDE peptidase domain-containing protein 1
(PPPDE1), also known as PNAS-4, is a recently identified protein
through large-scale genome sequencing (1). PPPDE1 consists of 194 amino acids and
contains a highly conserved DUF862 peptidase domain that is found
in plants and animals, suggesting it has fundamental roles in
biological evolution (2).
Bioinformatics analysis has shown that PPPDE1 is a potential factor
in an ubiquitin-related signaling system (3). Our previous study showed that
overexpression of PPPDE1 could induce apoptosis of human lung
adenocarcinoma A549 cells (4). In
addition, mRNA microinjection of PPPDE1 in zebrafish and Xenopus
embryos resulted in developmental defects, indicating essential
roles of PPPDE1 in embryonic development (5,6).
Previously, we demonstrated that PPPDE1 was mainly
located at the Golgi apparatus in the cytoplasm and presented a
wide distribution in the majority of tissues, including brain,
lung, kidney, liver, colon, prostate, cervix, ovary, breast and
muscle tissue (7). We further found
that PPPDE1 exhibited decreased expression of varying degrees in
certain tumors, such as pancreatic and skin cancer, which suggested
an involvement of PPPDE1 in these types of cancer (7). Pancreatic cancer is a common carcinoma
with the highest mortality rate, and it is necessary to reveal its
molecular mechanisms in carcinogenesis (8). Numerous studies have shown that
WNT/β-catenin signaling is important in the progression of
pancreatic cancer (9,10). In this process, translocation of
β-catenin into the nucleus is regarded as an essential prerequisite
for the transcription of a series of oncogenes including c-myc,
cyclin D1 and MMP-7 (11,12). As a member of the same family as
β-catenin, plakoglobin also possesses the ability to regulate
cancer progression, although its roles remain controversial
(13,14).
In our primary investigations, we found that PPPDE1
could affect the stability of desmosomes. Therefore, we aimed to
identify the distribution of plakoglobin and β-catenin, the two
important components of desmosomes, under the conditions of various
PPPDE1 expression levels in pancreatic ductal adenocarcinoma. By
means of analysis based on a tissue library containing 127 samples,
the present study aimed to enhance the understanding of the roles
of PPPDE1 and its implications in progression of pancreatic
cancer.
Materials and methods
Antibodies
The rabbit polyclonal anti-human antibody against
PPPDE1 was purchased from Proteintech (20517-1-AP; Chicago, IL,
USA), while polyclonal rabbit anti-human antibodies against
plakoglobin (sc-7900) and β-catenin (sc-7199) were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
polyclonal goat anti-rabbit secondary antibody conjugated with
horseradish peroxidase immunoglobulin G (sc-2030) was also obtained
from Santa Cruz Biotechnology, Inc. These antibodies were used
according to their respective manufacturer’s instructions.
Immunohistochemistry
The clinical specimens, including pancreatic ductal
adenocarcinoma and paired normal tissues, were obtained from 96
pancreatic ductal adenocarcinoma patients (age range, 31–74 years)
at the West China Hospital of Sichuan University (Chengdu, China).
The study was approved by the institutional ethics committee of
Sichuan University (Chengdu, China). All patient provided written
informed consent to participate in the study. The sections were
stained using an avidin-biotin-peroxidase complex (ABC) method and
visualized with diaminobenzidine (DAB; Beyotime, Haimen, China),
according to the manufacturer’s instructions. Briefly, the sections
were deparaffinized in xylene and rehydrated through graded alcohol
rinses. Antigen unmasking was performed by immersing slides into
boiling TE-EDTA (pH 9.0) buffer and then maintaining them at
boiling temperature for 15 min. After cooling, the sections were
incubated in 3% H2O2 for 10 min to quench
endogenous peroxidase activity. Subsequently, the sections were
blocked in 5% normal goat serum for 1 h at room temperature, and
incubated with primary antibodies for 3 h at room temperature.
Subsequently, the sections were washed with PBS and incubated with
biotinylated secondary antibodies for 30 min at room temperature.
Finally, the sections were treated with ABC reagents and developed
with DAB staining.
