Introduction
Lung cancer is a leading cause of cancer-related
mortality worldwide. From a histological perspective, the field of
lung cancer treatment has been relatively static for several
decades. Yet, several studies have shown that the histological
subtyping of non-small cell lung cancer (NSCLC) is extremely
important in predicting response rates, progression-free survival
and specific drugs toxicities (1–3). For
example, NSCLC subtypes differ significantly with respect to the
prevalence of specific molecular alterations, including the
epidermal growth factor receptor (EGFR) gene (3). Consequently, treatments are selected
according to the histological subtypes of NSCLC on a daily
basis.
Although novel diagnostic procedures, improved
imaging modalities and new immunostaining techniques have improved
histological accuracy, pathological examination occasionally fails
to subtype NSCLC, leading to the rather non-specific diagnosis of
NSCLC not otherwise specified (NOS). NOS diagnoses are often the
consequence of small sample sizes and highly heterogeneous tumors,
which limit the consistency and accuracy of subtyping using
bronchoscopic biopsies. It has been reported that NOS is an
unfavorable independent prognostic factor among stage IV NSCLCs, as
NOS is associated with an aggressive tumor biology (4). However, the prognostic value of NOS in
resectable NSCLC has not been studied.
Thoracic surgeons typically select from among the
available surgical procedures according to the lung tumor type. For
instance, limited resection has recently been recommended for early
lung cancer that is peripherally located and exhibits a glass
ground opacity (associated with minimum invasive adenocarcinoma) on
thin-section computed tomography (CT) (5). Conversely, limited resection may be
inappropriate as a curative surgery for certain aggressive tumors,
even those that are small (6). The
NOS subtype is occasionally encountered pre-operatively, yet no
surgical consensus has been established for NOS tumors.
Therefore, the present study sought to assess the
association between a pre-operative NOS subtype and the prognosis
of candidates for resectable NSCLC. Accordingly, this study aimed
to retrospectively determine whether pre-operative NOS can provide
prognostic information for patients who undergo surgical resection
for NSCLC. Additionally, the study sought to clarify the
association between a pre-operative NOS classification and the
pathological features of the resected specimen.
Materials and methods
Patients
The clinical data of 2,519 patients with primary
NSCLC who underwent complete surgical resection at the Kobe
University Hospital and Hyogo Cancer Center (Kobe, Hyogo, Japan)
between January 2001 and December 2011 was retrospectively
examined. In total, 20 patients were excluded due to incomplete
data. Of the 2,499 remaining patients, 2,309 had undergone a
pre-operative bronchoscopy to establish the tumor malignancy, and
1,913 of these were diagnosed with NSCLC (396 patients were
excluded due to pre-operative biopsy results that were ‘negative’
or ‘suspicious’ for malignancy). The 1,913 patients included in the
present study were divided into two groups: Cases diagnosed as NOS
(the NOS group) and cases with confirmed specific histological
subtypes (the confirmed group), and their clinical features and
outcomes were compared. The Kobe University Hospital and Hyogo
Cancer Center institutional review boards approved the study and
each participant provided informed consent. All patients were
operated on with curative intent. The candidates for limited
resection, such as segmentectomy and wedge resection, were selected
by the judgment of the surgeon responsible, who considered
resectability and the ability to obtain enough surgical margins
from the tumor. Patients with salivary gland-type tumors,
carcinoids and small cell carcinoma were excluded. All patients
treated with induction therapy were also excluded. Medical records
provided data on patient age, gender, body mass index (BMI),
smoking status, respiratory function, stage, surgery, pathological
findings, adjuvant therapy and prognosis. Contrast-enhanced CT
scans of the chest, abdomen and head, bone scintigraphy and
positron emission-CT since 2006 were executed routinely for
pre-operative evaluation. Staging was determined according to the
new International Union Against Cancer Staging System (7).
Diagnostic techniques
During the bronchoscopy sessions, cytological and
histological diagnostic procedures were performed whenever
feasible. The diagnostic results of cytological materials
(transbronchial needle aspiration or transbronchial brushing
cytology) were obtained for all cases. Histological diagnosis
(bronchoscopic biopsy) was performed wherever sufficient tumor
tissue material was available. If necessary, immunostaining was
also performed to maximize the diagnostic accuracy using biopsy
material.
Sample analysis
All samples were reviewed by two expert
pathologists. Carcinomas diagnosed using pre-operative
transbronchial samples were classified as adenocarcinomas, squamous
cell carcinomas or NSCLC-NOS depending on the cytological
diagnosis. These carcinomas were also classified as
adenocarcinomas, squamous cell carcinomas, large cell carcinomas,
combined tumors, adenosquamous cell carcinomas or NSCLC-NOS by
histological examination. Surgical specimens were morphologically
classified according to the 2004 World Health Organization
classification criteria (8).
