Introduction
Approximately 85% of human cancers restore telomeric
DNA base pairs, which have been lost, in order to preserve
fertility by activating telomerase (1,2).
Telomeres are repetitive DNA sequences (TTAGGG)n present at the
termini of chromosomes, which prevent chromosomes from fusing with
each other or rearranging. Telomerase is a ribonucleoprotein
complex that counterbalances telomere loss by elongating telomeric
DNA repeats; this maintains the chromosomal integrity and cells
divide continuously in the M phase (3,4). In
addition, telomerase is significantly associated with tumor size
and aggressiveness, as well as genomic instability and prognosis in
the majority of human cancers (5–8).
Telomerase consists of two critical components, the RNA subunit
(human telomerase RNA component; hTERC), which serves as a template
for telomere elongation, and a catalytic subunit (human telomerase
reverse transcriptase; hTERT), that provides reverse transcriptase
activity (9,10). hTERC is hypothesized to be expressed
ubiquitously in all tissues (11),
whereas hTERT expression significantly correlates with telomerase
activity and appears to be a limiting determinant of enzyme
function (12,13).
The accumulation of telomerase in the nucleus is
mediated by telomerase Cajal body protein 1 (TCAB1), which is a
WD40 repeat protein (also denoted as wdr79 and WRAP53). TCAB1
exists in the active telomerase holoenzyme and it has been
demonstrated that TCAB1 is required in HeLa and super telomerase
(S-T) cells for the trafficking of telomerase to the Cajal bodies
(CBs) during the S phase of the cell cycle (14–16),
the phase in which telomere DNA is replicated (17). Previous studies have shown that
TCAB1 is a constitutive component of CBs, which has been detected
within CBs in a panel of cancer and primary cell lines, including:
U2OS, osteosarcoma; H1299, lung cancer; HCT116, colon cancer;
HeLa-PV, cervical cancer; HEK293, embryonic kidney cancer; MCF-7,
mammary epithelial cells; and HDF, human diploid fibroblasts.
Knockdown of TCAB1 impairs the growth of these cancer cells and
results in mislocalization of telomerase from CBs to the nucleoli,
thus inhibiting the ability of telomerase to restore the telomere
length. This consequently leads to massive cell death with several
morphological changes in certain cultured cancer cells (18–22).
Annually, more than one million individuals succumb
to lung cancer worldwide, thus, it is the leading cause of cancer
mortality in humans. Non-small cell lung cancer (NSCLC) accounts
for 80% of all the lung cancer types, with adenocarcinoma as the
major subtype (23). Since 1995, a
paradigm shift in the management of advanced-stage NSCLC has
occurred and an expanding array of molecular markers are being used
to individualize cancer therapy. As a telomerase holoenzyme
component in the telomere synthesis pathway, TCAB1 stably
associates with the active telomerase enzyme and directs it through
CBs to the telomere in the S phase (20). It was hypothesized in the present
study that TCAB1 exhibits the same function in A549 lung
adenocarcinoma cells; therefore, to validate this hypothesis a
preliminary study was conducted. This demonstrated the function of
TCAB1 small interfering (si)RNA in the downregulation of TCAB1
expression in proliferation and enzymatic activities in A549 lung
adenocarcinoma cells, without altering telomerase activity.
Telomerase/telomere miscolocalization and the consequent cell cycle
arrest were further investigated, and the results indicated the
possibility of TCAB1 as a potential target during lung
adenocarcinoma therapy.
Materials and methods
Antibodies
The following three antibodies served as the primary
antibodies for western blot (WB) analysis: Monoclonal anti-β-actin
(A5441; Sigma-Aldrich, St. Louis, MO, USA); anti-TCAB1 (ab99376;
Abcam, Cambridge, MA, USA); and hTERT (RabMAb®; 1531-1;
Abcam). The secondary antibodies used for WB analysis were as
follows: Anti-rabbit IgG (#7074; Cell Signaling Technology, Inc.,
Danvers, MA, USA) and anti-mouse IgG horse radish
peroxidase-conjugated (#7076; Cell Signaling Technology, Inc.). For
immunofluorescence (IF), the hTERT (RabMAb®; 1531-1;
Abcam) and anti-telomeric repeat-binding factor (TRF)-2 (4A794;
ab-13579; Abcam) antibodies served as the primary antibodies. Goat
polyclonal secondary antibodies against rabbit IgG-H&L
(fluorescein-isothiocyanate; ab6717; Abcam) and mouse IgG-H&L
(cyanine 3; ab97035; Abcam) served as the secondary antibodies.
Cell culture, siRNA oligonucleotides,
plasmids and transfection
A549 cells obtained from the Cell Bank of the
Chinese Academy of Sciences (Shanghai, China) were cultured in
F-12K (1237832; Gibco-BRL, Carlsbad, CA, USA) with 10% fetal calf
serum (1133067; Gibco-BRL) at 37°C in a humidified incubator with
an atmosphere of 5% CO2. Lipofectamine 2000 (11668-019;
Invitrogen Life Technologies, Carlsbad, CA, USA) was used as the
transfection reagent. TCAB1 siRNA (sc-93974; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) was used to knock down
TCAB1 and control siRNA (sc-37007; Santa Cruz Biotechnology, Inc.)
served as the negative control. Diluted siRNA and control siRNA in
F-12K at a concentration of 40 nM were remixed and incubated for 30
min prior to each transfection.