Image analysis
Sections were counterstained with hematoxylin and
imaged by using a high resolution digital microscope (Leica DM2500;
Leica Microsystems GmbH, Wetzlar, Germany). The staining of
sections was calculated by Leica Application Suit software (Leica
Microsystems GmbH). Briefly, the values of staining intensity were
measured and subjected to calibration. The staining was divided
into four scales according to the size of intensity values (0–5%,
negative; 6–30%, mild; 31–70%, moderate; and 71–100%, strong). The
percentage of positively stained cells represents the ratio of
stained cancer cells/total cancer cells. For section analysis,
total staining was scored as the product of the staining intensity
score (negative, 0; mild, 1; moderate, 2; and strong, 3), and the
score for the percentage of positively stained cells were recorded
according to an ordered categorical scale (0–5%, 0; 6–30%, 1;
31–70%, 2; and 71–100%, 3), resulting in a scale of 0–9 (15). Sections were assessed separately by
two experienced pathologists. The discrepancy between the two
pathologists was resolved by careful evaluation and discussion
until consensus was reached.
Results
PPPDE1 expression analysis
A total of 96 pancreatic ductal carcinoma tissue
samples and 31 normal pancreatic tissue samples were collected and
examined to assess the expression levels of PPPDE1 by means of
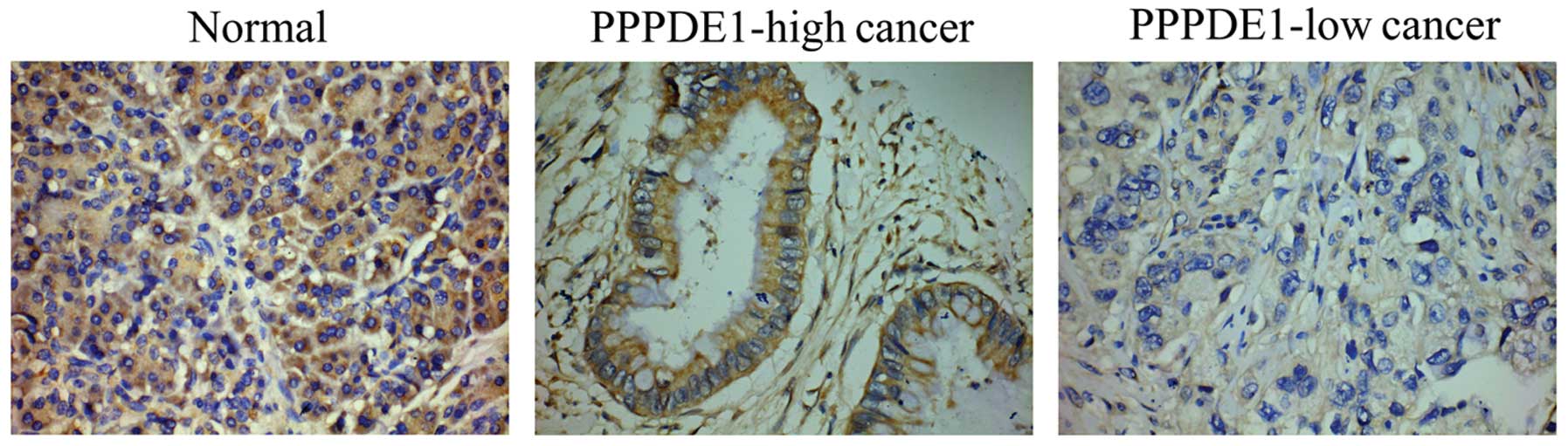
immunohistochemistry. Generally, strong PPPDE1 staining was evident
in the normal tissues, whereas weak PPPDE1 staining was observed in
the cancer tissues (Fig. 1). PPPDE1
was mainly located in the cytoplasm of the normal and cancer
tissues, with almost no staining in the plasma membrane and the
nucleus (Fig. 1). Notably, low
PPPDE1 staining score was more evident in the poorly differentiated
cancer tissues (Table I). These
results confirmed that PPPDE1 presented decreased expression in
pancreatic ductal adenocarcinoma, showing the lowest expression in
poorly differentiated cancer tissues.
 | Table ISubcellular distributions of
plakoglobin and β-catenin under different PPPDE1 expression
levels. |
Table I
Subcellular distributions of
plakoglobin and β-catenin under different PPPDE1 expression
levels.