Representative case
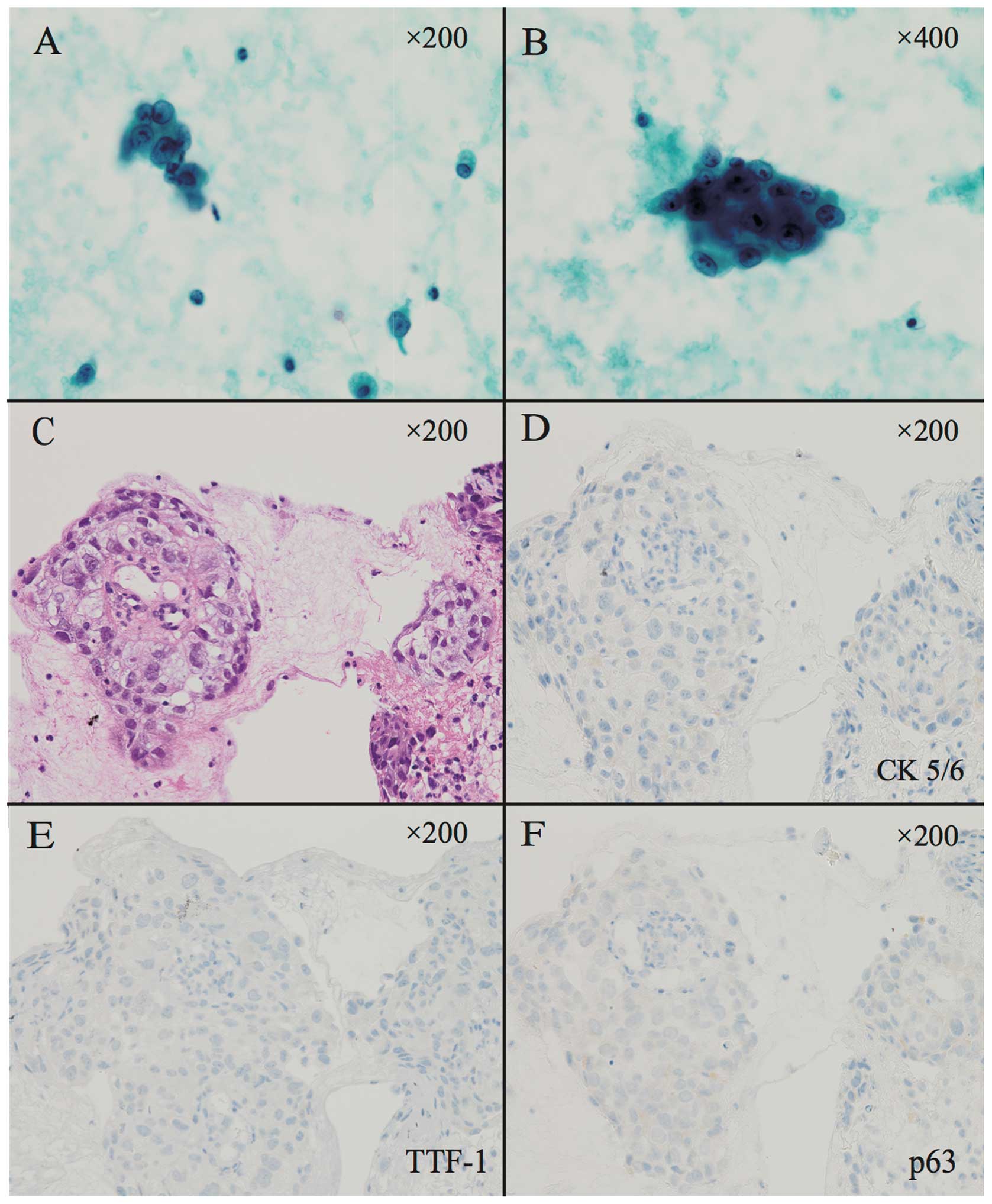
A representative NOS case is shown in Fig. 1. As this case was subtyped as
NSCLC-NOS according to bronchial smear and biopsy material,
immunohistochemistory (IHC) was additionally performed. The IHC
results were considered to indicate NSCLC-NOS if they included
negative findings for thyroid transcription factor-1 (TTF-1),
cytokeratin (CK)5/6 and p63 (9).
The majority of pulmonary adenocarcinomas expressed TTF-1, whereas
the majority of squamous cell carcinomas expressed CK5/6 and
p63.
Follow-up
Post-operative follow-up generally proceeded as
follows. During the 2 years after surgical intervention, systemic
and local examinations were performed every six months, including
blood tests, chest and abdominal CT, magnetic resonance imaging and
bone scintigrams. Between three and five years post-surgery, these
intensive examinations were performed every year. To check for
tumor recurrence and determine survival, observational follow-up
was continued indefinitely or for at least five years.
Statistical analysis
Statistical analyses were performed using JMP
software, version 8 (SAS Institute, Cary, NC, USA). Differences
between the NOS and confirmed groups were analyzed using Student’s
t-test and the χ2 test with regard to gender, age, BMI,
smoking status, respiratory function, size of tumor, surgical
procedure, histological subtype and pathological stage between the
NOS and confirmed groups. With respect to surgical procedures,
segmentectomy and wedge resection were considered to be limited
resection. The duration of overall survival was defined as the
interval between the day of the surgery and the date of mortality
(by any cause) or the last recorded follow-up. Disease-free
survival was defined as the interval between resection and the
proven detection of recurrence or metastases. Disease-free survival
and overall survival were estimated using the Kaplan-Meier method,
and differences in survival distributions were evaluated using the
log-rank test. The Cox proportional hazards model was used to
evaluate the association between the prognostic factors and
survival rate following pulmonary resection, in terms of hazards
ratios and 95% confidence intervals. P<0.05 was used to indicate
a statistically significant difference.