Cell lysis and WB analysis
Protein was extracted according to the standard
instructions for the radioimmunoprecipitation assay lysis buffer
(P0013B; Beyotime Institute of Biotechnology, Shanghai, China). The
concentration was determined using the DCTM protein
assay reagents package (Bio-Rad, Hercules, CA, USA). Next, 30 μg of
each sample was loaded onto a 12% polyacrylamide gel, which was
electrophoresed in electrophoresis buffer (Thermo Fisher
Scientific, Rockford, IL, USA) at 60 V for 30 min until the xylene
cyanol dye reached the end of the stacking gel produced in-house
(double-distilled H2O, 3.4 ml; 30% acrylic bicone 0.83
ml, 1M Tris HCL (pH 6.8), 0.63 ml; 10% sodium dodecyl sulfate, 0.05
ml, 10% ammonium persulfate, 0.05 ml and
tetramethylethylenediamine, 0.005 ml). The voltage was subsequently
increased to 90 V until the xylene cyanol dye was 1 cm from the
bottom of the gel. The protein was transferred to a polyvinylidene
fluoride membrane (Seebio Biotech, Inc., Shanghai, China) according
to the standard instructions and blocked with 3% bovine serum
albumin (BSA) [9408-46-8; Sangon Biotech (Shanghai) Co., Ltd.,
Shanghai, China] in Tris-buffered saline with Tween 20 (TBST) for 1
h prior to incubation with the primary antibody (1:1,000) overnight
at 4°C. The membranes were washed with TBST three times and
incubated with diluted secondary antibody (1:1,000). Signals were
detected using Pierce enhanced chemiluminescence (Pierce
Biotechnology, Inc., Rockford, IL, USA) plus Western Blotting
Substrate (PI32132; Thermo Fisher Scientific) and the membranes
were visualized on a Kodak XBT-1 film (Kodak, Rochester, NY, USA).
All reactions were performed in triplicate.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted using TRIzol reagent
(11596026; Invitrogen Life Technologies) according to the standard
instructions. cDNA synthesized from RNA (1 μg) was used as a
template for the RT reaction (R001B; Takara Bio, Inc., Shiga,
Japan), which was followed by PCR analysis. The primer sequences
were as follows: Forward, 5′-ctc cat cct ggc ctc gct gt-3′ and
reverse, 5′-gct gtc acc ttc acc gtt cc-3′ for human actin F; and
forward, 5′-aac cgt cag gag ccc act ta-3′ and reverse, 5′-gga gac
acc gct tgg aac ta-3′ for TCAB1. RT-PCR was performed under the
following conditions: 95°C for 5 min, followed by 30 cycles of 95°C
for 30 sec, 55–60°C for 30 sec and 72°C for 30 sec, with a final
step of 72°C for 10 min. A total of 10 μl PCR products from each
sample were loaded onto a 1.0% agarose gel and electrophoresed at
110 V for 30 min in 0.5X TBE electrophoresis buffer (00124374;
Thermo Fisher Scientific). The gel was visualized and the images
were captured using an ultraviolet (UV) transilluminator (170–8170,
Bio-Rad). All reactions were performed in triplicate.
Telomerase activity assay
A549 cell pellets were subjected to lysis in 300 μl
3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate lysis
buffer (provided in the kit) and protein concentration
standardization was performed using the Bio-Rad Protein Assay
reagent (Bio-Rad). For each sample, 1.5 μg cell extract was added
to the TRAPeze telomerase detection kit assay (S7707; Millipore,
Billerica, MA, USA). The gels were stained with GelRed (41003;
Biotium, Inc., Hayward, CA, USA) and visualized with a UV
transilluminator. Appropriate positive and negative
heat-inactivated cell extracts were set up with test samples and
all reactions were performed in triplicate.
Immunofluorescence assay
Cells were fixed in ice-cold 4% (v/v)
paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4) for 15
min at room temperature and washed twice with ice-cold PBS. The
samples were incubated for 10 min with PBS containing 0.25% Triton
X-100 (room temperature) and washed three times with PBS for 5 min
each. The samples were immediately incubated with 1% BSA
(9408-46-8; Sangon Biotech) in PBS with Tween 20 (PBST) for 30 min
for blocking at room temperature, followed by two primary
antibodies (1:1,000) diluted in 3% BSA in PBST (w/v). The cells
were subsequently incubated with two primary antibodies at 4°C
overnight, washed with PBS three times for 5 min each and incubated
with the secondary antibodies (1:1,000) in 1% BSA in PBST for 1 h
at room temperature in the dark. Finally, the cells were washed
three times with PBS for 5 minutes and visualized at room
temperature using a Carl Zeiss microscope (Carl Zeiss AG, Jena,
Germany). The exposure times between treatments were consistent and
the image brightness and contrast were adjusted using Adobe
Photoshop (Adobe Systems Inc., San Jose, CA, USA) for
presentation.
Cell cycle and apoptosis analysis
For the cell cycle assay, the cells were collected
and washed with PBS. The cell pellets were obtained by
centrifugation at 10 × g (Maxi Mix II rocker rotator - M37610-33CN,
Thermo Fisher Scientific) and the supernatant was discarded. Next,
1 ml of DNA staining solution (Multisciences Biotech Co., Ltd,
China) was added and blended by vortexing (Maxi Mix II rocker
rotator - M37610-33CN Thermo Fisher Scientific) for 10 sec. In
preparation for flow cytometry, the cells were incubated for 30 min
at room temperature in the dark. For the apoptosis assay, the cells
were resuspended in 0.5 ml binding buffer (Santa Cruz
Biotechnology, Inc.), containing Annexin V (1:50) and 40 ng/sample
of propidium iodide (BD Biosciences, Franklin Lakes, NJ, USA) and
incubated for 30 min at 37°C in the dark, prior to flow cytometry.
At least 10,000 cells were analyzed for each sample and the
experiments were repeated three times.