| Pancreatic ductal
adenocarcinoma |
|---|
|
|
|---|
| Low PPPDE1 | High PPPDE1 |
|---|
| Staining score | ≤3 | >3 |
| Sample (n) | 44 | 52 |
| Poor
differentiation | 73% (32/44) | 23% (12/52) |
| Membrane plakoglobin
(<30%) | 57% (25/44) | 27% (14/52) |
| Nucleus β-catenin
(>30%) | 64% (28/44) | 19% (10/52) |
Plakoglobin distribution in association
with PPPDE1 expression
The tissue samples that were analyzed to assess
PPPDE1 expression were also subjected to examination of plakoglobin
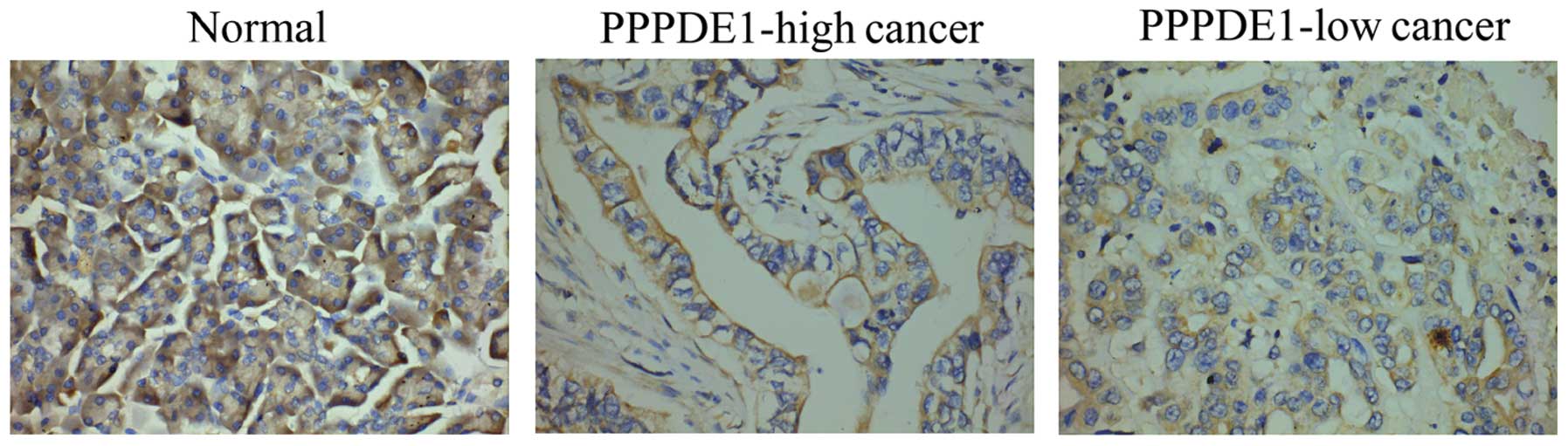
expression, by means of immunohistochemistry. Generally,
plakoglobin expression was decreased in the cancer tissues compared
with that in the normal tissues (Fig.
2). The cellular location of plakoglobin distribution was then
analyzed, revealing that plakoglobin was mainly distributed in the
membrane and cytoplasm border of normal cells, but was less evident
in the membrane of cancer cells (Fig.
2).
In order to analyze the implications of PPPDE1
expression in pancreatic ductal carcinoma, the cancer tissues were
further classified into two groups, those with high (staining
score, >3) levels of PPPDE1 expression (PPPDE1-high cancer) and
those with low (staining score, ≤3) PPPDE1 expression levels
(PPPDE1-low cancer) (Table I). A
greater percentage of cells exhibited low membrane plakoglobin
expression (<30%) in PPPDE1-low cancer compared with that in
PPPDE1-high cancer (Table I and
Fig. 2). However, cytoplasmic
staining of plakoglobin remained evident in PPPDE1-low cancer,
although its total abundance was markedly decreased compared with
that of normal cells (Fig. 2).
These results demonstrated that the membrane expression of
plakoglobin shows an identical change to PPPDE1 levels in
pancreatic ductal carcinoma.
