Introduction
Fulminant acute pancreatitis (FAP) is a subgroup of
severe AP, with rapid progression to multiorgan failure within 72 h
and a high level of mortality. The common causes of pancreatitis
include cholelithiasis, alcoholism, hyperlipidemia, overeating and
idiopathic factors. In addition, pancreatic carcinoma and related
chemotherapeutic agents are rare, but potentially serious causes,
with low reported incidence rates of 0.4 (1) and 0.1–2% (2), respectively. The chemotherapeutic
agents reported to induce pancreatitis include capecitabine,
paclitaxel, bortezomib, vinorelbine and ifosfamide (3–14). The
majority of these agents, however, are mild and self-limiting. The
current study presents a case of a 62-year-old female with
pancreatic carcinoma who developed FAP following the initial course
of gemcitabine and capecitabine therapy. Patient provided written
informed consent.
Case report
A 62-year-old female presented to the China-Japan
Friendship Hospital (Beijing, China) with abdominal distension that
had persisted for two months. Laboratory tests demonstrated an
abnormal elevation of tumor markers, in particular that of
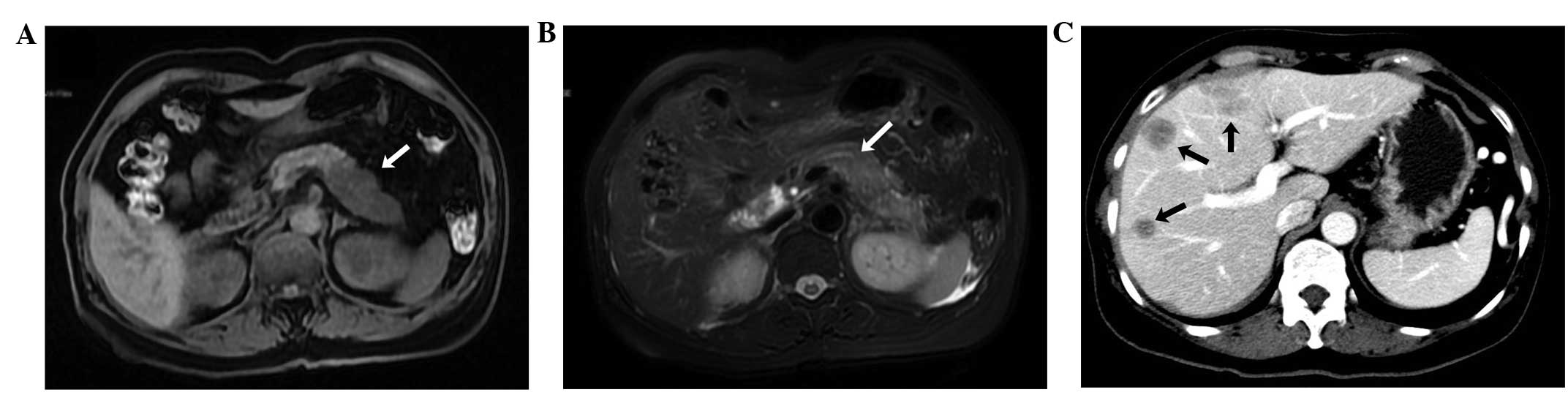
carbohydrate antigen 19–9 (>1,000 U/ml). Abdominal computed
tomography revealed a low density mass located in the body and tail
of the pancreas, as well as multiple round hypodense regions in the
liver. Pancreatic cancer was suspected, and therefore, a magnetic
resonance cholangiopancreatography (MRCP) was performed. The
results indicated that the pancreatic body and tail were distended
and non-homogeneous, with abnormal signal intensity, abrupt
interruption of the pancreatic duct and multiple nodular abnormal
signals in the liver (Fig 1). A
diagnosis of pancreatic carcinoma with liver metastasis was
determined without pathological confirmation.