Densitometry and statistical
analysis
ImageJ software (National Institutes of Health,
Bethesda, MD, USA) was used to quantify the band intensity. Data
are presented as intensities relative to the indicated loading
control and as the mean ± standard deviation of at least three
independent experiments. Statistical comparisons were performed
using Graph Pad Prism software version 5.01 (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
TCAB1 siRNA depletes TCAB1 protein
without altering hTERT expression
TCAB1 expression was investigated in A549 cells. The
protein and total RNA were isolated from A549 cells treated with
TCAB1 siRNA and control siRNA and the levels of TCAB1 and hTERT
were analyzed by WB analysis and RT-PCR. TCAB1 expression was
downregulated by ~45% without altering hTERT expression (Fig. 1A and B). Telomeric repeat
amplification protocol (TRAP) was also conducted to analyze
telomerase activity and the results showed that the telomerase
activity remained unchanged compared with the control groups
(Fig. 1C).
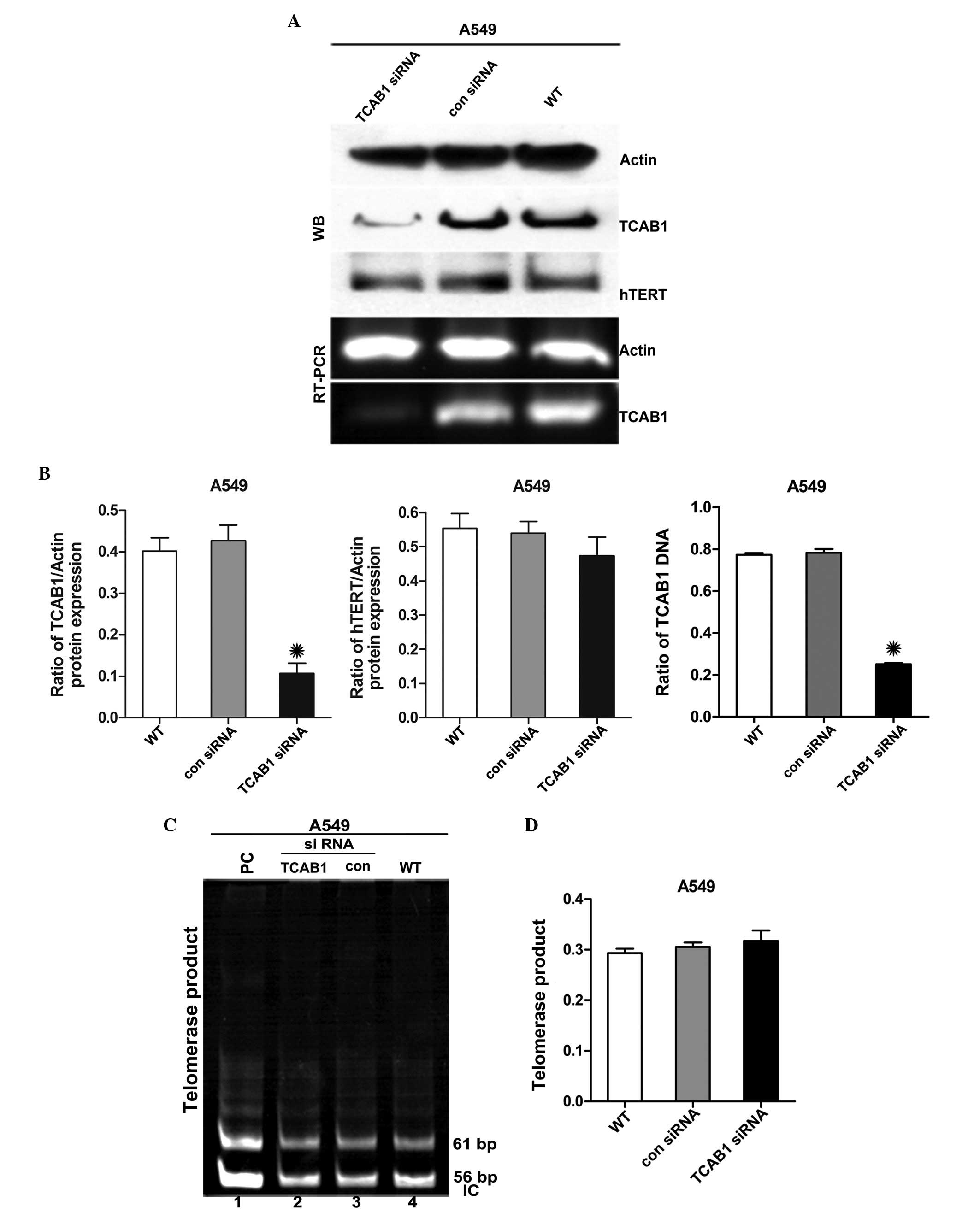 | Figure 1Effect of TCAB1 siRNA on the
expression of TCAB1 and hTERT. (A) WB and RT-PCR analyses
demonstrate the effect of TCAB1 siRNA transfection on TCAB1 and
hTERT expression in A549 cells. (B) Densitometric quantification of
the relative TCAB1 and hTERT levels of the cells. Levels of TCAB1
and hTERT were normalized to actin levels. Each bar represents
triplicate analyses of the mean ± SD. *P<0.05 vs. con
siRNA and WT (n=3). (C) A TRAP assay was performed to evaluate the
effect of TCAB1 siRNA treatment on the activity of telomerase in
A549 cells. The 56-bp bands represent the IC. (D) The bar graph
presents the densitometry-quantified data of the TRAP products in
the single lane/PC (line 1) of the TRAP reaction ratios from three
independent experiments. Telomerase activities were quantified by
comparing the mean band intensity of each lane with the PC. The PC
mean band intensity was defined as 100% telomerase-positive. Each
bar represents triplicate analyses of the mean ± SD.