Results
Included patients
Of 2,519 patients with primary NSCLC who underwent
complete surgical resection between January 2001 and December 2011,
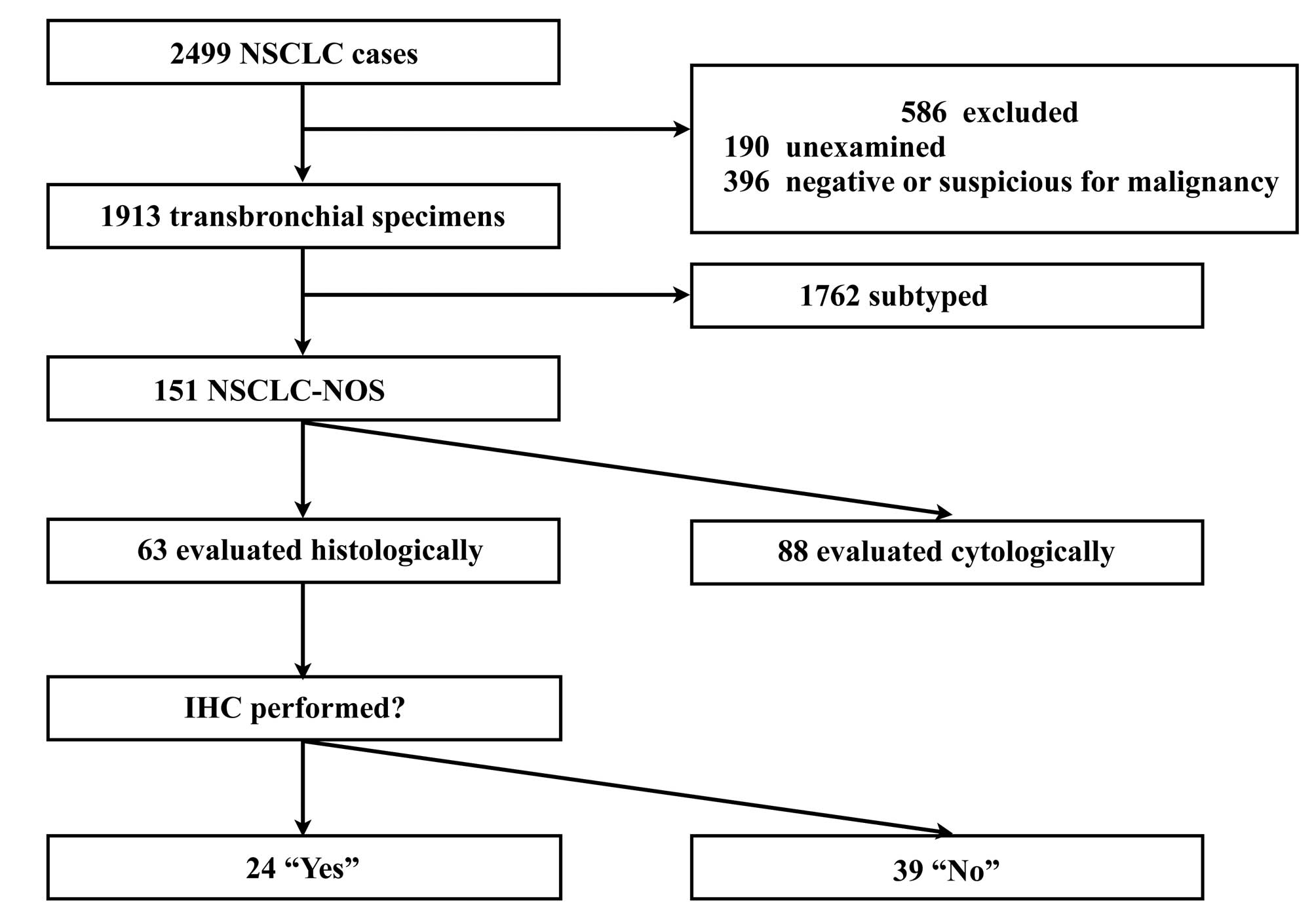
1,913 satisfied the inclusion criteria. Fig. 2 presents a flow chart of the
inclusion and exclusion criteria and diagnostic procedures. The
initial sample included 1,662 males and 837 females, with a median
age of 69 years (range, 30–91 years). Resected tumors were 3–170 mm
in size (median, 28 mm).
Diagnosis of NOS
Of the included cases, 151 (7.9%) were
pre-operatively diagnosed as NOS. Table
I presents the association between NOS findings and
clinicosurgical factors. The NOS subtype was more frequently
observed among male patients, smokers and patients with chronic
obstructive pulmonary disease (COPD). In total, 57 (37.7%) patients
received adjuvant chemotherapy in the NOS group, whereas 502
(28.4%) patients received adjuvant chemotherapy in the confirmed
group.
 | Table IClinicosurgical characteristics of the
study population. |
Table I
Clinicosurgical characteristics of the
study population.
| Factor | NOS group | Confirmed group | P-value |
|---|
| Total, n | 151 | 1762 | |
| Gender, n (M/F) | 127/24 | 1194/568 | <0.001 |
| Age, years (mean ±
SD) | 69±9 | 68±9 | 0.453 |
| BMI (mean ± SD) | 22.4±2.8 | 22.3±3.0 | 0.521 |
| Smoking status,
n | | | <0.001 |
| Smoker | 130 | 1211 | |
| Non-smoker | 21 | 551 | |
| FEV1.0, liters (mean
± SD) | 2.22±0.60 | 2.19±0.61 | 0.090 |
| FEV1.0/FVC, % (mean ±
SD) | 69.8±12.6 | 73.0±10.6 | 0.020 |
| Size of tumor, mm
(mean ± SD) | 35.3±16.0 | 33.9±16.6 | 0.410 |
| Procedure, n | | | 0.159 |
| Pneumonectomy | 0 | 15 | |
| Lobectomy | 120 | 1353 | |
| + extended
resection | 14 | 116 | |
| Segmentectomy | 12 | 197 | |
| Wedge resection | 5 | 81 | |
| Adjuvant
chemotherapy, n (yes/no) | 57/94 | 502/1260 | 0.003 |
A total of 88 NOS cases (58.3%) were diagnosed using
cytomorphology alone. The remaining 63 NOS cases were evaluated
histologically. IHC was performed in 24 of the histologically
evaluated cases.