On admission, the blood cell count and liver, kidney
and pancreas function tests were normal. On May 15th, 2013, the
patient, with a body surface area of 1.50 m2, received a
chemotherapy treatment regimen consisting of 1.2 g gemcitabine
intravenously on days one and eight, and 1,000 mg capecitabine
orally twice a day, on days two to 15, of a 21 day cycle. However,
during the course of the treatment, due to an elevated level of
alanine transaminase (ALT) and a low white blood cell and
neutrophil cell count, capecitabine was discontinued on the fifth
day. Reduced glutathione and Leucogen were prescribed throughout
the duration of the treatment. The subsequent gemcitabine treatment
was delayed until the 11th day when the laboratory results had
returned to normal, with the exception of the ALT level (65 IU/l;
normal range, 0–40 IU/l). Following this cycle, the patient was
discharged in a good condition. Two days later, on May 30th, the
patient developed unexplained abdominal pain, which was followed by
vomiting undigested food and clear gastric contents. Accompanied by
profuse sweating, the pain worsened, particularly in the epigastric
region, after a further two hours. The patient vomited once more,
with ~2,000 ml of watery, hemorrhagic substances. Gradually, the
patient became limp, weak and confused and was subsequently visited
the Emergency Department of the China-Japan Friendship Hospital.
The patient’s vital signs were measured and observed to be
abnormal, with a body temperature of 38.3°C, a pulse rate of
145/min, a respiratory rate of 40/min, blood pressure of 53/30 mmHg
and oxygen saturation at 60%. A normal level of consciousness was
observed and heart and lung examinations were normal. The abdomen
was flat with marked tenderness of the mid-upper section, where
muscular spasms and rebound tenderness were observed. The
laboratory tests revealed the following serum levels (normal ranges
provided in parentheses): Amylase, 346 IU/l (28–100 IU/l); lipase,
936 IU/l (0–160 IU/l); and creatinine, 140.5 μmol/l (35–106
μmol/l). The blood cell count and liver function test results are
shown in Fig. 2. Hypovolemic shock,
myelosuppression and liver and kidney dysfunction were diagnosed,
in addition to suspected AP. With continuous electrocardiography
monitoring, the use of oxygen masks and protective isolation, the
patient was admitted to the Department of Traditional Chinese
Medicine Oncology and administered intravenous fluids, dopamine to
maintain blood pressure, proton-pump inhibitors, antibiotics,
hemocoagulase and recombinant human granulocyte colony-stimulating
factor. However, the patient did not improve and gradually fell
into a coma, with a body temperature of 39°C, a rigid abdominal
bulge and a urine volume of <200 ml since the attack. At 13 h
post-admission, the serum amylase levels had increased to 938 IU/l,
while the serum lipase levels had decreased to 412 IU/l. The urine