*P<0.05 vs. con siRNA and WT (n=3). TCAB1, telomerase
Cajal body protein 1; con siRNA, control small interfering RNA; WT,
wild-type; WB, western blot; hTERT, telomerase reverse
transcriptase; RT-PCR, reverse transcription polymerase chain
reaction; PC, positive control; IC, internal control; SD, standard
deviation; TRAP, telomeric repeat amplification protocol. |
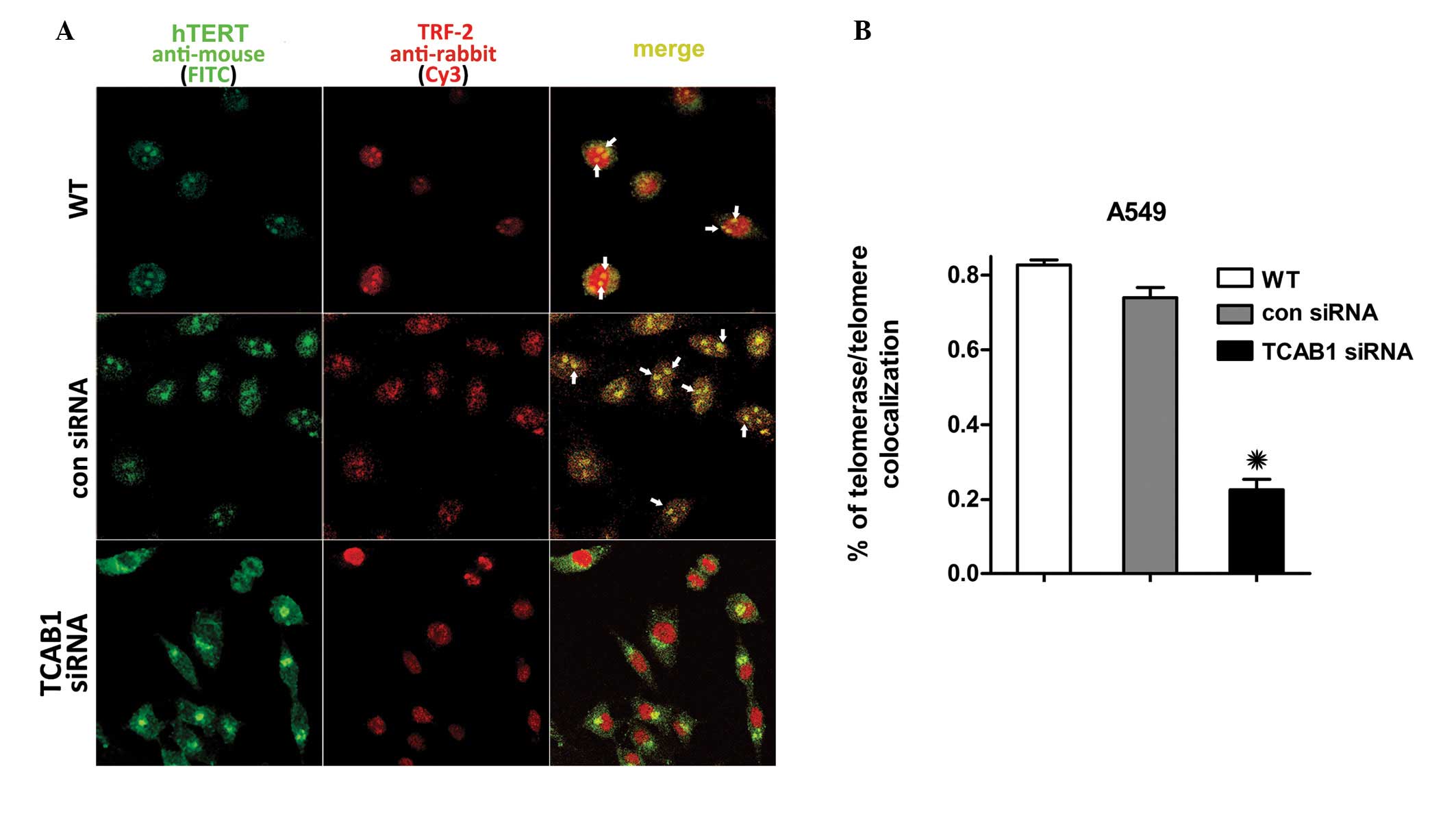
TCAB1 silencing inhibits hTERT
localization
The IF revealed that TCAB1 depletion suppressed
telomerase trafficking to telomeres in A549 cells. hTERT and TRF-2
are essential subunits of telomerase and telomeres without which
they are unable to form. In contrast to the control groups, TCAB1
depletion also suppressed hTERT accumulation at the TRF-2 of the
telomeres. The hTERT signal surrounds the membrane of the nucleus
in TCAB1-depleted A549 cells, however, does not enter the nucleus,
therefore, almost no foci were observed with hTERT/TRF-2
colocalization (Fig. 2A). A total
of 100 cells selected randomly from three random fields of each
group were scored. hTERT in the cells of the control groups was
found to accumulate in the nucleoli and colocalize with TRF-2 more
frequently than that in the TCAB1 siRNA-treated cells (Fig. 2B). These results indicated that A549
cells that lack TCAB1 are less likely to transport telomerase to
telomeres, which demonstrates the effect of TCAB1 depletion on
telomerase recruitment to the telomere.