Tumor histology and staging
Table II presents
the distribution of histologies and pathological stages in the NOS
and confirmed groups. In the NOS group, the histopathological types
were ultimately determined on the basis of the resected specimens;
60 (39.7%) adenocarcinomas, 42 (27.8%) squamous cell carcinomas, 19
(12.6%) pleomorphic cell carcinomas, 12 (7.9%) large cell
neuroendocrine cell carcinomas, 8 (5.3%) adenosquamous carcinomas,
8(5.3%) large cell carcinomas and 2 (1.3%) sarcomatoid carcinomas.
Pleomorphic cell carcinoma, large cell neuroendocrine cell
carcinoma, large cell carcinoma and adenosquamous carcinoma were
significantly more common in the NOS group than in the confirmed
group (P<0.001, P=0.002, P=0.019 and P=0.014, respectively). The
NOS group included 39 (25.8%) stage IA, 51 (33.8%) stage IB, 24
(15.9%) stage IIA, 14 (9.3%) stage IIB and 23 (15.2%) stage IIIA
cases. No NOS cases were stages IIIB or IV. The pathological stage
distribution did not differ significantly between the NOS and
confirmed groups (P=0.127).
 | Table IIHistopathological characteristics. |
Table II
Histopathological characteristics.
| Factor | NOS group | Confirmed group | P-value |
|---|
| Total, n | 151 | 1762 | |
| Pathological stage, n
(%) |
| IA | 39 (25.8) | 604 (34.3) | 0.127 |
| IB | 51 (33.8) | 479 (27.1) | |
| IIA | 24 (15.9) | 270 (15.3) | |
| IIB | 14 (9.3) | 141 (8.0) | |
| IIIA | 23 (15.2) | 249 (14.1) | |
| IIIB | 0 (0.0) | 9 (0.5) | |
| IV | 0 (0.0) | 10 (0.6) | |
| Vessel invasion, n
(Yes/no) | 99/52 | 965/797 | <0.001 |
| Lymphatic invasion, n
(Yes/no) | 56/95 | 721/1041 | 0.350 |
| Pleural invasion, n
(P0/P1/P2/P3) | 88/37/7/19 | 1149/330/135/148 | 0.053 |
| Histology, n (%) |