amylase level was 2,157 IU/l (normal range, <460 IU/l) and the
procalcitonin concentration was >200 ng/ml (normal range,
<0.5 ng/ml). Ultrasonography revealed large amounts of
peritoneum effusion, a poorly visualized pancreas and an empty
bladder. Due to these observations, FAP, severe infections and
acute renal failure (ARF) were diagnosed. In spite of an attempted
rescue of 12 h, the patient succumbed to the disease. Following
this, an abdominocentesis was performed and ~300 ml dark red,
fetid, chylous ascites was withdrawn. Two days later, a small
quantity of gram-negative bacillus growth was identified in an
aerobic cultivation of the blood.
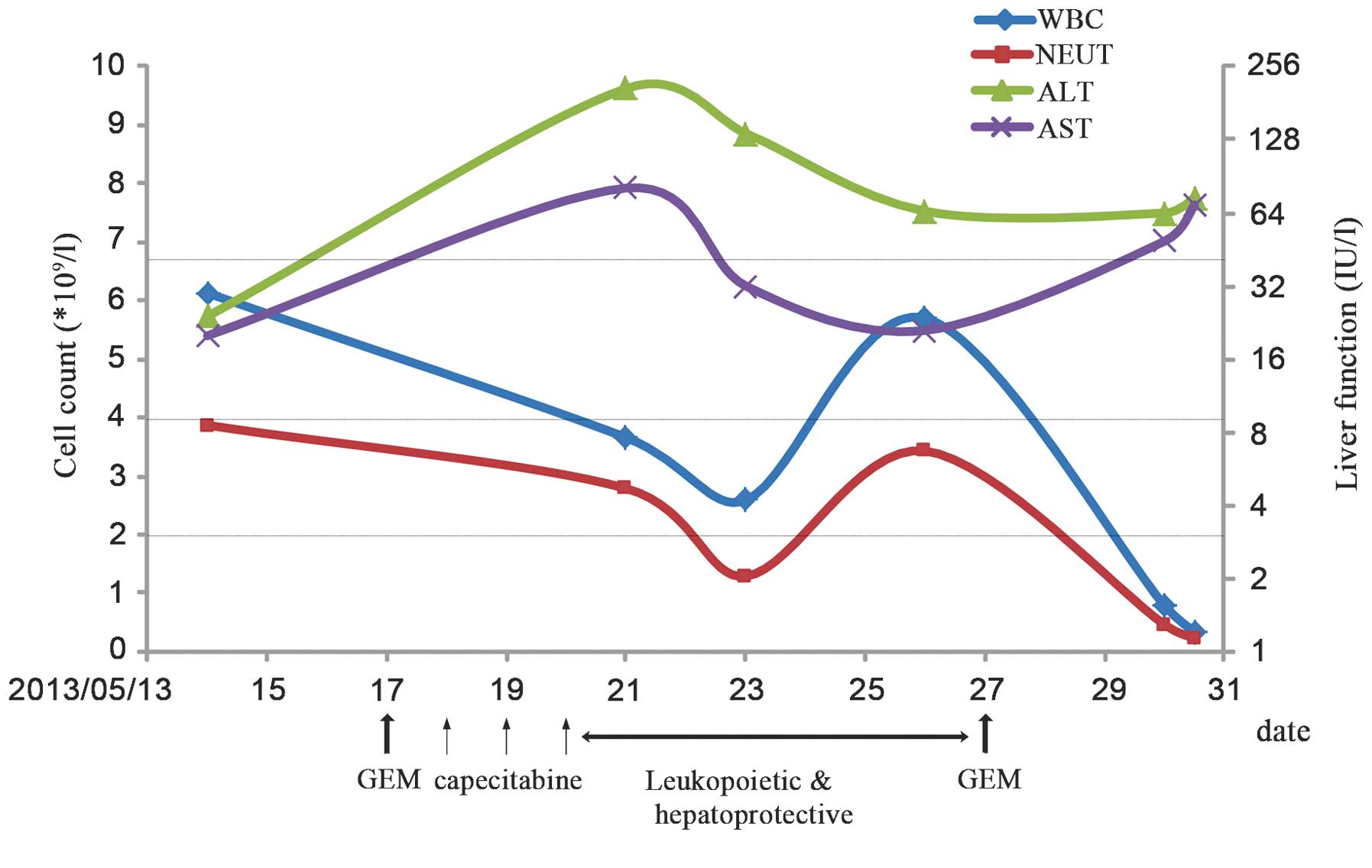 | Figure 2Chemotherapy progress. The green and
purple lines represent the upper limit of normal ALT and AST levels
(40 IU/l), respectively, with respect to the secondary axis
denoting liver function. The blue and red lines represent the lower
limit of normal WBC (4×109/l) and NEUT
(2×109/l) levels, with respect to the primary axis. GEM,
gemcitabine, WBC, white blood cell; NEUT, neutrophil, ALT, alanine
transaminase; AST, aspartate transaminase. |
Discussion
In the present study, AP with severe epigastric
pain, vomiting and elevated amylase and lipase levels, occurred
within two days of completion of the first cycle of chemotherapy.
The AP was supervened by shock, hypoxemia and the early stages of
ARF, and resulted in mortality. All these symptoms meet the
criteria for the diagnosis of FAP. The common etiologies of
pancreatitis were eliminated, and a correlation between the
incidence of FAP, and pancreatic cancer or chemotherapy, or both
was highly suspected. In this case, the interruption of the
pancreatic duct observed on MRCP may have been a risk factor for
pancreatitis. Previously reported cases of chemotherapy-induced AP
have a high variability with respect to the dose administered,
onset time following the final dose, whether or not the drug has
been used previously and the rechallenge result (Table I). AP is an infrequent complication
of chemotherapeutic agents, however, without rechallenge, the
diagnosis of chemotherapy-induced pancreatitis is difficult. A
number of the reported cases were not rechallenged with the
associated chemotherapeutic agents due to being deemed unsafe
(5,7,9,10,12,14).
While the same chemotherapy was continued with a second episode of
AP in the other studies (4,6,8,11,13).
For example, in 2010, Yucel and Warmerdam (4) reported the case of a 40-year-old
female who developed AP one day following the completion of the
third course of capecitibine. The patient recovered five days later
owing to the proper treatment. Subsequently, the patient was
administered the next course of capecitibine (rechallenge) and
again, AP occurred. Therefore, it became clear that capecibine was
the cause of AP onset. Only one reported case was rechallenged
without the occurrence of AP (3).