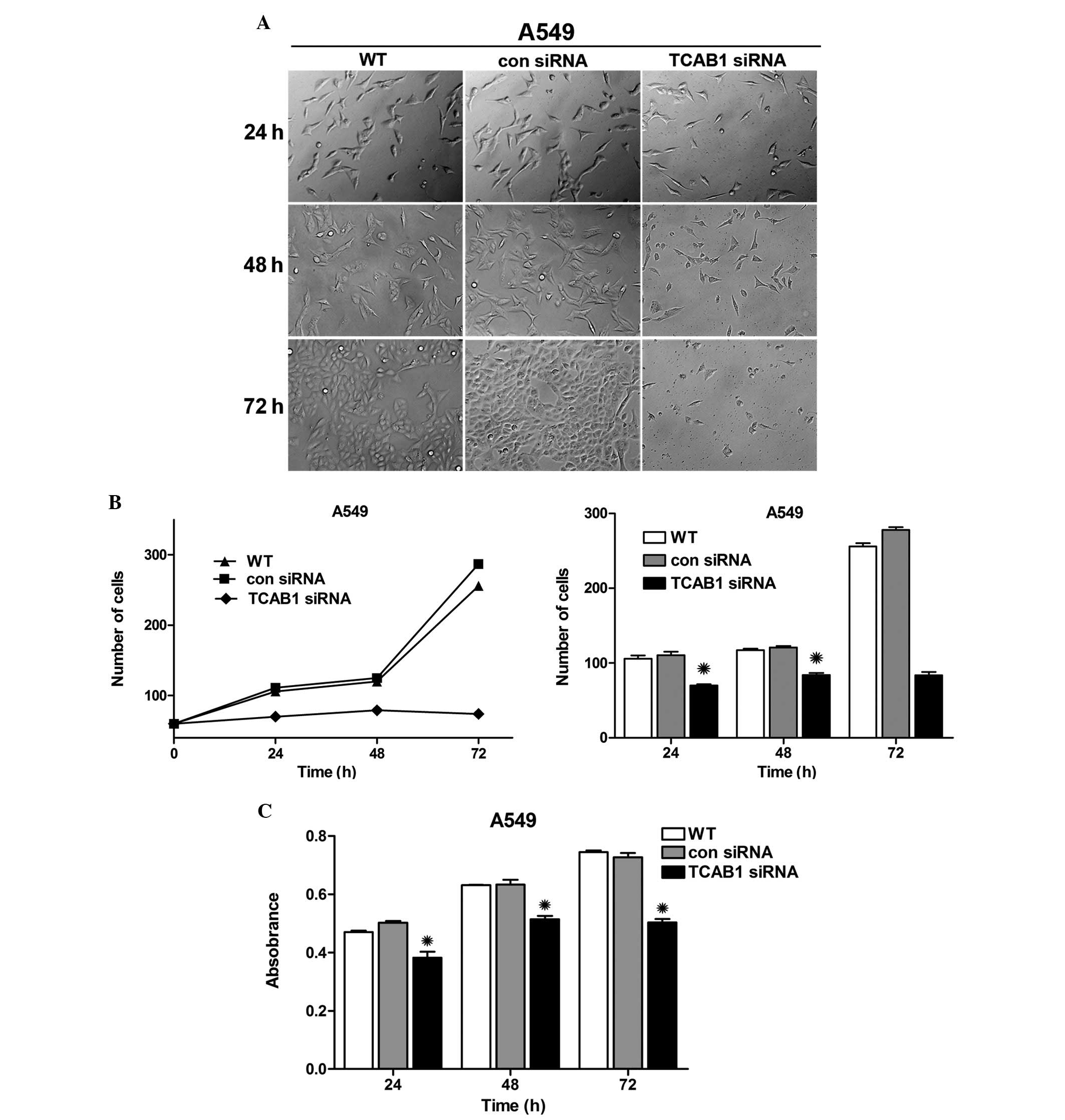
Inhibitory effect of TCB1 siRNA on the
proliferation of A549 cells
To investigate the impact of TCAB1 depletion in lung
adenocarcinoma cells, A549 cells were transfected with TCAB1 siRNA
and images were captured using an inverted microscope
(magnification, ×40; Olympus Corporation, Tokyo, Japan) separately
at 24, 48 and 72 h. Notably, the graphs revealed an evident
distinction between cell densities, particularly in the group
treated with TCAB1 siRNA (Fig 3A).
The graph and bar chart demonstrate a growth trend and cell number
difference in each group (Fig 3B),
which exhibited an evident weakness in the reproductive capacity of
TCAB1-depleted cells. Simultaneously, the activity of cellular
enzymes was evaluated by MTT assay and the results were compared to
reveal a decreased absorbance in TCAB1-depleted cells, which
indicated decreased activity of cellular enzymes (Fig 3C). These results indicated that TCAB1
siRNA effectively inhibits the proliferation of A549 cells.