| Adenocarcinoma | 60 (39.7) | 1144 (64.9) | <0.001 |
| Squamous cell
carcinoma | 42 (27.8) | 481 (27.3) | 0.969 |
| Adenosquamous
carcinoma | 8 (5.3) | 34 (1.9) | 0.014 |
| Large cell
carcinoma | 8 (5.3) | 29 (1.6) | 0.019 |
| Large cell
neuroendocrine carcinoma | 12 (7.9) | 47 (2.7) | 0.002 |
| Pleomorphic cell
carcinoma | 19 (12.6) | 23 (1.3) | <0.001 |
| Sarcomatoid
carcinoma | 2 (1.3) | 4 (0.2) | 0.074 |
Follow-up and overall survival
Of the 1,370 patients in the study who were not
known to have succumbed, 908 (66.2%) were lost to follow-up during
the initial five-year post-operative periods, and the remaining 462
(33.8%) were followed up for over five years. In the group of
patients that remained (NOS and confirmed groups), the median
duration of follow-up was 40.8 months (range, 0.4–145 months).
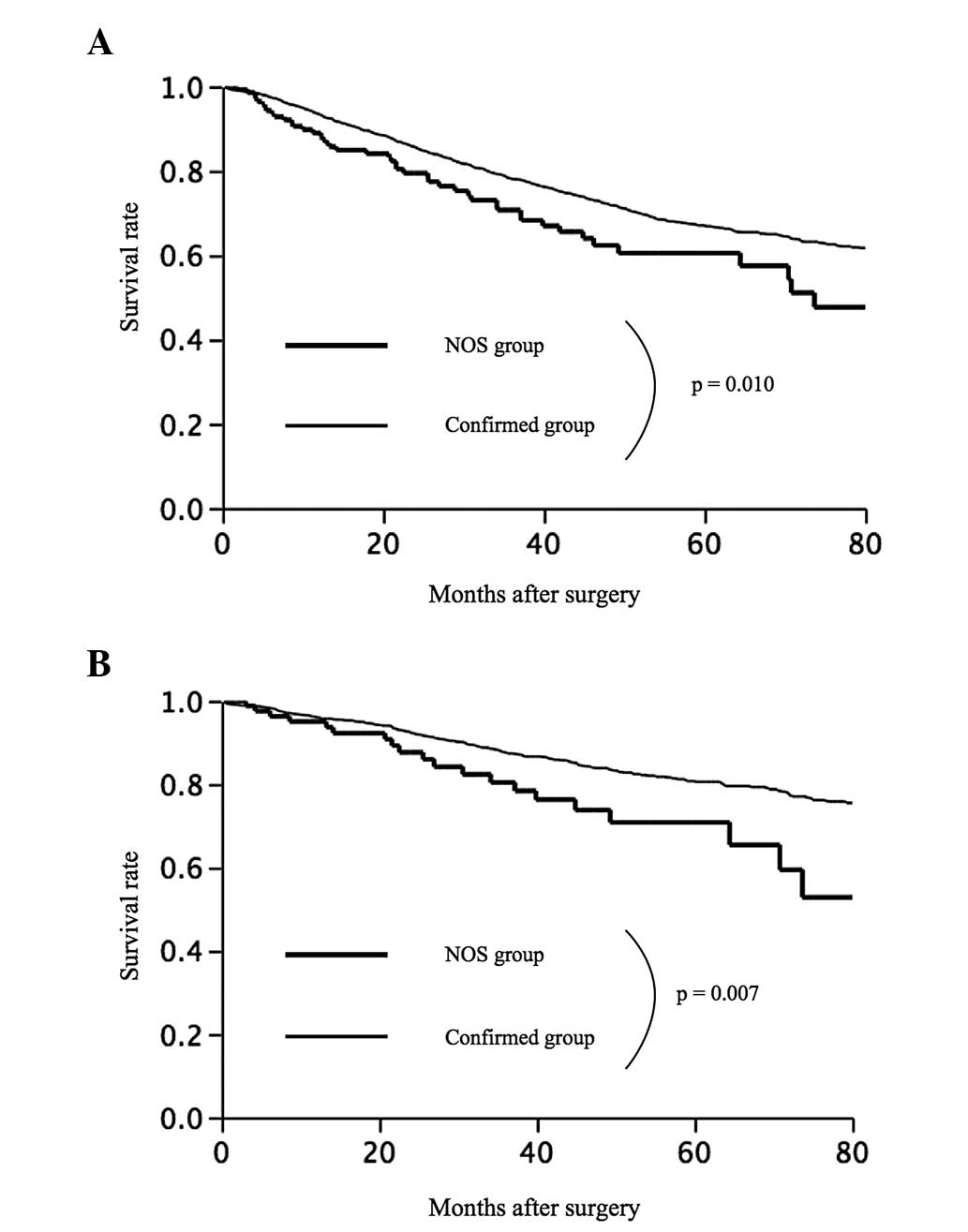
Fig. 3 summarizes the overall
survival rates observed in the study. The five-year survival rates
were 60.5% in the NOS group and 67.1% in the confirmed group
(Fig. 3A). Overall survival was
significantly poorer in the NOS group than in the confirmed group
(P=0.010). Among the 1,168 patients with stage I disease, the
five-year survival rates were 70.8% in the NOS group and 80.7% in
the confirmed group (P=0.007) (Fig.
3B).
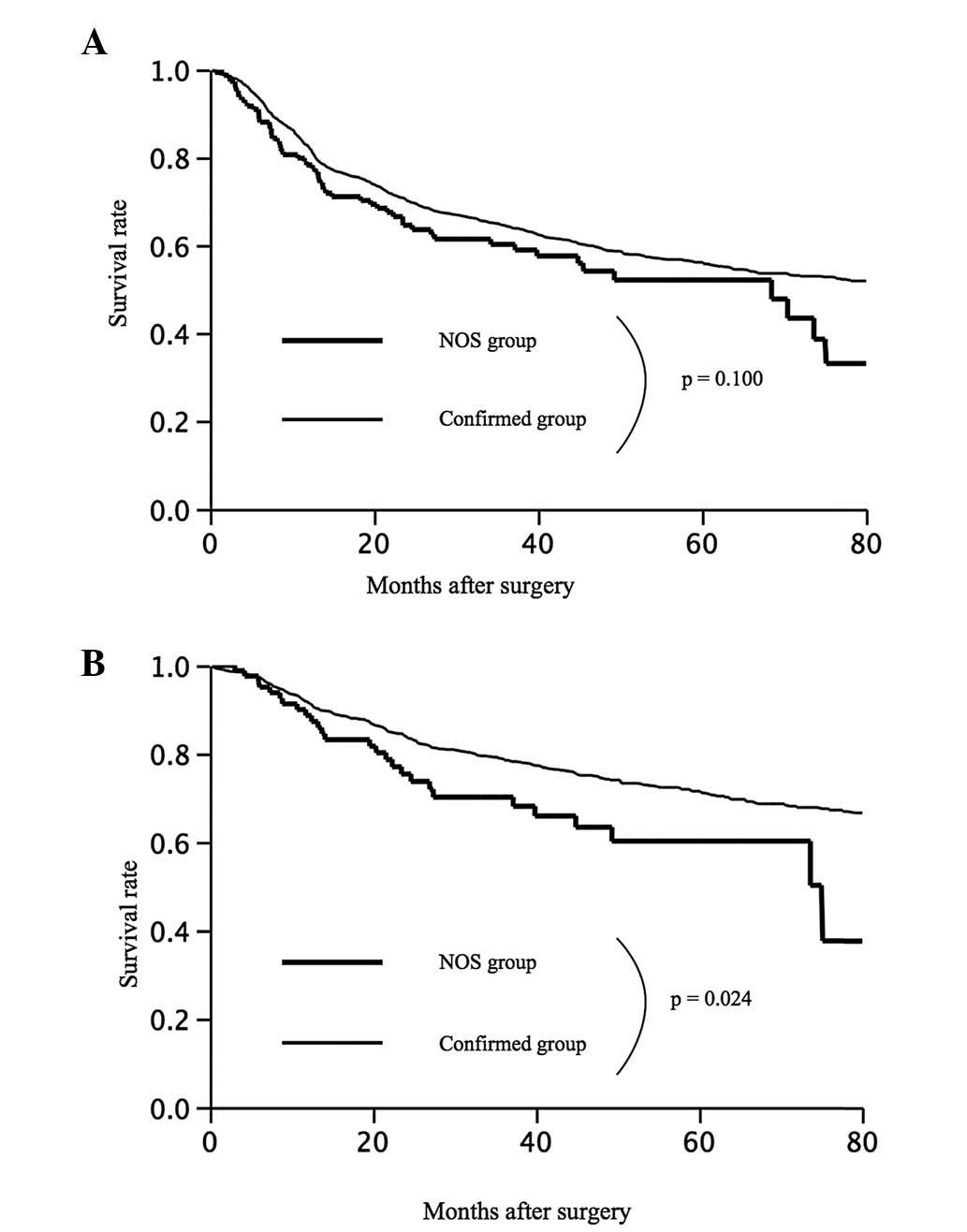
Disease-free survival
The five-year disease-free survival rates were 52.1%
in the NOS group and 60.0% in the confirmed group (P=0.100)
(Fig. 4A). The disease-free
survival rate did not significantly differ between these two
groups, but tended to be worse in the NOS group. Among the patients
with stage I disease, the five-year survival rates were 71.3% in
the NOS group and 60.2% in the confirmed group (P=0.020) (Fig. 4B).