The patient was a 47-year-old female who developed pancreatitis
approximately two months following treatment with capecitabine
(2,500 mg/m2/day). After healing, the patient was
rechallenged with capecitabine at a much lower dose of 1,450
mg/m2/day for 14 days. Throughout this course, the
results of the laboratory tests remained normal. Therefore, further
investigation is required to determine whether or not capecitabine
is the cause of AP, as well as if the onset is in a dose-related
manner. The chemotherapeutic agents that the current patient
received were gemcitabine and capecitabine, however, the drug that
caused the episode of AP and how it was caused remain unclear. A
total of three cases of capecitabine-induced AP have been
previously published (3–5). A study by Chan et al (5) proposed that capecitabine-induced
hypertriglyceridaemia leading to AP was a possibility. However, in
the present patient, all blood lipid levels between admission and
mortality were normal. The treatment with capecitabine was
discontinued after three days due to the intolerance of the patient
to this multidrug therapy. However, the episode occurred two days
following the second infusion of gemcitabine. On consideration of
the onset time, it is more likely that gemcitabine was the
causative agent in this instance of FAP. A literature review by
Badalov et al (15) revealed
that no previous studies have been published with regard to
instances of gemcitabine-induced pancreatitis. It is possible that
additional cases may be diagnosed if amylase and lipase levels are
monitored routinely throughout the duration of gemcitabine-based
chemotherapy. With regard to the pathogenesis, if the first episode
of AP arises one day following the completion of chemotherapy, it
may be due to an allergic reaction or direct toxicity (13). The analysis of mechanism for the
incidence of FAP in the present case is as follows: i) Pancreatic
cancer with interruption of the pancreatic duct, where the
pancreatic juice is not excreted easily; ii) an allergic response
or a direct toxic effect due to the chemotherapeutic agents; and
iii) digestive disorders due to chemotherapy, nausea and
vomiting-induced bile reflux. These, and other unknown
possibilities, lead to the activation of trypsin, followed by
inflammation of pancreatic tissue caused by their own digestion.
Additionally, severe infections secondary to myelosuppression may
further aggravate the condition, leading to mortality.
Clinicians should be aware that life-threatening FAP may occur in
patients with pancreatic cancer receiving chemotherapy. Amylase and
lipase levels should be checked routinely during or after
chemotherapy if abdominal pain, nausea and vomiting appear.
Furthermore, once chemotherapy-induced AP has been observed, the
patient should not be rechalleged with the chemotherapy.
 | Table IMain characteristics of the reported
cases of chemotherapy-induced AP. |
Table I
Main characteristics of the reported
cases of chemotherapy-induced AP.
| First author/s, year
(ref.) | Age/gender | Diagnosis | Drugs/dose,
mg/m2/days | Prior use | Onset after final
dose, days | Highest serum
amylase/lipase level, IU/l | Rechallenge | Outcome/duration,
days |
|---|
| Jones and Valero,
2003 (3) | 47/F | Breast cancer with
pleural metastasis |
Capecitabine/2,500/14 | Yes | 60 | 1,157/115 | Negativea | Improved/9 |
| Yucel and Warmerdam,
2010 (4) | 40/F | Duodenal cancer |
Capecitabine/1,250/14 | Yes | 1 | 760/NA | Positive | Improved/5 |
| 1c | 140/NA | Improved/5 |
| Chan et al,
2012 (5) | 42/F | Colon cancer |
Capecitabine/2,000/14 | Yes | 2 | 2,046/NA | No attempt | Improved/21 |
| Hoff et al,