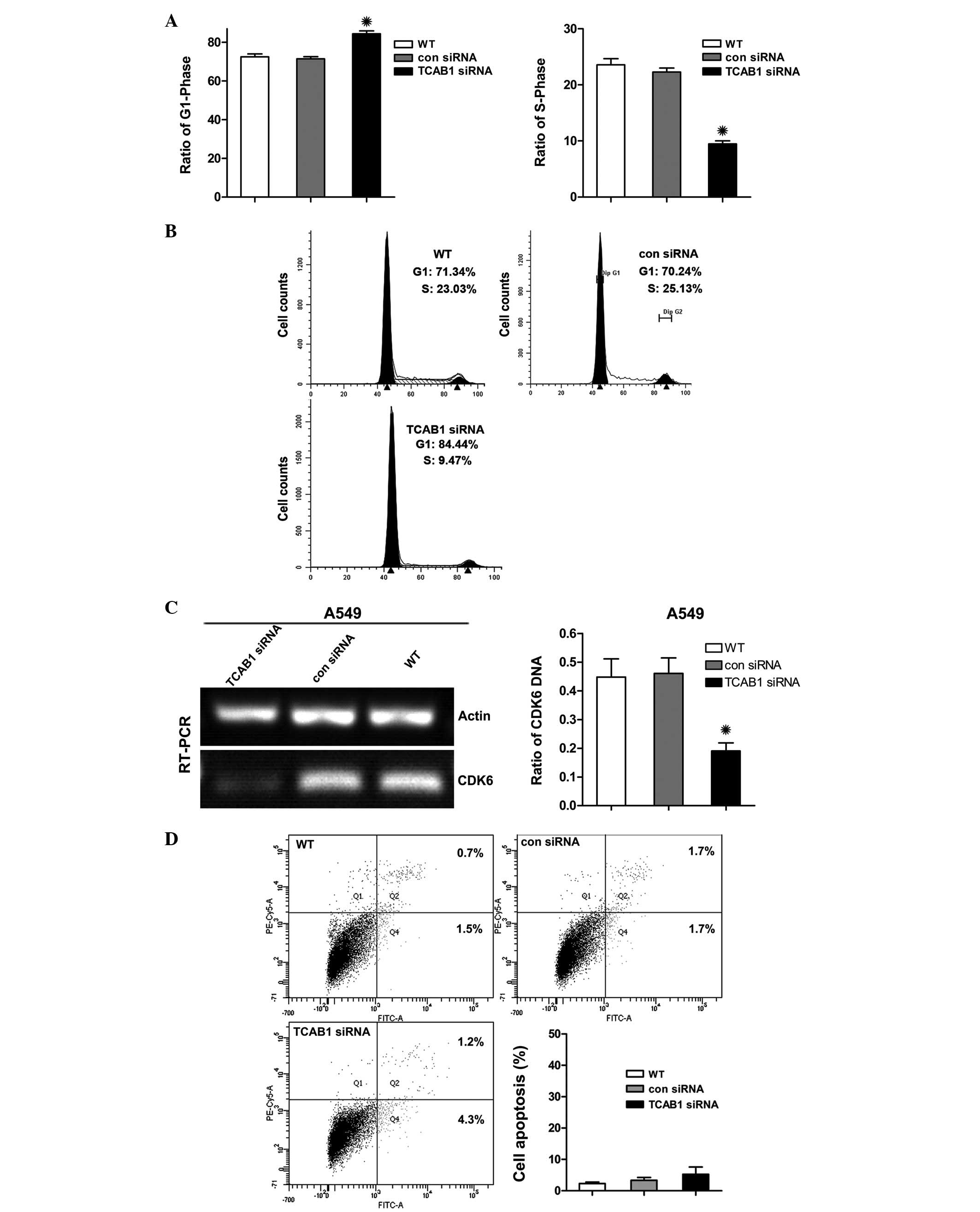
TCAB1 depletion arrests the G1
cell phase without inducing apoptosis
Further study was conducted to elucidate the
mechanism of action for the antiproliferative activity of TCAB1
depletion on A549 cells. Flow cytometry was used to analyze the
A549 cell cycle distribution and apoptosis. Cancer cells are
generally immortal and divide uncontrollably, and the replication
of telomeres occurs in the S phase (24). Thus, it was predicted that the
number of A549 cells would increase in the G1 phase and
decrease in the S phase as a result of TCAB1 depletion. Consistent
with this hypothesis, TCAB1 siRNA was found to induce G1
cell cycle arrest, and the percentage of cells with G1
DNA content increased from 70 to 88% and the percentage of cells in
the S phase decreased from 25 to 9%, when compared with that of the
control groups (Fig. 4A). However,
cells were not found to accumulate in the sub G1 peak
(Fig. 4B). In addition, the
G1 cell cycle checkpoint protein, cyclin-dependent
kinase 6 (CDK6), was suppressed (Fig.
4C). Accordingly, the Annexin V staining apoptosis assay of
A549 cells treated with TCAB1 siRNA demonstrated no evidence of
apoptotic cell death (Fig. 4D).
These observations indicated that the antiproliferative effects of
TCAB1 siRNA in A549 cells is caused by cell cycle arrest without
the induction of apoptosis.
Discussion
Up to 90% of cancers exhibit activated telomerase
that permits cell immortalization and leads to tumorigenesis.
Furthermore, the localization of telomerase in CBs in the S phase
of the cell cycle is an indispensable step in telomere synthesis.
TCAB1 is required in this step in HeLa and S-T cells, however, its
depletion has not previously been investigated in A549 lung
adenocarcinoma cells. In this initial study, the expression of
TCAB1 in TCAB1 siRNA treated and untreated A549 cells was examined
and was further integrated with the results regarding TCAB1
expression in NSCLC cell lines. As predicted, TCAB1 was present in
the A549 cell line and was downregulated by transfection with TCAB1
siRNA. A previous study has identified that TCAB1 is required for
the delivery of telomerase to the nucleus for telomere replication
in certain human cancer cells, and that TCAB1 knockdown impairs the
growth of these cancer cells (20).
However, to evaluate the efficacy and to further elucidate the
mechanism of TCAB1 in A549 cells, the current study investigated
telomerase/telomere colocalization in TCAB1-depleted A549 cells. IF
demonstrated that the hTERT (telomerase) adheres to the membrane of
the nucleus and fail to associate with the TRF-2 (telomere) in
TCAB1 siRNA-treated cells. Consistently, the graphs of cell density
demonstrated that TCAB1 depletion exhibits a potent
antiproliferative effect on A549 cells, in addition to decreasing
the activity of cellular enzymes in the MTT assay. This
antiproliferative effect may be due to the telomerase/telomere
miscolocalization caused by TCAB1 depletion. In addition, recent
studies have revealed that telomerase (hTERT) depletion in mouse
lymphomas results in the emergence of the alternative lengthening
of telomere (ALT) (25). ALT is a
telomere maintenance mechanism, which exists in telomerase-negative
neoplastic and non-neoplastic human cells, characterized by
homologous recombination (26–28).