β-catenin distribution in association
with PPPDE1 expression
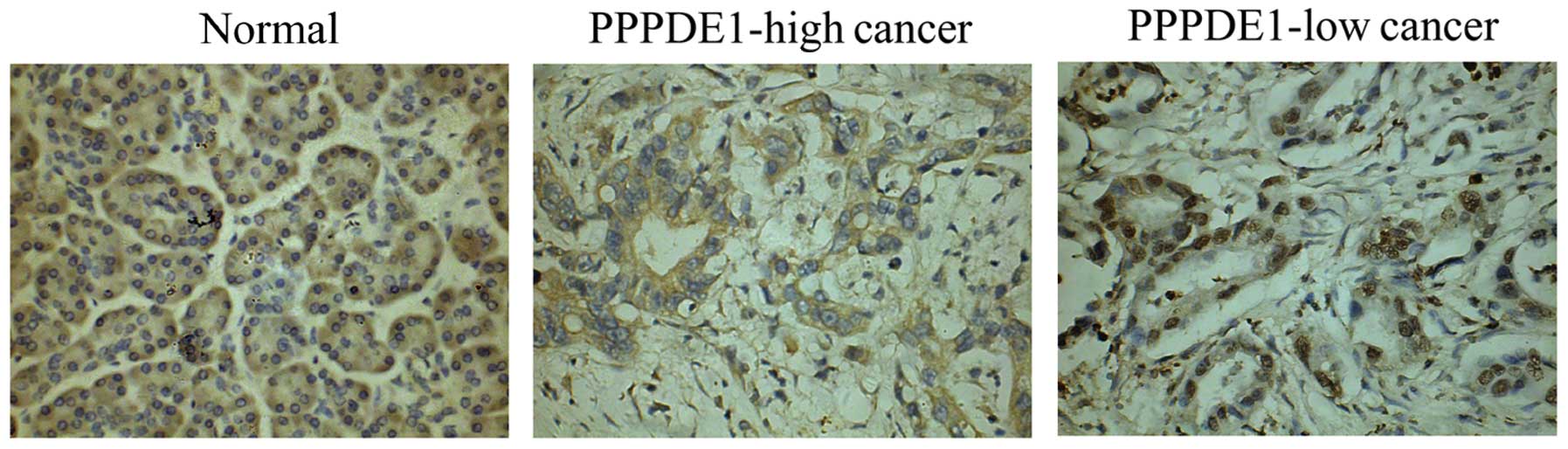
The distribution of β-catenin in association with
PPPDE1 expression in pancreatic ductal carcinoma was also
investigated. Immunohistochemical analysis revealed that β-catenin
was mainly distributed along the cytoplasm border in normal cells.
However, β-catenin staining was most frequently observed in the
cytoplasm in cancer cells. In certain representative cancer samples
(Table I), β-catenin expression was
identified in nucleus (Fig. 3).
High nuclear β-catenin expression (>30%) was more frequently
observed in PPPDE1-low cancer compared with that of PPPDE1-high
cancer (Table I and Fig. 3). These results demonstrated
β-catenin translocation into the nucleus was more likely to occur
under the conditions of low PPPDE1 expression in pancreatic ductal
carcinoma.
Discussion
As a recently identified protein, the exact
functions of PPPDE1 remain unclear. Our previous study found that
PPPDE1 was located at the Golgi apparatus of HeLa cells, suggesting
that it may be involved in post-translational modification in the
process of protein synthesis (7).
Amino acid analysis has demonstrated that PPPDE1 possesses high
sequence homology to DESI-1, a deSUMOylase involving in protein
modification (16). As a reverse
process of SUMO, deSUMOylation mainly participates in protein
stability, translocation and expression (16,17).
The substrate of DESI-1 has been identified to be BZEL, but the
targets of PPPDE1 remain unknown. We previously identified that
PPPDE1 upregulation could lead to the weak stability of vimentin,
suggesting that PPPDE1 may be involved in cytoskeleton metabolism
(18). Plakoglobin and β-catenin
are both important cytoskeleton-related proteins, and have been
demonstrated to play important roles in cancer progression
(19). In the present study,
PPPDE1-low pancreatic cancer was observed to present characteristic
distribution of the two cytoskeleton proteins, loss of membrane
plakoglobin and translocation of β-catenin into nucleus. These
findings were consistent with the high malignancy of poorly
differentiated pancreatic cancer, in which PPPDE1 expression was
greatly decreased. Therefore, our data provided insights into the
role of PPPDE1 in the progression of pancreatic cancer.
Plakoglobin is a component of adherens junctions and
desmosomes, and plays a pivotal role in the regulation of cell-cell
adhesion. Previous studies have identified conflicting results
regarding the function of plakoglobin in cancer progression
(13,14). Numerous studies have demonstrated
that plakoglobin is able to regulate the invasive properties of
cancer cells (20–22). Several types of cancer, such as
breast, prostate, lung, bladder, skin, thyroid, oral and pharyngeal
cancer, have been found to exhibit decreased plakoglobin expression
and increased the likelihood of metastasis and/or a poor prognosis
(19–21).