Subtyping
To assign cases to the NOS subtype, cytological and
histological methods were relied upon. The association between the
different diagnostic methods and the survival differences were
analyzed in order to determine any correlations. Cytologically
diagnosed NOS cases (n=88) exhibited a 65% five-year survival rate,
whereas histologically diagnosed NOS cases (n=63) exhibited a 50.4%
five-year survival rate, but this difference was not significant
(P=0.378).
Clinical variables
Additionally investigations were made into the
associations between the clinical variables and overall survival in
the total population (Table III).
According to univariate analyses, NOS, age, gender, BMI,
pathological stage, COPD, smoking status, histology, vessel
invasion, lymphatic invasion and pleural invasion were each
significantly associated with post-operative prognosis.
Multivariate Cox regression analysis indicated that NOS, age,
gender, BMI, pathological stage, histology, vessel invasion,
lymphatic invasion and pleural invasion were independent prognostic
factors in all the tested patients.
 | Table IIIUnivariate and multivariate analyses
of factors associated with prognosis. |
Table III
Univariate and multivariate analyses
of factors associated with prognosis.
| Factor | Hazard ratio | P-value |
|---|
| Univariate
analysis |
| NOS (Yes vs.
no) | 1.47 (1.07–1.96) | 0.016 |
| Age, years (≥65 vs.
<65) | 1.39 (1.16–1.68) | <0.001 |
| Gender (male vs.
female) | 2.01 (1.64–2.49) | <0.001 |
| BMI (≥22 vs.
<22) | 0.78 (0.66–0.93) | 0.005 |
| P-stage (I vs.
II–IV) | 3.00 (2.53–3.57) | <0.001 |
| Surgical procedure
(non-limited vs. limited) | 1.22
(0.97–1.55) | 0.080 |
| COPD (FEV1.0% ≤70
vs. >70) | 1.40
(1.18–1.66) | <0.001 |
| Smoking status
(Yes vs. no) | 1.81
(1.49–2.21) | <0.001 |
| Histology (Sq vs.
non-Sq) | 1.66
(1.39–1.98) | <0.001 |
| Vessel invasion
(Yes vs. no) | 2.17
(1.83–2.58) | <0.001 |
| Lymphatic invasion
(Yes vs. no) | 2.36
(1.98–2.79) | <0.001 |
| Pleural invasion
(P1–3 vs. P0) | 2.25
(1.85–2.73) | <0.001 |
| Adjuvant
chemotherapy (Yes vs. no) | 0.86
(0.70–1.04) | 0.140 |
| Multivariate
analysis |
| NOS (Yes vs.
no) | 1.40
(1.02–1.86) | 0.041 |
| Age, years (≥65
vs. <65) | 1.55
(1.28–1.88) | <0.001 |
| Gender (male vs.
female) | 1.51
(1.16–1.96) | 0.002 |
| BMI (≥22 vs.
<22) | 0.82
(0.69–0.97) | 0.025 |
| P-stage (I vs.
II–IV) | 2.22
(1.84–2.69) | <0.001 |
| COPD (FEV1.0% ≤70
vs. >70) | 1.02
(0.84–1.23) | 0.818 |
| Smoking status
(Yes vs. no) | 1.11
(0.86–1.45) | 0.409 |
| Histology (Sq vs.
non-Sq) | 1.23
(1.02–1.49) | 0.028 |
| Vessel invasion
(Yes vs. no) | 1.27
(1.06–1.56) | 0.012 |
| Lymphatic invasion
(Yes vs. no) | 1.55
(1.28–1.88) | <0.001 |
| Pleural invasion
(P1–3 vs. P0) | 1.24
(1.02–1.49) | 0.041 |
Discussion
As novel, molecular targeted agents have
type-specific efficacy and adverse effects, accurate identification
of the primary lung cancer type is a necessity. For example, among
patients with lung cancer who are treated with bevacizumab, those
with squamous cell carcinoma are at increased risk from
life-threatening hemorrhage (1). A
recent study showed that combined cisplatin and pemetrexed
treatment resulted in statistically greater survival rates compared
with combined cisplatin and gemcitabine, but only for
adenocarcinomas and large cell carcinomas (not for squamous cell
carcinomas) (2). Moreover, the
response to the EGFR-tyrosine kinase inhibitors, gefitinib and
erlotinib, is strongly associated with the adenocarcinoma subtype
(3). These studies pioneered the
use of the histological subtypes as key determinants of treatment
strategies for advanced NSCLC. The most current Multidisciplinary
Classification of Lung Adenocarcinoma, jointly issued by the
International Association for the Study of Lung Cancer, the
American Thoracic Society and the European Respiratory Society,
recommends that NOS be assigned as infrequently as possible
(10,11). However, a NOS classification is
unavoidable in specific cases, as routine morphology and
immunohistochemistry cannot differentiate certain tumor cells.