The ALT mechanism exists in NSCLC and thus, leads to aggressive
malignant properties and the acquisition of resistant mechanisms to
counteract telomerase deficiency in late generations of tumor cells
(25,29). The association between TCAB1 and
telomerase is not fully understood and therefore, to exclude the
possibility of telomerase alternation and emergence of ALT in
TCAB1-depleted A549 cells, the current study identified the
disassociation between TCAB1 depletion and telomerase activity in
A549 cells by WB and TRAP. Consistent with the unaltered hTERT
expression identified by WB analysis, the results of TRAP also
revealed unaltered telomerase activity. Furthermore, the
considerable infertility of TCAB1-depleted A549 cells showed no
sign of activating the ALT mechanism. These results are consistent
with with a study reporting that TCAB1 only functions as a
telomerase holoenzyme component in the telomere synthesis pathway
following the assembly of the telomerase complex, which contains
TERT, TERC and dyskerin (20).
Based on the potent antiproliferative activity, the
current study investigated the mechanism of TCAB1 siRNA treatment
in the regulation of cell proliferation in A549 cells. Generally,
cell cycle arrest and apoptosis are the most common causes of
antiproliferation of cells and thus, whether TCAB1-depletion
regulates cell cycle progression or induces cellar apoptosis was
also investigated. Notably, the cell cycle assay revealed that a
greater percentage of cells remain in the G1 phase, with
a reduced percentage of cells in the S phase following TCAB1 siRNA
treatment. However, the sub-G1 peak, which is indicative
of apoptotic cell death, was not detected. Accordingly, Annexin V
staining of TCAB1-depleted cells revealed no evidence of apoptotic
cell death. Additionally, G1-acting CDK6 expression was
investigated and found to be significantly reduced in response to
TCAB1 siRNA treatment, indicating that the downregulation of CDK6
may result in the suppression of the cyclin D1-CDK6 complex, which
eventually leads to G1 phase arrest. These results
indicate that the inhibitory effect of TCAB1 depletion on the
proliferation of A549 cells is evoked by cell cycle arrest at the
G1 phase without causing evident apoptotic cell
death.
The cell cycle is a process that involves DNA
replication and cell division (30). DNA replication occurs during the S
phase, and division occurs in the G2 and M phases;
arrest in any of the phases may inhibit cell proliferation.
Furthermore, metabolic activities of DNA at the chromosome ends,
where telomeres exist, are important throughout cell cycle
progression (31). Telomeric DNA is
arranged into folded structures in the G1 phase, which
unfold to replicate during the process of DNA replication following
the recruitment of telomerase to the telomere in the S phase
(32). Thus, the present study
identified that the infertility of TCAB1 siRNA-treated A549 cells
is evoked by G1 phase arrest due to TCAB1 depletion,
which reduces the presence of telomerase at the telomeres (shown by
IF) in the S phase when telomere DNA replication occurs.
In conclusion, the results of the present study
demonstrated the function of TCAB1 depletion via decreased
telomerase/telomere colocalization proportions, infertility, and
reduced activity of cellular enzymes in A549 cells, without
downregulating telomerase expression and activity. In addition, it
was found that the antiproliferative effect of TCAB1 depletion is
evoked by G1 phase cell cycle arrest without inducing
apoptotic cell death. These results highlight the essential
function of TCAB1 within A549 cells. However, the function of TCAB1
in the recruitment of the telomerase complex from the cytoplasm to
CBs and restoration of telomere length remains unclear. Recent
studies have identified TCAB1 as an essential factor for CBs
maintenance and that TCAB1 knockdown prevents the formation of
novel CBs (18). Stern et al
(33) revealed that depletion of
CBs also reduces telomerase foci at telomeres. These observations
further establish TCAB1 as a significant factor for telomerase
recruitment and telomere synthesis. Another study has revealed that
the oligonucleotide-binding-fold domain of the telomere-binding
protein, TPP1, recruits telomerase to the telomeres through a
TERT-associated mechanism (34).
The manner in which TCAB1 acts as a cofactor in this mechanism for
telomerase trafficking and their connection, as well as the
telomere length response in TCAB1-depleted A549 cells, remain to be
identified and require further investigation.
Briefly, ‘oncogene addiction’ is defined as the
dependence of a cancer cell on one pathway or overactive gene for
its survival and/or growth, which provides cancer-specific
weaknesses that can be targeted during anticancer therapy (35). In the present study, such properties
were identified with regard to the TCAB1 protein. Specifically,
TCAB1 is a potential oncogene that is essential for A549 cell
survival and, thus, is a notable target for therapeutic
intervention in lung adenocarcinoma.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 31170720).
References
|
1
|
Greider CW and Blackburn EH:
Identification of a specific telomere terminal transferase activity
in Tetrahymena extracts. Cell. 43:405–413. 1985.
|
|
2
|
Shay JW and Bacchetti S: A survey of
telomerase activity in human cancer. Eur J Cancer. 33:787–791.
1997.
|
|
3
|
Cui W, Wylie D, Aslam S, et al:
Telomerase-immortalized sheep fibroblasts can be reprogrammed by
nuclear transfer to undergo early development. Biol Reprod.
69:15–21. 2003.
|
|
4
|
Bolzán AD: Chromosomal aberrations
involving telomeres and interstitial telomeric sequences.
Mutagenesis. 27:1–15. 2012.
|
|
5
|
Granger MP, Wright WE and Shay JW:
Telomerase in cancer and aging. Crit Rev Oncol Hematol. 41:29–40.
2002.
|
|
6
|
Hoos A, Hepp HH, Kaul S, Ahlert T, Bastert
G and Wallwiener D: Telomerase activity correlates with tumor
aggressiveness and reflects therapy effect in breast cancer. Int J
Cancer. 79:8–12. 1998.
|
|
7
|
Yashima K, Milchgrub S, Gollahon LS, et
al: Telomerase enzyme activity and RNA expression during the
multistage pathogenesis of breast carcinoma. Clin Cancer Res.
4:229–234. 1998.
|
|
8
|
Mokbel K and Williams NJ: Telomerase and
breast cancer: from diagnosis to therapy. Int J Surg Investig.