By contrast, certain studies have demonstrated that
plakoglobin exerts oncogenic activity through the triggering
Tcf/Lef transcription signal, presenting similar activity to that
of β-catenin (13,14,23).
Notably, plakoglobin is primarily localized at the plasma membrane,
with some perinuclear distribution in the cytoplasm (14). These contradictions regarding the
roles of plakoglobin may be due to its differential subcellular
localization. In cancer cells, the desmosomes are often destructed,
resulting in the translocation of membrane plakoglobin into the
cytoplasm (19). The decreased
membrane plakoglobin can result in the loss of cell-cell contact
and promote cell migration; however, the cytoplasm plakoglobin can
liberate β-catenin from the constrained state in the cytoplasm and
lead to translocation of excess β-catenin into the nucleus.
Therefore, plakoglobin displays oncogenic activity through its
cytoplasm accumulation to regulate the β-catenin signal, although
membrane plakoglobin has been identified to be greatly decreased in
cancer cells (13).
Although β-catenin is a cytoskeleton-related
protein, it has been a focus of research mainly due to the
WNT/β-catenin pathway, an important signaling pathway involved in
development and carcinogenesis (11,24).
Previous studies regarding the regulation of β-catenin
translocation into the nucleus have revealed interactions between
plakoglobin and β-catenin (25,26).
Due to the high sequence homology between plakoglobin and
β-catenin, both proteins can bind proteins such as E-cadherin, Axin
and APC (13,14,25,26).
This competitive binding causes the liberation of β-catenin from
the Axin/APC complex and leads to β-catenin accumulation in
cytoplasm. Consequently, the excess cytoplasmic β-catenin is able
to translocate into the nucleus and subsequently interact with the
Tcf/Lef transcription factors, which triggers the expression of a
series of oncogenes, such as c-myc, cyclin D1 and MMP-7. In the
present study, it was identified that the cellular distribution of
plakoglobin and β-catenin may be regulated by PPPDE1 expression in
pancreatic ductal carcinoma. Although the mechanisms whereby PPPDE1
expression is decreased in pancreatic ductal carcinoma remain
unclear, the intracellular distributions of plakoglobin and
β-catenin in association with PPPDE1 expression suggested there may
be certain connections among these proteins.
In summary, the findings of the present study
suggested that there may be associations between PPPDE1 expression
and the distribution of plakoglobin and β-catenin expression in
pancreatic ductal adenocarcinoma. The characteristic cellular
distributions of plakoglobin and β-catenin in pancreatic cancer
have provided insights into the role of PPPDE1 in pancreatic cancer
progression. The molecular mechanisms underlying the characteristic
distributions in association with PPPDE1 expression require further
investigation on the interacting proteins of PPPDE1 with regard to
their interactions in the future.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81101530).
References
|
1
|
Gregory SG, Barlow KF, McLay KE, et al:
The DNA sequence and biological annotation of human chromosome 1.
Nature. 441:315–321. 2006.
|
|
2
|
Lai CH, Chou CY, Ch’ang LY, Liu CS and Lin
W: Identification of novel human genes evolutionarily conserved in
Caenorhabditis elegans by comparative proteomics. Genome
Res. 10:703–713. 2000.
|
|
3
|
Iyer LM, Koonin EV and Aravind L: Novel
predicted peptidases with a potential role in the ubiquitin
signaling pathway. Cell Cycle. 3:1440–1450. 2004.
|
|
4
|
Yan F, Gou L, Yang J, et al: A novel
pro-apoptosis gene PNAS4 that induces apoptosis in A549 human lung
adenocarcinoma cells and inhibits tumor growth in mice. Biochimie.