1997 (6) | 74/F | Breast cancer with
liver metastasis | Paclitaxel/1,75/3
h | No | 1 | 168/503 | Positive | Improved/23 |
| 1c | 128/586 | Deceased/16 |
| Kumar et al,
2003 (7) | 36/F | Ovarian cancer, stage
III | Paclitaxel/1,75/3
h | No | 10 | 1,831/NA | No attempt | Improved/5 |
| Elouni et al,
2010 (8) | 58/F | Myeloma |
Bortezomib/1.3/2b | No | 2 | 67/493 | Positive | Improved/8 |
| 1c | NA/477 | Improved/6 |
| Solakoglu et
al, 2013 (9) | 67/M | Multiple myeloma | Bortezomib/NA/NA | No | 4 | 354/523 | No attempt | Improved/3 |
| Tester et al,
1997 (10) | 40/F | Advanced breast
cancer |
Vinorelbine/NA/NA | Yes | 21 | 109/469 | No attempt | Improved/11 |
| Izraeli et al,
1994 (11) | 16/F | Osteosarcoma with
multiple lung, kidney and liver metastases | Ifosfamide/1,800/3 or
1,200/3 | Yes | 1 | 1378/554 | Positive | Improved/10 |
| 1c | 1,956/8,340 | Improved/10 |
| Gerson et al,
1997 (12) | 65/F | Small cell lung
cancer with right adrenal and brain metastasis |
Ifosfamide/1,200/2 | No | 2 | 452/402 | No attempt | Improved/6 |
| Hung et al,
2007 (13) | 9/M | Localized
osteosarcoma | Ifosfamide/3,000/5 or
2,400/5 | Yes | 1 | 1,173/5,027 | Positive | Improved/10 |
| 48c | 879/11,610 | Improved/18 |
| Grag et al,
2010 (14) | 7/F | Wilms tumor, stage
4 |
Ifosfamide/1,500/3 | Yes | 1 | 1,600/NA | No attempt | Improved/3 |
Acknowledgements
This study was funded by the ‘12th Five-Year’ Plan
research projects supported by the National Science and Technology
Program (grant no. 2012BAJ18B05).
References
|
1
|
Cheng L and Wang XP: Changes in etiology,
diagnosis and therapeutics in patients with acute pancreatitis
during the past ten years: A retrospective study of 725 cases. Wei
Chang Bing Xue. 5:280–283. 2004.(In Chinese).
|
|
2
|
Nitsche CJ, Jamieson N, Lerch MM and
Mayerle JV: Drug induced pancreatitis. Best Pract Res Clin
Gastroenterol. 24:143–155. 2010.
|
|
3
|
Jones KL and Valero V:
Capecitabine-induced pancreatitis. Pharmacotherapy. 23:1076–1078.
2003.
|
|
4
|
Yucel H and Warmerdam LV:
Capecitabine-induced pancreatitis. J Oncol Pharm Pract. 16:133–134.
2010.
|
|
5
|
Chan HY, Ng CM, Tiu SC, Chan OK and Shek
CC: Hypertriglyceridaemia-induced pancreatitis: a contributory role
of capecitabine? Hong Kong Med J. 18:526–529. 2012.
|
|
6
|
Hoff PM, Valero V, Holmes FA, Whealin H,
Hudis C and Hortobagyi GN: Paclitaxel-induced pancreatitis: a case
report. J Natl Cancer Inst. 89:91–93. 1997.
|
|
7
|
Kumar DM, Sundar S and Vasanthan S: A case
of paclitaxel-induced pancreatitis. Clin Oncol (R Coll Radiol).
15:352003.
|
|
8
|
Elouni B, Ben Salem C, Zamy M, Sakhri J,
Bouraoui K and Biour M: Bortezomib-induced acute pancreatitis. JOP.
11:275–276. 2010.
|
|
9
|
Solakoglu T, Akyol P, Guney T, et al:
Acute pancreatitis caused by bortezomib. Pancreatol. 13:189–190.
2013.
|
|
10
|
Tester W, Forbes W and Leighton J:
Vinorelbine-induced pancreatitis: a case report. J Natl Cancer
Inst. 89:16311997.
|
|
11
|
Izraeli S, Adamson PC, Blaney SM and Balis
FM: Acute pancreatitis after ifosfamide therapy. Cancer.
74:1627–1628. 1994.
|
|
12
|
Gerson R, Serrano A, Villalobos A,
Sternbach GL and Varon J: Acute pancreatitis secondary to
ifosfamide. J Emerg Med. 15:645–647. 1997.
|
|
13
|
Hung MC, Hung GY, Lin PC, Tiu CM and Tien
YC: Acute pancreatitis associated with ifosfamide. J Chin Med
Assoc. 70:176–179. 2007.
|
|
14
|
Grag R, Agarwala S and Bhatnagar V: Acute
pancreatitis induced by ifosfamide therapy. J Pediatr Surg.
45:2071–2073. 2010.
|
|
15
|
Badalov N, Baradarian R, Iswara K, Li J,
Steinberg W and Tenner S: Drug-induced acute pancreatitis: an
evidence-based review. Clin Gastroenterol Hepatol. 5:648–661.
2007.
|