2:85–88. 2000.
|
|
9
|
Feng J, Funk WD, Wang SS, et al: The RNA
component of human telomerase. Science. 269:1236–1241. 1995.
|
|
10
|
Cech TR, Nakamura TM and Lingner J:
Telomerase is a true reverse transcriptase. A review. Biochemistry
(Mosc). 62:1202–1205. 1997.
|
|
11
|
Avilion AA, Piatyszek MA, Gupta J, Shay
JW, Bacchetti S and Greider CW: Human telomerase RNA and telomerase
activity in immortal cell lines and tumor tissues. Cancer Res.
56:645–650. 1996.
|
|
12
|
Ito H, Kyo S, Kanaya T, Takakura M, Inoue
M and Namiki M: Expression of human telomerase subunits and
correlation with telomerase activity in urothelial cancer. Clin
Cancer Res. 4:1603–1608. 1998.
|
|
13
|
Kyo S, Kanaya T, Takakura M, Tanaka M and
Inoue M: Human telomerase reverse transcriptase as a critical
determinant of telomerase activity in normal and malignant
endometrial tissues. Int J Cancer. 80:60–63. 1999.
|
|
14
|
Cristofari G, Adolf E, Reichenbach P, et
al: Human telomerase RNA accumulation in Cajal bodies facilitates
telomerase recruitment to telomeres and telomere elongation. Mol
Cell. 27:882–889. 2007.
|
|
15
|
Zhu Y, Tomlinson RL, Lukowiak AA, Terns RM
and Terns MP: Telomerase RNA accumulates in Cajal bodies in human
cancer cells. Mol Biol Cell. 15:81–90. 2004.
|
|
16
|
Tomlinson RL, Abreu EB, Ziegler T, et al:
Telomerase reverse transcriptase is required for the localization
of telomerase RNA to cajal bodies and telomeres in human cancer
cells. Mol Biol Cell. 19:3793–3800. 2008.
|
|
17
|
Arnoult N, Schluth-Bolard C, Letessier A,
et al: Replication timing of human telomeres is chromosome
arm-specific, influenced by subtelomeric structures and connected
to nuclear localization. PLoS Genet. 6:e10009202010.
|
|
18
|
Mahmoudi S, Henriksson S, Weibrecht I, et
al: WRAP53 is essential for Cajal body formation and for targeting
the survival of motor neuron complex to Cajal bodies. PLoS Biol.
8:e10005212010.
|
|
19
|
Tycowski KT, Shu MD, Kukoyi A and Steitz
JA: A conserved WD40 protein binds the Cajal body localization
signal of scaRNP particles. Mol Cell. 34:47–57. 2009.
|
|
20
|
Venteicher AS, Abreu EB, Meng Z, et al: A
human telomerase holoenzyme protein required for Cajal body
localization and telomere synthesis. Science. 323:644–648.
2009.
|
|
21
|
Egan ED and Collins K: An enhanced H/ACA
RNP assembly mechanism for human telomerase RNA. Mol Cell Biol.
32:2428–2439. 2012.
|
|
22
|
Smogorzewska A, van Steensel B, Bianchi A,
et al: Control of human telomere length by TRF1 and TRF2. Mol Cell
Biol. 20:1659–1668. 2000.
|
|
23
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008.
|
|
24
|
Helmstetter CE: DNA synthesis during the
division cycle of rapidly growing Escherichia coli B/r. J
Mol Biol. 31:507–518. 1968.
|
|
25
|
Hu J, Hwang SS, Liesa M, et al:
Antitelomerase therapy provokes ALT and mitochondrial adaptive
mechanisms in cancer. Cell. 148:651–663. 2012.
|
|
26
|
Henson JD, Neumann AA, Yeager TR and
Reddel RR: Alternative lengthening of telomeres in mammalian cells.
Oncogene. 21:598–610. 2002.
|
|
27
|
Slatter TL, Tan X, Yuen YC, et al: The
alternative lengthening of telomeres pathway may operate in
non-neoplastic human cells. J Pathol. 226:509–518. 2012.
|
|
28
|
Lundblad V and Blackburn EH: An
alternative pathway for yeast telomere maintenance rescues
est1-senescence. Cell. 73:347–360. 1993.
|
|
29
|
Bryan TM, Englezou A, Dalla-Pozza L,
Dunham MA and Reddel RR: Evidence for an alternative mechanism for
maintaining telomere length in human tumors and tumor-derived cell
lines. Nat Med. 3:1271–1274. 1997.
|
|
30
|
Pajalunga D, Mazzola A, Franchitto A,
Puggioni E and Crescenzi M: The logic and regulation of cell cycle
exit and reentry. Cell Mol Life Sci. 65:8–15. 2008.
|
|
31
|
Cesare AJ and Karlseder J: A three-state
model of telomere control over human proliferative boundaries. Curr
Opin Cell Biol. 24:731–738. 2012.
|
|
32
|
Verdun RE and Karlseder J: The DNA damage
machinery and homologous recombination pathway act consecutively to
protect human telomeres. Cell. 127:709–720. 2006.
|
|
33
|
Stern JL, Zyner KG, Pickett HA, Cohen SB
and Bryan TM: Telomerase recruitment requires both TCAB1 and Cajal
bodies independently. Mol Cell Biol. 32:2384–2395. 2012.
|
|
34
|
Zhong FL, Batista LF, Freund A, Pech MF,
Venteicher AS and Artandi SE: TPP1 OB-fold domain controls telomere
maintenance by recruiting telomerase to chromosome ends. Cell.
150:481–494. 2012.
|
|
35
|
Schildkraut JM, Goode EL, Clyde MA, et al:
Australian Ovarian Cancer Study Group: Single nucleotide
polymorphisms in the TP53 region and susceptibility to invasive
epithelial ovarian cancer. Cancer Res. 69:2349–2357. 2009.
|