Sigel et al (12) found that
NOS was diagnosed in 12% of cytology and 6% of biopsy specimens.
Where paired specimens were available (representing the two
methods), the prevalence of NOS decreased to 4%. In the present
study, it was found that 7.9% of cases were classified as NOS, a
rate comparable to that previously reported (4,12,13).
NOS is generally diagnosed using cytology or biopsy
specimens, and not by surgically resected specimens. For the cases
of advanced-stage NSCLC, resected specimens were unavailable in the
present study. Consequently, the true histology or correlation
between the histological subtypes and the prognosis of the NOS
patients could not be determined. Therefore, the study was limited
to the resected cases. To the best of our knowledge, the present
study is the first to examine whether pre-operative NOS can provide
prognostic information for patients who undergo surgical resection
for NSCLC.
We hypothesize that there are two principal causes
of a NOS diagnosis. First is the nature of the biopsy itself; it
can be difficult to obtain more than a scant bronchial specimen,
which lacks distinctive features. In the present study, all
transbronchial procedures were performed using a conventional
bronchoscope under radiographic guidance. However, several recent
studies have indicated that endobronchial ultrasound-guided
transbronchial biopsy (EBUS-TBNA) is a widely accepted method for
diagnosing thoracic tumors (14,15).
The EBUS-TBNA scope can be used to assess and diagnose
intrapulmonary lesions not visible through a conventional
bronchoscope, as long as they are within the reach of the EBUS-TBNA
scope. Consequently, EBUS-TBNA provides relatively high yields for
diagnosing lung tumors. However, the EBUS-TBNA scope and other
novel devices often fail to recover tumoral specimens if the tumor
is located in the peripheral lung parenchyma or if the tumor
interior is necrotic. By excluding the 396 (15.7%) cases of
suspicious and negative results in the present study, the effect of
the variations in transbronchial procedure was minimized.
Second, the NOS subtype may be assigned due to the
poor differentiation of certain tumor cells. Pleomorphic cell
carcinoma, large cell carcinoma, large cell neuroendocrine
carcinoma and adenosquamous carcinoma are classified as
poorly-differentiated tumors. In the present study, these tumors
were found to be particularly likely to be pre-operatively
diagnosed as NOS. Pleomorphic carcinoma accounted for 12.6% of the
cases in the NOS group, even though the true prevalence of
pleomorphic carcinoma has been reported to be only 1.6% (16). Due to their heterogeneity and
poorly-differentiated tumor cells, these tumor types are difficult
to diagnose on pre-operative pathological examination.
Consequently, resected specimens were necessary to achieve
definitive diagnoses. Additionally, these subtypes are associated
with a poor prognosis even if the disease is diagnosed at early
stages and resected (16,17). The poor prognosis of the NOS group
in the present series appears to be affected by the characteristics
of these tumor cells.
It has been reported that sublobar resection,
including segmentectomy and wedge resection, is not inferior to
lobectomy for patients with small-sized NSCLC. Studies by Okada
et al (18,19) indicated that sublobar resection
should be considered as an alternative surgical option for stage IA
NSCLC tumors that are ≤2 cm in size, even for low-risk patients.
Conversely, in the case of certain aggressive tumors, sublobar
resection may be inappropriate for curative surgery. Indeed,
Varlotto et al (20) showed
that, among patients with stage I NSCLC, sublobar resection is
associated with a greater risk of local recurrence than lobectomy,
particularly for patients with poorly-differentiated tumors or
tumors of >2 cm in size. Hattori et al (6) showed that sublobar resection is not
feasible for purely solid tumors, particularly those with a high
maximum standardized uptake value, including small lung cancers.
The present results indicate that the NOS classification is
associated with poor survival, even for stage I cases. Moreover,
the pathological diagnosis of the resected specimens indicated that
poorly-differentiated tumors, such as pleomorphic cell carcinoma,
are significantly more frequent in NOS patients; a finding that is
concordant with the poor prognosis observed for these patients.
Therefore, NOS cases may not be good candidates for sublobar
resection.
In the present study, 88 cases (58.3%) were
diagnosed on the basis of cytomorphology alone and the remaining 63
cases were evaluated histologically. Recent clinical observations
of patients with advanced lung cancer have motivated pathologists
to exert the additional effort that is necessary to distinguish
between the histological subtypes, improving the overall quality of
subtyping. In comparison, cytological diagnoses of squamous and
non-squamous lung cancer subtypes have only a fair degree of
accuracy (21). Moreover,
independent pathological review is not available to all oncologists
in daily practice, limiting the further subclassification of NSCLC
following the initial diagnosis. Several recommendations for the
pre-operative evaluation of patients with resectable NSCLC do not
indicate definitive pre-operative histological subtyping (22). In the present study, the prognosis
did not depend on the mode of NOS diagnosis (cytological or
histological), indicating that pre-operative NOS had the role of a
prognostic factor regardless of the two differing diagnostic
modes.
There are certain limitations to the present study.
First, the study data was analyzed retrospectively and without
central pathological review, although all diagnoses were reviewed
by two expert pathologists. Second, although sublobar resection may
be inappropriate for curative surgery in the early stage of NOS
cases, the prognoses of the NOS patients undergoing sublobar
resection was not evaluated due to the small sample sizes. This
matter should be formally investigated and discussed in a larger
population in the future.
In conclusion, the present study found that
pre-operative NOS diagnosis was associated with significantly
poorer survival among patients with NSCLC, even those with stage I
disease. In conjunction with other clinicopathological parameters,
NOS can be a useful prognostic factor when selecting a treatment
strategy for NSCLC.