91:502–507. 2009.
|
|
5
|
Yao S, Xie L, Qian M, et al: Pnas4 is a
novel regulator for convergence and extension during vertebrate
gastrulation. FEBS Lett. 582:2325–2332. 2008.
|
|
6
|
Yan F, Ruan XZ, Yang HS, et al:
Identification, characterization, and effects of Xenopus
laevis PNAS-4 gene on embryonic development. J Biomed
Biotechnol. 2010:1347642010.
|
|
7
|
He Y, Wang J, Gou L, et al: Comprehensive
analysis of expression profile reveals the ubiquitous distribution
of PPPDE peptidase domain 1, a Golgi apparatus component, and its
implications in clinical cancer. Biochimie. 95:1466–1475. 2013.
|
|
8
|
Zhu M, Xu Z, Wang K, Wang N and Li Y:
microRNA and gene networks in human pancreatic cancer. Oncol Lett.
6:1133–1139. 2013.
|
|
9
|
Morris JP IV, Cano DA, Sekine S, Wang SC
and Hebrok M: Beta-catenin blocks Kras-dependent reprogramming of
acini into pancreatic cancer precursor lesions in mice. J Clin
Invest. 120:508–520. 2010.
|
|
10
|
Kobayashi T, Shimura T, Yajima T, et al:
Transient gene silencing of galectin-3 suppresses pancreatic cancer
cell migration and invasion through degradation of β-catenin. Int J
Cancer. 129:2775–2786. 2011.
|
|
11
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012.
|
|
12
|
Noubissi FK, Elcheva I, Bhatia N, et al:
CRD-BP mediates stabilization of betaTrCP1 and c-myc mRNA in
response to beta-catenin signalling. Nature. 441:898–901. 2006.
|
|
13
|
Aktary Z and Pasdar M: Plakoglobin: role
in tumorigenesis and metastasis. Int J Cell Biol.
2012:1895212012.
|
|
14
|
Zhurinsky J, Shtutman M and Ben-Ze’ev A:
Plakoglobin and beta-catenin: protein interactions, regulation and
biological roles. J Cell Sci. 113(Pt 18): 3127–3139. 2000.
|
|
15
|
Gou L, Wang W, Tong A, et al: Proteomic
identification of RhoA as a potential biomarker for proliferation
and metastasis in hepatocellular carcinoma. J Mol Med (Berl).
89:817–827. 2011.
|
|
16
|
Shin EJ, Shin HM, Nam E, et al:
DeSUMOylating isopeptidase: a second class of SUMO protease. EMBO
Rep. 13:339–346. 2012.
|
|
17
|
Wang Y and Dasso M: SUMOylation and
deSUMOylation at a glance. J Cell Sci. 122(Pt 23): 4249–4252.
2009.
|
|
18
|
Gou LT, Tong AP, Yan F, et al: Altered
protein-expressing profile in hPNAS4-induced apoptosis in A549
human lung adenocarcinoma cells. J Cell Biochem. 108:1211–1219.
2009.
|
|
19
|
Dusek RL and Attardi LD: Desmosomes: new
perpetrators in tumour suppression. Nat Rev Cancer. 11:317–323.
2011.
|
|
20
|
Wei Q, Hariharan V and Huang H: Cell-cell
contact preserves cell viability via plakoglobin. PLoS One.
6:e270642011.
|
|
21
|
Bailey CK, Mittal MK, Misra S and
Chaudhuri G: High motility of triple-negative breast cancer cells
is due to repression of plakoglobin gene by metastasis modulator
protein SLUG. J Biol Chem. 287:19472–19486. 2012.
|
|
22
|
Yin T, Getsios S, Caldelari R, et al:
Mechanisms of plakoglobin-dependent adhesion: desmosome-specific
functions in assembly and regulation by epidermal growth factor
receptor. J Biol Chem. 280:40355–40363. 2005.
|
|
23
|
Maeda O, Usami N, Kondo M, et al:
Plakoglobin (gamma-catenin) has TCF/LEF family-dependent
transcriptional activity in beta-catenin-deficient cell line.
Oncogene. 23:964–972. 2004.
|
|
24
|
Gavert N and Ben-Ze’ev A: beta-Catenin
signaling in biological control and cancer. J Cell Biochem.
102:820–828. 2007.
|
|
25
|
Choi HJ, Gross JC, Pokutta S and Weis WI:
Interactions of plakoglobin and beta-catenin with desmosomal
cadherins: basis of selective exclusion of alpha- and beta-catenin
from desmosomes. J Biol Chem. 284:31776–31788. 2009.
|
|
26
|
Solanas G, Miravet S, Casagolda D, et al:
beta-Catenin and plakoglobin N- and C-tails determine ligand
specificity. J Biol Chem. 279:49849–49856. 2004.
|