Acknowledgements
The authors would like to thank Dr Toshiko Sakuma,
(Department of Pathology, Hyogo Cancer Center, Akashi, Hyogo,
Japan) for providing technical assistance in the evaluation of the
immunostained samples.
References
|
1
|
Johnson DH, Fehrenbacher L, Novotny WF, et
al: Randomized phase II trial comparing bevacizumab plus
carboplatin and paclitaxel with carboplatin and paclitaxel alone in
previously untreated locally advanced or metastatic non-small-cell
lung cancer. J Clin Oncol. 22:2184–2191. 2004.
|
|
2
|
Scagliotti G, Hanna N, Fossella F, et al:
The differential efficacy of pemetrexed according to NSCLC
histology: a review of two Phase III studies. Oncologist.
14:253–263. 2009.
|
|
3
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009.
|
|
4
|
Ou SH and Zell JA: Carcinoma NOS is a
common histologic diagnosis and is increasing in proportion among
non-small cell lung cancer histologies. J Thorac Oncol.
4:1202–1211. 2009.
|
|
5
|
Suzuki K, Asamura H, Kusumoto M, Kondo H
and Tsuchiya R: ‘Early’ peripheral lung cancer: prognostic
significance of ground glass opacity on thin-section computed
tomographic scan. Ann Thorac Surg. 74:1635–1639. 2002.
|
|
6
|
Hattori A, Suzuki K, Matsunaga T, et al:
Is limited resection appropriate for radiologically ‘solid’ tumors
in small lung cancers? Ann Thorac Surg. 94:212–215. 2012.
|
|
7
|
Sobin L and Wittekind CH: TNM
Classification of Malignant Tumours. 6th edition. Wiley-Liss; New
York: pp. 99–103. 2002
|
|
8
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: World Health Organization Classification of
Tumours. Pathology & Genetics. Tumours of the Lung, Pleura,
Thymus and Heart. IARC Press; Lyon: 2004
|
|
9
|
Warth A, Muley T, Herpel E, et al:
Large-scale comparative analyses of immunomarkers for diagnostic
subtyping of non-small-cell lung cancer biopsies. Histopathology.
61:1017–1025. 2012.
|
|
10
|
Travis WD, Rekhtman N, Riley GJ, et al:
Pathologic diagnosis of advanced lung cancer based on small
biopsies and cytology: a paradigm shift. J Thorac Oncol. 5:411–414.
2010.
|
|
11
|
Travis WD, Brambilla E, Noguchi M, et al:
International association for the study of lung cancer/american
thoracic society/european respiratory society international
multidisciplinary classification of lung adenocarcinoma. J Thorac
Oncol. 6:244–285. 2011.
|
|
12
|
Sigel CS, Moreira AL, Travis WD, et al:
Subtyping of non-small cell lung carcinoma: a comparison of small
biopsy and cytology specimens. J Thorac Oncol. 6:1849–1856.
2011.
|
|
13
|
da Cunha Santos G, Lai SW, Saieg MA, et
al: Cyto-histologic agreement in pathologic subtyping of non small
cell lung carcinoma: review of 602 fine needle aspirates with
follow-up surgical specimens over a nine year period and analysis
of factors underlying failure to subtype. Lung Cancer. 77:501–506.
2012.
|
|
14
|
Yasufuku K, Nakajima T, Chiyo M, Sekine Y,
Shibuya K and Fujisawa T: Endobronchial ultrasonography: current
status and future directions. J Thorac Oncol. 2:970–979. 2007.
|
|
15
|
Nakajima T, Yasufuku K, Fujiwara T, et al:
Endobronchial ultrasound-guided transbronchial needle aspiration
for the diagnosis of intrapulmonary lesions. J Thorac Oncol.
3:985–988. 2008.
|
|
16
|
Yuki T, Sakuma T, Ohbayashi C, et al:
Pleomorphic carcinoma of the lung: a surgical outcome. J Thorac
Cardiovasc Surg. 134:399–404. 2007.
|
|
17
|
Raveglia F, Mezzetti M, Panigalli T, et
al: Personal experience in surgical management of pulmonary
pleomorphic carcinoma. Ann Thorac Surg. 78:1742–1747. 2004.
|
|
18
|
Okada M, Nishio W, Sakamoto T, et al:
Effect of tumor size on prognosis in patients with non-small cell
lung cancer: the role of segmentectomy as a type of lesser
resection. J Thorac Cardiovasc Surg. 129:87–93. 2005.
|
|
19
|
Okada M, Koike T, Higashiyama M, Yamato Y,
Kodama K and Tsubota N: Radical sublobar resection for small-sized
non-small cell lung cancer: a multicenter study. J Thorac
Cardiovasc Surg. 132:769–775. 2006.
|
|
20
|
Varlotto JM, Medford-Davis LN, Recht A, et
al: Identification of stage I non-small cell lung cancer patients
at high risk for local recurrence following sublobar resection.
Chest. 143:1365–1377. 2013.
|
|
21
|
Sakr L, Roll P, Payan MJ, et al:
Cytology-based treatment decision in primary lung cancer: is it
accurate enough? Lung Cancer. 75:293–299. 2012.
|
|
22
|
Scott WJ, Howington J, Feigenberg S,
Movsas B and Pristers K; American College of Chest Physicians.
Treatment of non-small cell lung cancer stage I and stage II: ACCP
evidence-based clinical practice guidekines (2nd edition). Chest.
132(3 Suppl): 234S–242S. 2007.
|